Abstract
The antitumor effects of nonsteroidal anti-inflammatory drugs (NSAID) are assumed to be due to the inhibition of COX activity, but COX-independent mechanisms may also play an important role. NSAID-activated gene (NAG-1/GDF15) is induced by NSAIDs and has antitumorigenic activities. To determine the contribution of COX-2 inhibition and NAG-1/GDF15 expression to the prevention of colon carcinogenesis by NSAIDs, we evaluated several sulindac derivatives [des-methyl (DM)-sulindac sulfide and its prodrug DM-sulindac] that do not inhibit COX-2 activity. Sulindac sulfide and DM-sulindac induced the expression of NAG-1/GDF15 in HCT116 cells as determined by quantitative real-time PCR and Western blot. We fed APC/Min mice with 320 ppm of sulindac and doses of DM-sulindac. Only sulindac significantly inhibited tumor formation in APC/Min mice. To determine the pharmacokinetic properties of sulindac and DM-sulindac in vivo, wild-type C57/B6 mice were fed with sulindac and DM-sulindac at 80, 160, and 320 ppm. High-performance liquid chromatography analysis revealed that the conversion of DM-sulindac to DM-sulindac sulfide (active form) was less efficient than the conversion of sulindac to sulindac sulfide (active form) in the mice. Lower levels of DM-sulindac sulfide accumulated in intestinal and colon tissues in comparison with sulindac sulfide. In addition, NAG-1/GDF15 was induced in the liver of sulindac-fed mice but not in the DM-sulindac–fed mice. Collectively, our results suggest that the tumor-inhibitory effects of sulindac in APC/Min mice may be due to, in part, NAG-1/GDF15 induction in the liver. Our study also suggests that pharmacologic properties should be carefully evaluated when developing drug candidates.
Introduction
Colorectal cancer is the third most common cancer and a leading cause of cancer mortality in both men and women in the United States. An estimated 142,570 cases of colorectal cancer and 51,370 deaths from this cancer were expected to occur in 2010 (1). A significant body of evidence from epidemiological, clinical, laboratory animal, and cell culture studies demonstrate that use of nonsteroidal anti-inflammatory drugs (NSAID), such as aspirin, piroxicam, celecoxib, or sulindac are effective at inhibiting the incidence and mortality of colorectal cancer (2, 3). NSAIDs have also been associated with a reduced risk of breast, esophageal, stomach, bladder, ovary, and lung cancers (4–6).
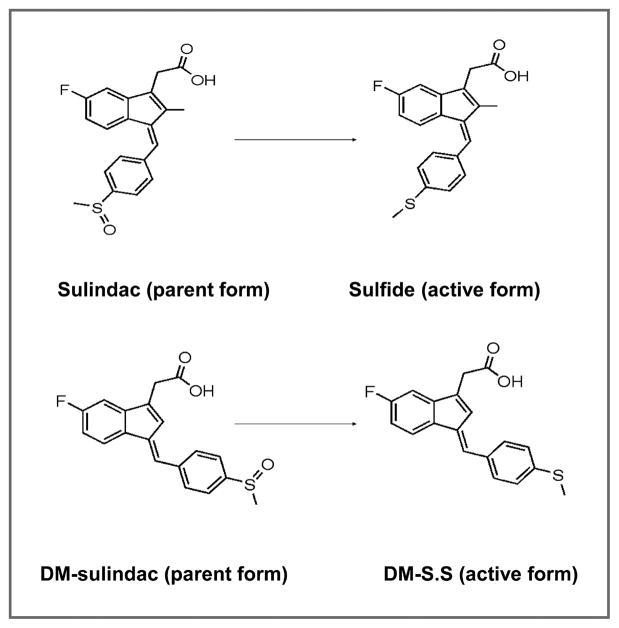
Among the NSAIDs that have been used as cancer chemopreventive agents, sulindac has been most extensively studied in both human and animals. Sulindac itself is a prodrug that is reduced by gut flora to the active metabolite, sulindac sulfide which is a potent COX inhibitor with anti-inflammatory and antitumorigenic activity (7; Fig. 1). In randomized clinical trials, sulindac is very effective in reducing the number and size of colorectal polyps in patients with familial adenomatous polyposis (FAP; refs. 8–10). Alterations of the adenomatous polyposis coli (APC) gene play a critical role in the development of FAP (11). The APC/Min mouse model is a well-established animal model of FAP of intestinal cancer and is commonly used to study the chemopreventive activity of various compounds in colorectal cancer (11). Sulindac effectively suppresses polyp formation in APC/Min mice and in other mouse models of intestinal cancer (12–14).
Figure 1.
Structures of the parent compounds sulindac and DM-sulindac, and their metabolites, sulindac sulfide and DM-sulindac sulfide (DM-S.S).
Despite extensive studies on the effectiveness of using NSAIDs as chemopreventive agents, the molecular mechanisms underlying the chemopreventive effects of NSAIDs are not completely understood. The cancer-preventive activity of NSAIDs has generally been attributed to the inhibition of COX-1/COX-2 activity and prostaglandin production. However, this concept is challenged by the fact that very high doses of sulindac are required to exhibit tumor inhibitory effects but only low doses are required for an inhibition of prostaglandin formation (15). Substantial evidence suggests that COX-independent mechanisms may be involved, and that these COX-independent effects may contribute to the chemopreventive activity of NSAIDs (15). For example, NSAIDs inhibit the growth of colon cancer cell lines that do not express COX-1 or COX-2 (16, 17) and inhibit growth of mouse embryo fibroblasts null for both COX-1 and COX-2 genes (18). Chiu et al. reported that the suppression of polyp growth by sulindac in the APC/Min mouse is independent of prostaglandin biosynthesis (12). Studies from this laboratory and other investigators suggest that NSAIDS primarily induce apoptosis independent of COX activity (19).
Our laboratory discovered that NSAIDs increase the expression of NSAID-activated gene (NAG-1/GDF15) independent of COX inhibition in human colorectal cancer cells (16). Sulindac sulfide is one of the most potent inducers of NAG-1/GDF15 expression in vitro and is the most potent COX inhibitor for the induction of NAG-1/GDF15 in human colorectal cells in culture. NAG-1/GDF15 is a member of the TGF-β superfamily that is formed as a proprotein, then cleaved and secreted (20). NAG-1/GDF15 has poorly understood biological activity. It exhibits proapoptotic, anti-inflammatory, antitumorigenic activities and inhibits intestinal tumor growth in animal and cell culture models (20). We have developed a transgenic mouse model that expresses human NAG-1 (hNAG-1) in most tissues including the intestinal tract. These mice are resistant to (azoxymethane) AOM-induced intestinal tumors (21). Crossing hNAG-1 transgenic mice with APC/Min mice results in APC/Min mice expressing hNAG-1 in the intestinal tract. These mice had fewer and smaller polyps than the wild-type APC/Min mouse not expressing hNAG-1 confirming that NAG-1 can attenuate intestinal polyp development (21).
