Abstract
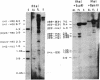
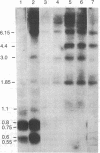
The cytosolic phosphenolpyruvate carboxykinase [PEPCK; GTP:oxaloacetate carboxy-lyase (transphosphorylating), EC 4.1.1.32] gene was isolated from a rat genomic library, and a map of the methylatable sites C-C-G-G and G-C-G-C has been constructed. The extent of methylation of 18 sites in the PEPCK gene in adult liver, kidney, spleen, and heart muscle and in fetal liver has been analyzed using the 5-methylcytosine sensitive enzymes Hpa II and Hha I. This analysis revealed extensive undermethylation of the PEPCK gene in the adult liver and kidney (PEPCK-expressing tissue), whereas the gene in adult spleen and heart muscle as well as in fetal liver (PEPCK-nonexpressing tissues) was heavily methylated. However, unlike the gene in the adult nonexpressing tissues, a region in the middle of the gene was found to be partially hypomethylated in fetal liver. This hypomethylation correlates with the competence of the fetal liver gene to be expressed. Treatment of fetuses by in utero injection of 5-azacytidine causes a hypomethylation-associated activation of the PEPCK gene. Taken together, the present findings suggest a sequential loss of methyl groups during development. When related to PEPCK gene expression, the sequential loss of methyl groups demonstrates an early stage prior to transcription characterized by hypomethylation of discrete sites and a later developmental hypomethylation of all sites associated with the mature active PEPCK gene around the time of birth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benvenisty N., Simchon E. B., Cohen H., Mencher D., Meyuhas O., Reshef L. Control of the activity of phosphoenolpyruvate carboxykinase and the level of its mRNA in livers of newborn rats. Effect of diabetes, glucose load and glucocorticoids. Eur J Biochem. 1983 May 16;132(3):663–668. doi: 10.1111/j.1432-1033.1983.tb07416.x. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Chick W., Gilbert W. Comparison of the methylation patterns of the two rat insulin genes. J Biol Chem. 1983 May 25;258(10):6645–6652. [PubMed] [Google Scholar]
- Cimbala M. A., Lamers W. H., Nelson K., Monahan J. E., Yoo-Warren H., Hanson R. W. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J Biol Chem. 1982 Jul 10;257(13):7629–7636. [PubMed] [Google Scholar]
- Colgan V., Elbrecht A., Goldman P., Lazier C. B., Deeley R. The avian apoprotein II very low density lipoprotein gene. Methylation patterns of 5' and 3' flanking regions during development and following induction by estrogen. J Biol Chem. 1982 Dec 10;257(23):14453–14460. [PubMed] [Google Scholar]
- Faliks D., Meyuhas O. Coordinate regulation of ribosomal protein mRNA level in regenerating rat liver. Study with the corresponding mouse cloned cDNAs. Nucleic Acids Res. 1982 Feb 11;10(3):789–801. doi: 10.1093/nar/10.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Ruiz J. P., Ingram R., Hanson R. W. Changes in hepatic messenger RNA for phosphoenolpyruvate carboxykinase (GTP) during development. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4189–4193. doi: 10.1073/pnas.75.9.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger R. M., Hazard-Leonards R. M., Samaha F., Hougan L. M., Lesk M. R., Thomsen G. H. Is hypomethylation linked to activation of delta-crystallin genes during lens development? Nature. 1983 Nov 3;306(5938):88–91. doi: 10.1038/306088a0. [DOI] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh L. S., Owens B. B., Hellewell O. S., Ingram V. M. DNA methylation in chicken alpha-globin gene expression. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5332–5336. doi: 10.1073/pnas.79.17.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Ballard J. Hormonal regulation of hepatic P-enolpyruvate carboxykinase (GTP) during development. Fed Proc. 1975 Feb;34(2):166–171. [PubMed] [Google Scholar]
- Hanson R. W., Fisher L., Ballard F. J., Reshef L. The regulation of phosphoenolpyruvate carboxykinase in fetal rat liver. Enzyme. 1973;15(1):97–110. [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Kunnath L., Locker J. Developmental changes in the methylation of the rat albumin and alpha-fetoprotein genes. EMBO J. 1983;2(3):317–324. doi: 10.1002/j.1460-2075.1983.tb01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. J., Bentle L. A., Lardy H. A. P-enolpyruvate carboxykinase ferroactivator. Distribution, and the influence of diabetes and starvation. J Biol Chem. 1978 Jan 10;253(1):116–124. [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- Mencher D., Cohen H., Benvenisty N., Meyuhas O., Reshef L. Primary activation of cytosolic phosphoenolpyruvate carboxykinase gene in fetal rat liver and the biogenesis of its mRNA. Eur J Biochem. 1984 May 15;141(1):199–203. doi: 10.1111/j.1432-1033.1984.tb08175.x. [DOI] [PubMed] [Google Scholar]
- Mencher D., Reshef L. Effect of triamcinolone on renal and hepatic phosphoenolpyruvate carboxykinase in the newborn rat. Changes in the rate of synthesis of the enzyme and in the activity of its translatable messenger RNA. Eur J Biochem. 1979 Mar;94(2):581–589. doi: 10.1111/j.1432-1033.1979.tb12928.x. [DOI] [PubMed] [Google Scholar]
- Mencher D., Shouval D., Reshef L. Premature appearance of hepatic phosphoenolpyruvate carboxykinase in fetal rats, not mediated by adenosine 3':5'-monophosphate. Eur J Biochem. 1979 Dec 17;102(2):489–495. doi: 10.1111/j.1432-1033.1979.tb04264.x. [DOI] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Cassio D., Levilliers J., Sala-Trepat J., Weiss M. C. Undermethylation at the 5' end of the albumin gene is necessary but not sufficient for albumin production by rat hepatoma cells in culture. Cell. 1982 Oct;30(3):825–833. doi: 10.1016/0092-8674(82)90287-2. [DOI] [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Roux J. M., Jahchan T., Fulchignoni M. C. Desoxyribonucleic acid and pyrimidine synthesis in the rat during intra-uterine growth retardation: responsiveness of several organs. Biol Neonate. 1975;27(3-4):129–140. doi: 10.1159/000240770. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein R., Sciaky-Gallili N., Razin A., Cedar H. Pattern of methylation of two genes coding for housekeeping functions. Proc Natl Acad Sci U S A. 1983 May;80(9):2422–2426. doi: 10.1073/pnas.80.9.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Szyf M., Avraham-Haetzni K., Reifman A., Shlomai J., Kaplan F., Oppenheim A., Razin A. DNA methylation pattern is determined by the intracellular level of the methylase. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3278–3282. doi: 10.1073/pnas.81.11.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel M., Gomez-Garcia M., Sala M., Sala-Trepat J. M. Changes in methylation pattern of albumin and alpha-fetoprotein genes in developing rat liver and neoplasia. Nucleic Acids Res. 1983 Jul 11;11(13):4335–4354. doi: 10.1093/nar/11.13.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- White R., Parker M. Developmental changes in DNA methylation around prostatic steroid-binding protein genes. J Biol Chem. 1983 Jul 25;258(14):8943–8948. [PubMed] [Google Scholar]
- Wilks A., Seldran M., Jost J. P. An estrogen-dependent demethylation at the 5' end of the chicken vitellogenin gene is independent of DNA synthesis. Nucleic Acids Res. 1984 Jan 25;12(2):1163–1177. doi: 10.1093/nar/12.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]