The prodrug sulindac fed to APC/Min mice also inhibits polyp formation. However, the contribution of NAG-1/GDF15 expression to the prevention of polyp formation by sulindac has not been determined. Studies in mice are made more difficult to interpret and extrapolate to humans because the basal expression of NAG-1/GDF15 is very different in mice as compared with humans. In mice, the highest basal expression is found in the liver with little expression observed in the intestinal tract. In contrast, in humans, very low expression is detected in the liver but high expression is observed in the prostate and epithelial cells including the intestinal epithelial cells (22–24). The expression of NAG-1/GDF15 in mouse tissues, particularly the intestinal tract after sulindac feeding, has not been fully investigated. In mouse models for intestinal cancer, ample evidence links the inhibition of COX to reduction in intestinal polyps. However, additional experimental evidence is needed to understand the importance of drug-induced increases in the expression of NAG-1/GDF15 in mouse models of intestinal cancer.
The gastrointestinal and cardiovascular side effects of COX inhibitors reduce their attractiveness as chemopreventive agents, a number of sulindac derivatives (both sulfides and sulfoxides) have been synthesized that lack COX inhibitory activity. If these compounds induce NAG-1/GDF15 expression, they may be useful tools to elucidate the contribution of NAG-1/GDF15 expression in mouse models. In this report, we have examined the induction of NAG-1/GDF15 by 2 sulindac des-methyl (DM) analogues (DM-sulindac and DM-sulindac sulfide; Fig. 1) that effectively induced NAG-1/GDF15 expression in vitro in colorectal cells. Sulindac inhibited polyp development in the APC/Min mouse model and induced hepatic NAG-1/GDF15 expression whereas DM-sulindac did not inhibit polyp formation nor induce NAG-1/GDF15 expression. Pharmacokinetic analysis showed sulindac was effectively converted to sulindac sulfide, the active metabolite, whereas DM-sulindac was rapidly excreted and not efficiently converted to the active DM-sulindac sulfide metabolite. This emphasizes the importance of examining the pharmacokinetics of model chemopreventive drugs designed on the results of in vitro experiments. Our study suggests that induced expression of NAG-1/GDF15 may play an important role in sulindac reduction of intestinal polyps in mice.
Materials and Methods
Materials
Sulindac sulfide and sulindac were purchased from Sigma. DM-sulindac and DM-sulindac sulfide were synthesized as described in the Chemical Synthesis Core Facility of the Vanderbilt Institute of Chemical Biology (25). Polyclonal β-actin antibody was obtained from Abcam. Human GDF15 (NAG-1/GDF15) ELISA kit was purchased from R&D Systems. RNeasy Mini RNA extraction kit and QIAshredder kit were purchased from Qiagen. The iScript cDNA synthesis kit was purchased from Bio-Rad. The bicinchoninic acid (BCA) assay kit was purchased from Pierce. The Western LighteningTM Plus-ECL Enhanced Chemiluminescence Substrate assay kit was purchased from PerkinElmer.
Cell culture
Human colorectal cancer cell line HCT116 and mouse colorectal cancer cell line CMT93 were purchased from the American Type Culture Collection. HCA-7 cell line was a generous gift from Susan Kirkland (University of London). The HCA-7 cell line has not been tested and authenticated in our laboratory. Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C in McCoy’s 5A medium supplemented with 10% FBS. For experiments, cells were seeded at 2 × 105 cells per well in 6-well plates and incubated for 24 hours in serum-containing medium. At 80% to 90% confluence, cells were treated with sulindac sulfide or DM compounds at indicated doses for 24 hours or for different time points for time-course study. Sulindac sulfide was dissolved in ethanol and the des-methyl analogs were dissolved in either ethanol or DMSO (dimethyl sulf-oxide) according to their solubility properties.
Real-time PCR
After cells were treated with doses of sulindac sulfide or DM compounds, total RNA was extracted by RNeasy Mini kit as described by Qiagen. The total RNA from duodenum, jejunum, ileum, colon, and liver tissues of mice were also extracted by RNeasy Mini kit. The Qiagen QIAshredder was used to homogenize samples from cell culture or animal tissues. Both the quantity and quality of total RNA were analyzed by the Agilent Bioanalyzer 2100 system. Total RNA (1 μg) was reverse transcribed with iScript cDNA synthesis kit. Real-time PCR was performed to determine the expression of NAG-1/GDF15 using SYBR Green PCR master mix (Bio-Rad) on an iCycler IQ Real-Time PCR detection system (Bio-Rad). The 5′ forward and 3′ reverse PCR primers for NAG-1/GDF15 were as follows: F, TGCCCGCCAGCTACAATC; R, TCTTTGGCTAACAAGTCATCATAGGT. β-Actin was used as the reference gene for all samples.
Western blot
Cells were seeded in 6-well plates as described above. After 24 hours of treatment with sulindac sulfide or DM compounds at indicate doses in serum-free medium, cells were washed twice with phosphate-buffered saline (PBS). After aspiration, about 75 μL of RIPA (radioimmunoprecipitation assay) buffer (PBS, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, 1× complete Min-tablet, 10 mmol/L of sodium fluoride, and 1 mmol/L of sodium orthovanadate) was added to each well. Cells were quickly scraped from the plate. Cell lysates were collected using standard methods and soluble protein concentrations were determined by BCA protein assay kit. A total of 25 μg of protein were loaded onto a 4% to 15% SDS-polyacrylamide gel and electrophoresed at 170 V for 1 hour. Separated proteins were transferred onto a PVDF (poly-vinylidene difluoride) membrane at 100 V for 30 minutes on ice. After transfer, membranes were blocked with 5% nonfat dry milk in 1× TBST (Tris-buffered saline with Tween; 50 mmol/L of Tris, pH 7.5, 150 mmol/L of NaCl, 0.1% Tween-20) at room temperature for 1 hour. The membrane was probed with NAG-1/GDF15 primary antibody as described previously. The secondary antibody was anti-rabbit-HRP from Cell Signaling. The membrane was stripped using Restore Western Blot Stripping Buffer according to manufacturer’s instruction. After stripping, the membrane was probed for β-actin for loading control. The signals were detected using the western lightning Plus-ECL–enhanced chemiluminescence substrate according to manufacturer’s instruction.
APC/Min mice study
Experiments were performed in male-specific, pathogen-free mice in accordance with the NIH Guidelines for the Use and Care of Laboratory Animals, and under an approved animal protocol. All procedures in this protocol are in compliance with the Animal Welfare Act Regulations, 9 CFR 1-4. Male APC/Min mice in a C57 BL/6 background were purchased from Jackson laboratory and maintained on a standard chow diet at Integrated Laboratory Systems, Inc. (ILS) at RTP, NC. APC/Min mice were randomized into 4 groups (8 mice per group). At 11 weeks of age, control AIN76 diet, AIN76 diet supplemented with sulindac (320 ppm), or AIN76 diet supplemented with DM-sulindac (320 and 640ppm) were administered to APC/Min mice for 7 weeks. The dosed diets were prepared at ILS. Feed consumption and body weight were measured twice per week, and fresh diets were provided at least twice per week. High-performance liquid chromatography (HPLC) analysis revealed no degradation or decomposition of sulindac or DM-sulindac in the diets during feeding experiment. Each animal was observed once daily. The animals were sacrificed 7 weeks after treatment. At necropsy, mice were euthanized by CO2 inhalation and confirmed by exsanguination, blood was drawn, and the intestines were removed. The intestines were rinsed with PBS and RNA later, and then were opened longitudinally and soaked in RNA later. Intestines were divided into 3 equal sections and pinned to an index card. The number, location, and size of the intestinal polyps were recorded. After counting polyp numbers and measuring polyp sizes, 5 representative polyps from each intestine were isolated and snap-frozen for biological analysis.
Pharmacokinetic study
A total of 48 C57/BL6 wild-type mice at 8 to 9 weeks of age were randomized into 16 groups (3 mice per group). Four groups of mice were treated with AIN76 control diet or ANI76 diet supplemented with 80, 160, and 320 ppm of sulindac for 14 days. A second set of 4 groups was on the same diet for 28 days rather than 14. A third set of 4 groups of mice were treated with AIN76 diet, AIN76 diet supplemented with 80, 160, and 320 ppm of DM-sulindac for 14 days. And a set of fourth 4 groups of mice were subjected to this DM-sulindac diet for 28 days instead of 14. Food consumption and body weight were measured twice per week and mice were observed once daily. No toxicity was observed in any groups during the treatments. Approximately 24 hours before necropsy, each individual mouse was placed in metabolism cage to collect urine. All mice were on diet continuously and there was no or little delay between blood collection and feeding. All the mice were bled within a small variable of time which was controlled between groups. At necropsy, mice were euthanized as described above and blood was drawn. Tissues from the 3 sections of the small intestines (duodenum, jejunum, and ileum), colon, and liver were isolated and snap-frozen for metabolism analysis and NAG-1/GDF15 induction analysis. For the study of the pharmacokinetic properties of higher doses of DM-sulindac, additional mice (3 per group) were treated with either the AIN76 control diet, or AIN76 diet supplemented with 640, 1,000, 1,500, 2,000, or 2,500 ppm of DM-sulindac for 14 days. There was no toxicity observed in the mice during treatment. Mice were sacrificed and samples were collected same as above.
Chromatography
Tissue, urine, and blood samples from dosed animals were prepared for HPLC analysis. Briefly, solid tissue samples were homogenized and then purified by either solid-phase extraction (SPE) or liquid–liquid extraction. Liquid samples were simply diluted. Detailed sample preparation procedures are described in Supplemental Data. All samples were subject to HPLC analysis as described in detail in Supplemental Data.
Statistical analysis
Data are all represented as means ± SD. Comparisons among multiple groups were performed by ANOVA with Tukey’s post hoc comparisons. A P value of < 0.05 was considered statistically significant. All the analyses were performed with the use of SAS Eguide software.
Results
Sulindac sulfide and DM-sulindac sulfide induce NAG-1/GDF15
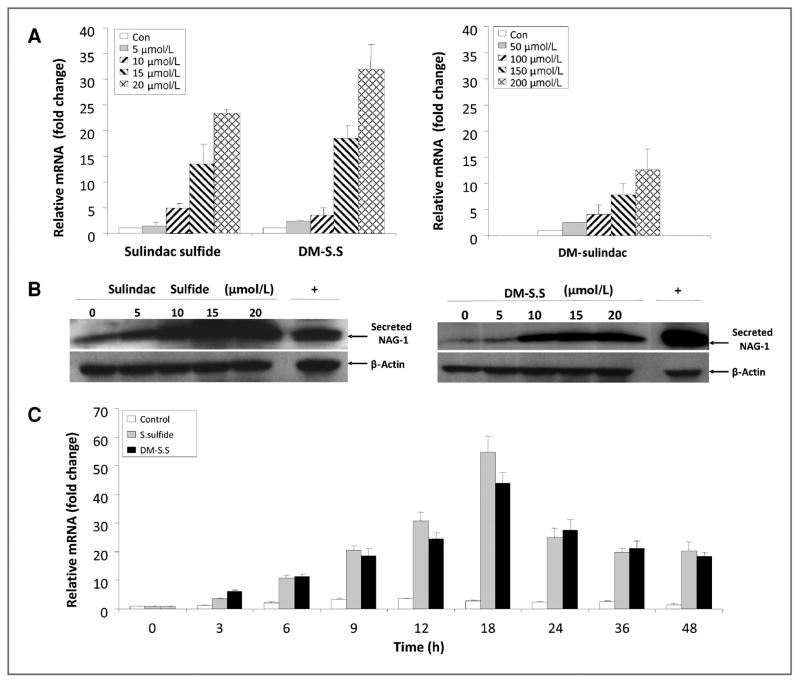
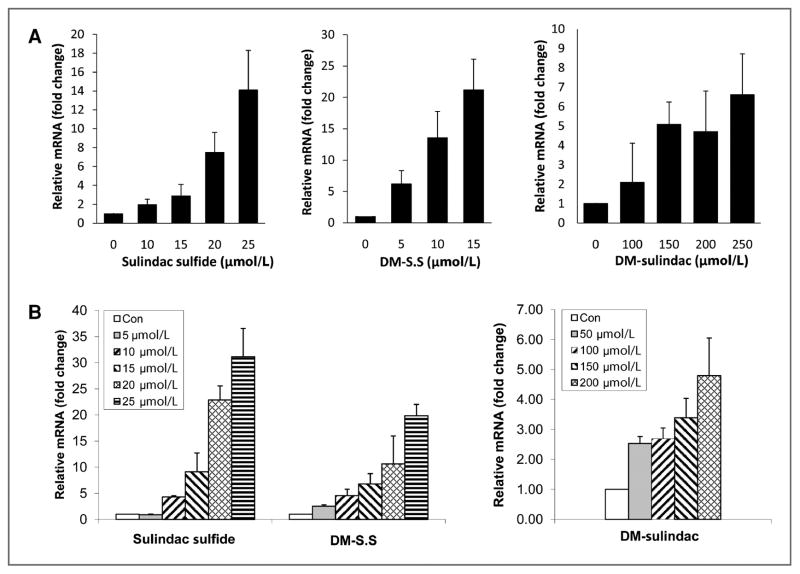
To determine the activity in inducing NAG-1/GDF15 expression, a total of 8 sulindac sulfide analogs were incubated in HCT116 cells and the expression of NAG-1/GDF15 was measured by quantitative real-time PCR (qRT-PCR) analysis. Seven of the 8 sulindac derivatives were indeed able to induce NAG-1/GDF15 mRNA upregulation in HCT116 cells with the most potent being DM-sulindac sulfide. The structures and NAG-1/GDF15-inducing activity of the analogs is shown in Supplemental Data Table 1. We investigated further the activity of DM-sulindac sulfide and compared it with sulindac sulfide with respect to concentration and time of increased expression. As expected, sulindac sulfide significantly increased the NAG-1/GDF15 expression at both the mRNA and protein levels in a dose-dependent manner at concentrations ranging from 5 to 20 μmol/L after 24 hours of treatment in HCT116 cells (Fig. 2A and B). Similar to sulindac sulfide, DM-sulindac sulfide (5–20 μmol/L) significantly upregulated NAG-1/GDF15 mRNA and protein expression in a dose-dependent manner in HCT116 cells after 24 hours of treatment (Fig. 2A and B). Both sulindac sulfide and DM-sulindac sulfide at concentrations that were higher than 20 μmol/L induced toxicity to HCT116 cells. DM-sulindac, the sulfoxide and parent compound of DM-sulindac sulfide only significantly induced NAG-1/GDF15 expression at much higher concentrations (50–200 μmol/L) with minimal toxicity to the cells (Fig. 2A). The induction of NAG-1/GDF15 mRNA upregulation by both sulindac sulfide and DM-sulindac sulfide was increased in a time-dependent manner in HCT116 cells. Both compounds at 20 μmol/L induced NAG-1/GDF15 mRNA upregulation within 3 hours and maximum expression was reached by 18 hours (Fig. 2C). The upregulation of NAG-1/GDF15 mRNA expression by the 2 compounds remained high after 48 hours. Thus, the sulindac metabolite, sulindac sulfide, and the metabolite of DM-sulindac, DM-sulindac sulfide, are similar with respect to the induction of NAG-1/GDF15. Like sulindac, DM-sulindac is not a COX inhibitor. However, whereas sulindac sulfide is a potent, nonselective inhibitor of COX-2, DM-sulindac sulfide is a weak selective inhibitor of COX-1; it does not inhibit COX-2 (26). Because HCT116 cells are devoid of COX-2 expression (27), we treated another human colon adenocarcinoma cell line, HCA-7, which highly expresses COX-2 (27), with several concentrations of sulindac sulfide and DM-sulindac sulfide for 24 hours. Both compounds were potent at inducing NAG-1/GDF15 expression in HCA-7 cells and thus the expression of COX-2 does not correlate with the sensitivity to sulindac sulfide induction of NAG-1/GDF15 (Fig. 3A). Sulindac sulfide and DM-sulindac sulfide also induced NAG-1/GDF15 in mouse colon cancer cells. CMT93 cells were incubated with sulindac sulfide, DM-sulindac, and DM-sulindac sulfide, and mNAG-1/GDF15 mRNA was measured by qRT-PCR. As shown in Figure 3B, DM-sulindac sulfide and sulindac sulfide increased the expression of mNAG-1/GDF15; but, DM-sulindac sulfide was less potent than observed in the human colorectal cell lines. As expected, DM-sulindac was a very poor inducer of NAG-1/GDF15 expression in the mouse cells (Fig. 3B). Collectively, these data suggest that although sulindac sulfide and DM-sulindac sulfide differ in COX inhibition, they exhibit similar activity for inducing NAG-1/GDF15 expression in vitro in cell lines.
Figure 2.
NAG-1/GDF15 is highly induced in HCT116 cells by sulindac sulfide and DM-sulindac sulfide. A, sulindac sulfide, DM-sulindac sulfide (left), and DM-sulindac (right) at indicated doses induced NAG-1/GDF15 mRNA expression in HCT116 cells in a dose-dependent manner. HCT116 cells were seeded at 2 × 105 cells per well in 6-well plates. At 80% to 90% confluence, cells were treated with vehicle control (0 μmol/L), sulindac sulfide (5, 10, 15, and 20 μmol/L), DM-sulindac sulfide (5, 10, 15, and 20 μmol/L), or DM-sulindac (50, 100, 150, and 200 μmol/L) for 24 hours in serum-free McCoy’s 5 medium. Cells were collected and subjected to real-time PCR analysis. Data were obtained from 3 independent experiments. B, Western blot analysis showing that sulindac sulfide or DM-sulindac sulfide at 5, 10, 15, and 20 μmol/L of treatment for 24 hours induced NAG-1/GDF15 upregulation compared with vehicle control in HCT116 cells. Cell lysates from HCT116 cells transiently transfected with NAG-1/GDF15 plasmid was used as positive control. β-Actin was used for loading control. C, sulindac sulfide and DM-sulindac sulfide treatment at 20 μmol/L induced NAG-1/GDF15 mRNA upregulation in a time-dependent manner. HCT116 cells at 80% confluence were treated with 20 μmol/L of sulindac sulfide or DM-sulindac sulfide for 0, 3, 6, 9, 12, 18, 24, 36, and 48 hours. Cells were collected and subjected to real-time PCR. HCT116 cells treated with vehicle control were also collected at the above indicated time points when collecting treated cells. The results represent mean ± SD.
Figure 3.
A, NAG-1/GDF15 is induced in COX-2–expressing HCA-7 cells by sulindac sulfide and DM-sulindac compounds. Sulindac sulfide, DM-sulindac sulfide, and DM-sulindac at indicated doses induced NAG-1/GDF15 mRNA expression in HCA-7 cells in a dose-dependent manner. B, NAG-1/GDF15 is highly induced in mouse colorectal cancer CMT93 cells by sulindac sulfide and DM-sulindac compounds. Sulindac sulfide, DM-sulindac sulfide (left), and DM-sulindac (right) at indicated doses induced mNAG-1/GDF15 mRNA expression in CMT93 cells in a dose-dependent manner. Cells were seeded at 2 × 105 cells per well in 6-well plates. At 80% to 90% confluence, cells were treated with vehicle control (0 μmol/L), sulindac sulfide, DM-sulindac sulfide, or DM-sulindac for 24 hours in serum-free McCoy’s 5 medium. Cells were collected and subjected to real-time PCR analysis. Data were obtained from 3 independent experiments. The results represent mean ± SD.
Sulindac feeding, but not DM-sulindac, inhibits polyp formation in APC/Min mice
Many studies have shown sulindac to be effective in the inhibition of polyp formation in the APC/Min mouse model. To determine whether DM-sulindac could elicit similar antitumorigenic activity as sulindac, APC/Min mice were fed with 320 ppm of sulindac, 320 and 640 ppm of DM-sulindac for 7 weeks. The 320 ppm of sulindac was chosen because this dose effectively inhibited polyp growth in APC/Min mice studies without causing toxicity to the mice as reported by many investigators. Initial toxicity studies suggested that the C57/BL6 wild-type mice could tolerate 640 ppm of DM-sulindac; therefore, 320 and 640 ppm doses of DM-sulindac were used. The general health of APC/Min mice was not affected by sulindac and DM-sulindac treatment and no statistical difference in body weight gain among groups was observed. However, the sulindac-feeding group consumed significantly more food than the controls whereas the mice fed 640 ppm of DM-sulindac ate less food (data not shown). The APC/Min mice maintained on the control diet developed 29.6 ± 13.6 polyps per mouse (Table 1). As expected, sulindac at 320 ppm significantly reduced polyp formation by 61% and only 11.4 ± 4.8 polyps per mouse were observed at necropsy. DM-sulindac had no effect on tumor inhibition at 320 ppm and only slightly but not significantly reduced polyp formation at 640 ppm. There was no significant difference in polyp sizes between the groups (data not shown) and polyps were observed throughout the 3 sections of the small intestines (duodenum, jejunum, and ileum), but were rarely observed in colon section of the mice. Thus, under these experimental conditions, sulindac was an effective inhibitor of polyp formation in the APC/Min mice, whereas DM-sulindac was not.
Table 1.
Polyp formation in APC/Min mice after treatment
| Treatment | Dose, ppm | n | Polyp count ± SD |
|---|---|---|---|
| Control | 0 | 8 | 29.6 ± 13.59 |
| Sulindac | 320 | 8 | 11.4 ± 4.8a |
| DM-sulindac | 320 | 7 | 27.4 ± 11.98 |
| DM-sulindac | 640 | 7 | 21.1 ± 10.22 |
Note: Values are means ± SD.
P < 0.05 versus controls.
Pharmacokinetic analysis of sulindac and DM-sulindac
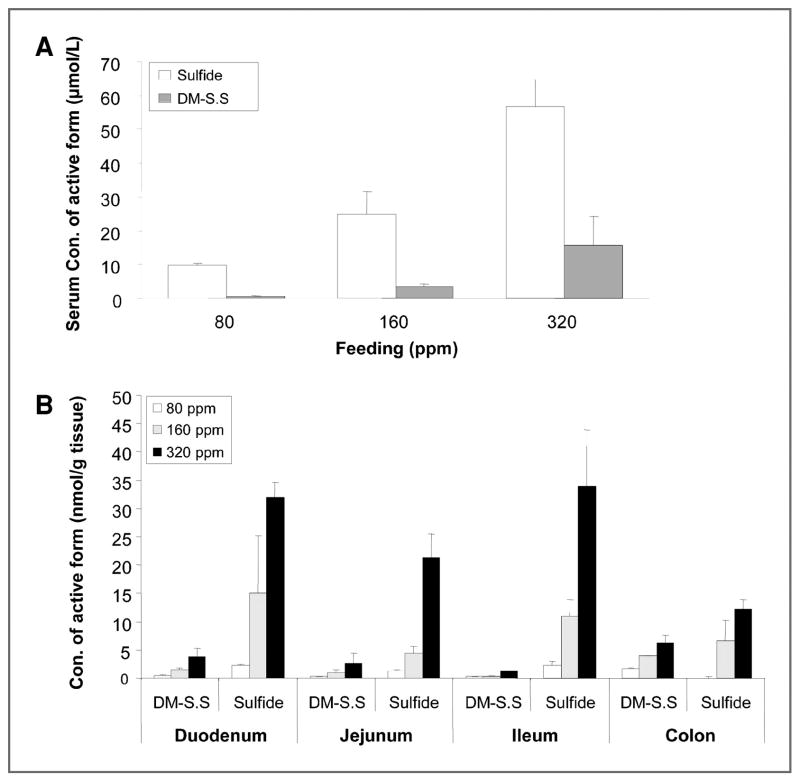
One possible explanation of the lack of chemopreventive activity for DM-sulindac is a difference in the pharmacokinetics and/or poor conversion of DM-sulindac to the active metabolite, DM-sulindac sulfide. Sulindac and DM-sulindac at 80, 160, and 320 ppm were fed to C57/BL6 wild-type mice for 14 and 28 days. HPLC analysis of the serum metabolites revealed that sulindac was metabolized into sulindac sulfone, a non-COX inhibitory metabolite, achieving serum concentrations of 66, 88, and 75 μmol/L, respectively with sulindac dosing at 80, 160, and 320 ppm for 14 days (data not shown). Sulindac sulfide, the active metabolite was detected in the serum at 19.9, 24.9, and 56.8 μmol/L, respectively after sulindac dosing at above doses after 14 days (Fig. 4A). Very small amounts of the sulindac and sulfone were detected in the urine samples, and no sulfide was detected in urine after sulindac dosing (data not shown). In contrast, only a small amount of DM-sulindac sulfide was detected in the serum with 0.5, 3.5, and 15.6 μmol/L after DM-sulindac dosing at 80, 160, and 320 ppm (Fig. 4A). Very small amounts of the parent DM-sulindac and DM-sulindac sulfone were detected in the serum after DM-sulindac dosing. However, urinary analysis demonstrated that majority of the parent compound, DM-sulindac, was excreted into the urine. In addition, higher doses of DM-sulindac (640, 1,000, 1,500, 2,000, and 2,500 ppm) failed to increase the level of active metabolite, the DM-sulindac sulfide in serum (data not shown). Pharmacokinetic analysis of the mice-fed sulindac or DM-sulindac for 28 days exhibited a similar metabolic pattern (data not shown). The high level of excretion of DM-sulindac into urine contrasts sharply with the disposition of sulindac and may account for its poor conversion to DM-sulindac sulfide.
Figure 4.
A, serum level of the active metabolite is substantially higher in sulindac-fed mice than DM-sulindac–fed mice after 14 days of feeding. C57/BL6 wild-type mice (3 per group) were fed with control AIN76 diet, or AIN76 diets supplemented with 80, 160, or 320 ppm of sulindac or DM-sulindac for 14 or 28 days. At necropsy, blood was collected and serum was isolated followed by HPLC analysis. Sulfide represents the active metabolite after sulindac feeding, and DM-S.S represents the active metabolite after DM-sulindac feeding. B, the tissue accumulation of the active metabolite is much higher in mice fed with sulindac than in mice fed with DM-sulindac for 14 days. At necropsy, sections of duodenum, jejunum, ileum, and colon tissues were excised and processed for HPLC analysis for tissue accumulation of the sulfur metabolites. Sulfide represents the active metabolite after sulindac feeding, and DM-S.S represents the active metabolite of DM-sulindac. Results from control diet feeding were not shown, and only results from 14 days feeding are shown. Levels of the metabolites of each analyte are given in nmol/g of wet tissue. The results represent mean ± SD (n = 3).
We also examined the accumulation of sulindac or DM-sulindac metabolites in small intestinal and colon tissues of the mice by HPLC-UV analysis. Similar to results from analysis of drug concentrations in the serum, we found that much higher concentrations of the sulindac sulfide as compared with DM-sulindac sulfide in the duodenum, jejunum, and ileum of small intestinal tissues (Fig. 4B). In colon tissue, only slightly higher concentrations of sulindac sulfide after sulindac feeding were detected than DM-sulindac sulfide after DM-sulindac feeding (Fig. 4B). Similarly, higher doses of DM-sulindac (640, 1,000, 1,500, 2,000, and 2,500 ppm) failed to increase the level of active metabolite, the DM-sulindac sulfide in small intestinal and colon tissues (data not shown). Thus, the lack of tumor inhibitory effects by DM-sulindac in the APC/Min study may due to the inefficiency in the metabolism of the parent compound, DM-sulindac, into the active compound, DM-sulindac sulfide, in mice.
NAG-1/GDF15 induction is observed in liver, but not in small intestinal and colon tissues
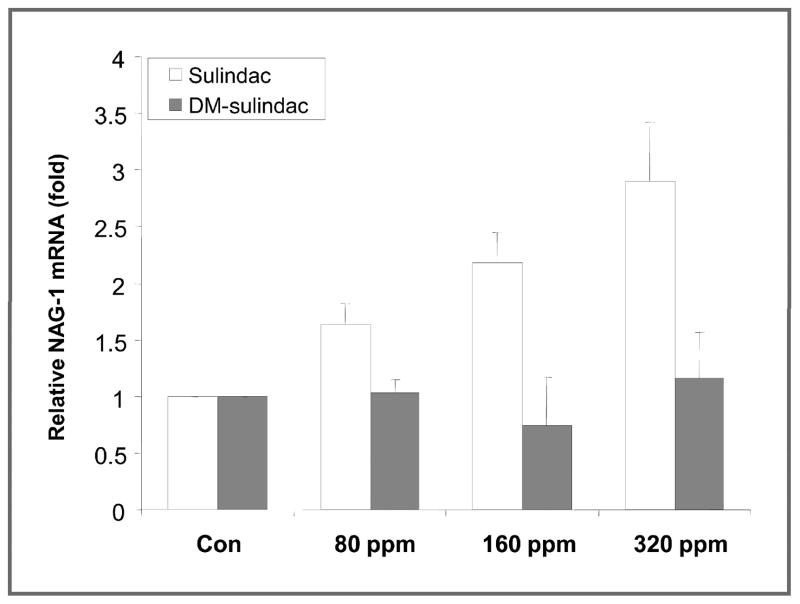
Analysis of the expression of NAG-1/GDF15 in the polyps and normal tissue from the APC/Min mice fed either sulindac or DM-sulindac was done by qRT-PCR because no suitable antibody or ELISA is available to measure the mNAG-1/GDF15 expression in tissue or circulating level in serum. The NAG-1/GDF15 expression in these intestinal tissues was very low and was not increased by either sulindac or DM-sulindac feeding. To investigate this more extensively, C57/BL6 wild-type mice were treated with several doses of sulindac or DM-sulindac. The expression of NAG-1/GDF15 in duodenum, jejunum, ileum, and colon tissues of the mice was measured by qRT-PCR. NAG-1/GDF15 expression was very low in small intestinal and colon tissues and no induction of NAG-1/GDF15 was detected in any of the intestinal tissues from the mice treated with sulindac or DM-sulindac as compared with the control group (data not shown). Because liver is the major organ expressing NAG-1/GDF15 in mice, we decided to examine whether hepatic NAG-1/GDF15 expression was altered by drug treatment. Measurement of mRNA levels of NAG-1/GDF15 indicated high basal liver expression of NAG-1/GDF15, which was significantly induced in dose-dependent manner. A 1.6-, 2.2-, and 2.9-fold increase was observed upon feeding with 80, 160, and 320 ppm of sulindac, respectively (Fig. 5). However, DM-sulindac feeding at all doses failed to induce NAG-1/GDF15 mRNA expression in mouse liver. Because NAG-1/GDF15 is a secreted protein and present in the serum, the increase in liver NAG-1/GDF15 expression suggest an increase in the concentration of circulating NAG-1/GDF15 in serum after sulindac feeding.
Figure 5.
NAG-1/GDF15 mRNA expression was upregulated upon sulindac feeding in liver tissue after 4 weeks of treatment. C57/BL6 wild-type mice (3 per group) were fed with control AIN76 diet, and AIN76 diets were supplemented with 80, 160, and 320 ppm of sulindac or DM-sulindac for 4 weeks. At necropsy, liver tissue was collected and subjected to RNA extraction followed by qRT-PCR analysis. The results represent mean ± SD (n = 3).
Discussion
NAG-1/GDF15, a member of the TGF-β superfamily, was identified by PCR-based subtractive hybridization using a NSAID-induced library (28). NAG-1/GDF15 has been given a variety of names including macrophage inhibitory cytokine 1 (MIC-1; ref. 29), growth differentiation factor 15 (GDF15; ref. 22), placental bone morphogenetic protein (PLAB; ref. 30), placental transformation growth factor β (PTGF-β; ref. 31), and prostate-derived factor (PDF; ref. 32). NAG-1/GDF15 may elicit diverse biological activity in different tissues or cell types. However, the precise biological functions of NAG-1/GDF15 are not well understood and its downstream signaling pathways have not been identified (33). A substantial number of in vitro studies have demonstrated that NAG-1/GDF15 expression is induced by a large number of antitumorigenic agents, such as NSAIDs (16), genistein (34), peroxisome proliferator-activated receptor γ ligands, such as troglitazone (35), retinoids (36), resveratrol (37), and diallyl disulfide (38). Further studies to determine the mechanisms for the induction of NAG-1/GDF15 by these chemicals showed the expression is regulated by 3 tumor suppressor pathways, p53, Egr-1, and AKT (39). The presence of p53-binding sites and an Egr-1 site, overlapping with Sp1-binding sites, were confirmed in previous studies (20). Previous studies from our laboratory have shown that hNAG-1 (human transgene) transgenic (NAG-Tg) mice developed about 50% fewer aberrant crypt foci and no tumors in intestinal tissues as compared with nontransgenic littermates after treatment with AOM (21). In addition, crossing NAG-Tg with APC/Min mice results in significantly reduced polyp formation (40%) compared with APC/Min littermates (21). A more recent study suggests that the NAG-Tg mice are less sensitive to urethane-induced lung tumorigenesis (40). The increase in the expression by cancer prevention drugs and chemicals coupled with the observed decrease in intestinal (21) and pulmonary tumors in NAG-1/GDF15 transgenic mouse suggest that NAG-1/GDF15 may act as a tumor suppressor gene. Despite a large body of evidence from in vitro studies suggesting the induction of NAG-1/GDF15 by sulindac or other chemopreventive agents may exert anti-inflammatory and antitumorigenic activities, limited in vivo animal studies are available to support this notion. Sulindac effectively inhibits tumor formation in many models, especially in the APC/Min colorectal cancer model and is one of the most potent NAG-1/GDF15 inducers in vitro. It is not clear whether NAG-1/GDF15 induction by sulindac sulfide observed in vitro also occurs in mouse models and whether NAG-1/GDF15 is essential or at least partially contributes to the tumor inhibitory effects of sulindac.
Prolonged use of NSAIDs, which is required for cancer prevention, is compromised by the side effects of these drugs. Therefore, we developed sulindac derivatives with greatly reduced COX inhibitory activity but with off-target effects comparable with sulindac sulfide. DM-sulindac sulfide was also able to induce NAG-1/GDF15 expression in HCT116 cells lacking COX-2 expression and in HCA-7 expressing COX-2. In HCT116 cells, which we used as a standardized cell assay to investigate NAG-1/GDF15 expression by chemicals, DM-sulindac sulfide is more potent than sulindac sulfide in increasing NAG-1/GDF15 expression. The NAG-1/GDF15 induction was also observed in mouse intestinal cells in culture. The structural similarity between sulindac sulfide and DM-sulindac sulfide (Fig. 1) may explain their similar effects in inducing NAG-1/GDF15 in vitro, which suggests that they may elicit similar biological functions such as tumor inhibition in vivo. Preliminary experiments indicated that DM-sulindac sulfide was degraded in diets so the sulfoxide was fed with the expectation that it would be reduced to the sulfide in vivo in a fashion analogous to sulindac.
At similar doses to sulindac (320 and 640 ppm), dietary DM-sulindac failed to inhibit tumor formation in APC/Min mice and increase the expression of NAG-1/GDF15 in all the tissue examined. In contrast, feeding sulindac, the prodrug for sulindac sulfide, at 320 ppm significantly reduced tumor formation by 61% in APC/Min mice compared with mice fed with control diet. Pharmacokinetic analysis revealed upon feeding equivalent doses of sulindac or DM-sulindac to mice, that sulindac is much more efficiently converted to the active form, sulindac sulfide, as determined in serum or intestinal tissues. However, DM-sulindac was poorly converted to DM-sulindac sulfide and only low levels were detected in serum or small intestinal tissues. The majority of the DM-sulindac was excreted in the urine, which probably accounts for its poor conversion to DM-sulindac sulfide in vivo. We also found that further increasing the doses of DM-sulindac failed to achieve equivalent concentrations of DM-sulindac sulfide to those observed on feeding 320 ppm of sulindac in mice. Thus, the ineffectiveness of DM-sulindac in inhibiting tumor formation in APC/Min mice appears to be due to the inadequate concentrations of the active form, DM-sulindac sulfide, in blood and tissue. However, APC/Min mice model is limited because tumors are rarely observed in colon tissue with majority found in small intestines. Interestingly, we observed that the levels of the active metabolites were higher in the colon tissue than the small intestine (Fig. 4B). These raise the question of whether DM-sulindac might still be active in the more representative mouse models of human colonic tumorigenesis in which tumors are induced in the colon such as AOM model. More studies will be conducted in future to address this question using AOM model.
Although DM-sulindac did not induce NAG-1/GDF15 expression in vivo and was ineffective in inhibiting tumor formation in APC/Min mice, analysis of NAG-1/GDF15 expression after sulindac treatment did provide some clue as to the contribution of NAG-1/GDF15 induction in sulindac-induced tumor inhibition in this mouse model. NAG-1/GDF15 is a secreted protein and present in circulating blood of normal patients at 400 to 600 pg/mL and can be as high at 20 ng/mL in certain disease states or in pregnancy. In the NAG-1/GDF15 transgenic mouse, a serum concentration of the human NAG-1/GDF15 from 20 to 40 ng/mL is observed. The expression of NAG-1/GDF15 is high in mouse liver and is likely the major source of increasing circulating NAG-1/GDF15 upon feeding with sulindac. Indeed, we observed that the mouse NAG-1/GDF15 was significantly induced in liver in a dose-dependent manner upon sulindac feeding. In contrast, NAG-1/GDF15 was below the limit of detection by real-time PCR in small intestinal and colon tissues and not induced by the sulindac feeding. In a previous study, feeding C57/BL6 mice treated with sulindac developed fewer tumors and NAG-1/GDF15 was induced in liver (24). Collectively, these observations suggest that NAG-1/GDF15 is increased in the liver tissue upon sulindac treatment, and that NAG-1 is then secreted from liver cells into circulation and transported to small intestinal tissue where it elicits its antitumorigenic activity. Unfortunately, we could not measure the circulating level of NAG-1/GDF15 in the serum of the APC/Min and C57/BL6 wild-type mice due to the lack of an antibody for the measurement of mouse NAG-1/GDF15. Interestingly, a recent study from Zimmers et al. supports the importance of circulating NAG-1/GDF15 in sulindac inhibition of polyps in the APC/Min mice (41). Crossing the APC/Min mouse with a NAG-1/GDF15−/− mice did not alter polyp formation, a finding in agreement with the lack of NAG-1/GDF15 expression in the intestinal tract. Sulindac reduced polyp formation by 4-fold in APC/Min mice which express wild-type NAG-1/GDF15. However, sulindac did not reduce polyp formation in NAG-1/GDF15−/− crossed with APC/Min mouse model (41). This finding indicates that NAG-1/GDF15 production is required for antitumorigenic activity of sulindac in the APC/Min mouse model. Although results from this research group demonstrated that NAG-1/GDF15 is essential for antitumorigenic activity of sulindac, further studies are needed to determine whether NAG-1/GDF15 is indeed induced in the NAG-1/GDF15+/+ APC/Min mice in future.
In summary, we conclude that NAG-1/GDF15 may contribute, in part, to sulindac-induced tumor inhibition in APC/Min mouse model with the liver tissue serving as a major source of secreted NAG-1/GDF15 upon sulindac treatment.
Supplementary Material
Acknowledgments
We thank Glenda Moser and Jeff Davis at Integrated Laboratory Systems, Inc for their work on animal feeding studies.
Grant Support
This research was supported by the intramural Research Program of the NIH, National Institute of Environmental Health Sciences; NIH grant CA89450, and funds from National Foundation for Cancer Research.
Footnotes
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cancer Facts and Figures 2010. American Cancer Society; http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. [Google Scholar]
- 2.Baron JA. Aspirin and NSAIDs for the prevention of colorectal cancer. Recent Results Cancer Res. 2009;181:223–9. doi: 10.1007/978-3-540-69297-3_21. [DOI] [PubMed] [Google Scholar]
- 3.Iwama T. NSAIDs and colorectal cancer prevention. J Gastroenterol. 2009;44(Suppl 19):72–6. doi: 10.1007/s00535-008-2265-7. [DOI] [PubMed] [Google Scholar]
- 4.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–52. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 5.Olsen JH, Friis S, Poulsen AH, Fryzek J, Harving H, Tjonneland A, et al. Use of NSAIDs, smoking and lung cancer risk. Br J Cancer. 2008;98:232–7. doi: 10.1038/sj.bjc.6604151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, et al. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117:141–50. doi: 10.1007/s10549-008-0228-6. [DOI] [PubMed] [Google Scholar]
- 7.Ciolino HP, Bass SE, MacDonald CJ, Cheng RY, Yeh GC. Sulindac and its metabolites induce carcinogen metabolizing enzymes in human colon cancer cells. Int J Cancer. 2008;122:990–8. doi: 10.1002/ijc.23218. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–5. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 9.Giardiello FM. NSAID-induced polyp regression in familial adenomatous polyposis patients. Gastroenterol Clin North Am. 1996;25:349–62. doi: 10.1016/s0889-8553(05)70251-x. [DOI] [PubMed] [Google Scholar]
- 10.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–9. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 11.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–60. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 12.Chiu CH, McEntee MF, Whelan J. Sulindac causes rapid regression of preexisting tumors in Min/+mice independent of prostaglandin biosynthesis. Cancer Res. 1997;57:4267–73. [PubMed] [Google Scholar]
- 13.Guillen-Ahlers H, Buechler SA, Suckow MA, Castellino FJ, Ploplis VA. Sulindac treatment alters collagen and matrilysin expression in adenomas of ApcMin/+mice. Carcinogenesis. 2008;29:1421–7. doi: 10.1093/carcin/bgn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon Y, Bottone FG, Jr, McEntee MF, Eling TE. Suppression of tumor cell invasion by cyclooxygenase inhibitors is mediated by thrombospondin-1 via the early growth response gene Egr-1. Mol Cancer Ther. 2005;4:1551–8. doi: 10.1158/1535-7163.MCT-05-0213. [DOI] [PubMed] [Google Scholar]
- 15.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res. 2009;2:572–80. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–8. [PubMed] [Google Scholar]
- 17.Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9:383–90. [PubMed] [Google Scholar]
- 18.Zhang X, Morham SG, Langenbach R, Young DA. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med. 1999;190:451–59. doi: 10.1084/jem.190.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek SJ, Eling TE. Changes in gene expression contribute to cancer prevention by COX inhibitors. Prog Lipid Res. 2006;45:1–16. doi: 10.1016/j.plipres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Eling TE, Baek SJ, Shim M, Lee CH. NSAID activated gene (NAG-1), a modulator of tumorigenesis. J Biochem Mol Biol. 2006;39:649–55. doi: 10.5483/bmbrep.2006.39.6.649. [DOI] [PubMed] [Google Scholar]
- 21.Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–60. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Bottner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1) Gene. 1999;237:105–11. doi: 10.1016/s0378-1119(99)00309-1. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20:3742–51. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, Eling TE. Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology. 2002;122:1388–98. doi: 10.1053/gast.2002.32972. [DOI] [PubMed] [Google Scholar]
- 25.Felts AS, Ji C, Stafford JB, Crews BC, Kingsley PJ, Rouzer CA, et al. Desmethyl derivatives of indomethacin and sulindac as probes for cyclooxygenase-dependent biology. ACS Chem Biol. 2007;2:479–83. doi: 10.1021/cb700077z. [DOI] [PubMed] [Google Scholar]
- 26.Walters MJ, Blobaum AL, Kingsley PJ, Felts AS, Sulikowski GA, Marnett LJ. The influence of double bond geometry in the inhibition of cyclooxygenases by sulindac derivatives. Bioorg Med Chem Lett. 2009;19:3271–4. doi: 10.1016/j.bmcl.2009.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Chan ST, Mahendran R. Nitric oxide induces cyclooxygenase expression and inhibits cell growth in colon cancer cell lines. Carcinogenesis. 2003;24:637–42. doi: 10.1093/carcin/bgg014. [DOI] [PubMed] [Google Scholar]
- 28.Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J Biol Chem. 2001;276:33384–92. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- 29.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–9. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys Acta. 1997;1354:40–4. doi: 10.1016/s0167-4781(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 31.Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, et al. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem. 2000;275:20127–35. doi: 10.1074/jbc.M909580199. [DOI] [PubMed] [Google Scholar]
- 32.Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, et al. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273:13760–7. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 33.Lim JH, Park JW, Min DS, Chang JS, Lee YH, Park YB, et al. NAG-1 up-regulation mediated by EGR-1 and p53 is critical for quercetin-induced apoptosis in HCT116 colon carcinoma cells. Apoptosis. 2007;12:411–21. doi: 10.1007/s10495-006-0576-9. [DOI] [PubMed] [Google Scholar]
- 34.Wilson LC, Baek SJ, Call A, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene (NAG-1) is induced by genistein through the expression of p53 in colorectal cancer cells. Int J Cancer. 2003;105:747–53. doi: 10.1002/ijc.11173. [DOI] [PubMed] [Google Scholar]
- 35.Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem. 2004;279:6883–92. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- 36.Newman D, Sakaue M, Koo JS, Kim KS, Baek SJ, Eling T, et al. Differential regulation of nonsteroidal anti-inflammatory drug-activated gene in normal human tracheobronchial epithelial and lung carcinoma cells by retinoids. Mol Pharmacol. 2003;63:557–64. doi: 10.1124/mol.63.3.557. [DOI] [PubMed] [Google Scholar]
- 37.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–34. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 38.Bottone FG, Jr, Baek SJ, Nixon JB, Eling TE. Diallyl disulfide (DADS) induces the antitumorigenic NSAID-activated gene (NAG-1) by a p53-dependent mechanism in human colorectal HCT 116 cells. J Nutr. 2002;132:773–8. doi: 10.1093/jn/132.4.773. [DOI] [PubMed] [Google Scholar]
- 39.Bauskin AR, Brown DA, Kuffner T, Johnen H, Luo XW, Hunter M, et al. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66:4983–6. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- 40.Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res. 2009;2:450–8. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmers TA, Gutierrez JC, Koniaris LG. Loss of GDF-15 abolishes sulindac chemoprevention in the ApcMin/+ mouse model of intestinal cancer. J Cancer Res Clin Oncol. 2010;136:571–6. doi: 10.1007/s00432-009-0691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.