Abstract
As an early responder to an inflammatory stimulus, neutrophils (PMNs) must exit the vasculature and migrate through the extravascular tissue to the site of insult, which is often remote from the point of extravasation. Following a central epithelial corneal abrasion, PMNs recruited from the peripheral limbal vasculature migrate into the avascular corneal stroma. In vitro studies suggest PMN locomotion over 2-D surfaces is dependent on integrin binding while migration within 3-D matrices can be integrin-independent. Electron micrographs of injured mouse corneas show migrating PMNs make extensive surface contact not only with collagen fibrils in the extracellular matrix (ECM), but also keratocytes. Evidence supporting involvement of integrins in corneal inflammation has prompted research and development of integrin blocking agents for use as anti-inflammatory therapies. However, the role of integrin binding (cell-cell; cell-ECM) during stromal migration in the inflamed cornea has previously not been clearly defined. In this study in vivo time lapse imaging sequences provided the means to quantify cell motility while observing PMN interactions with keratocytes and other stromal components in the living eye. The relative contribution of β1, β2 and β3 integrins to PMN locomotion in the inflamed mouse cornea was investigated using blocking antibodies against the respective integrins. Of the 3 integrin families (β1, β2 and β3) investigated for their potential role in PMN migration, only β1 antibody blockade produced a significant, but partial, reduction in PMN motility. The preferential migration of PMNs along the keratocyte network was not affected by integrin blockade. Hence, the dominant mechanism for PMN motility within the corneal stroma appears to be integrin-independent as does the restriction of PMN migration paths to the keratocyte network.
Keywords: cornea, neutrophil, migration, integrin, inflammation, keratocytes
1. Introduction
Corneal insult, such as a central corneal epithelial abrasion, evokes prompt and extensive inflammation with neutrophils (PMNs) providing the major early cellular response (reviewed in (Silva, 2010)). PMN infiltration into the injured cornea is necessary for efficient epithelial wound healing (Li et al., 2006). Upon extravasation from the limbal vessels, PMNs must make their way through the compact stroma to reach a remote site of injury. During the course of this journey they encounter an extracellular matix (ECM) of tightly-spaced sheets (lamellae) of predominantly parallel fibrils of type I collagen laid down in criss-crossed fashion with keratocytes interspersed between lamellae. Keratocytes form an intricate network of interconnected flattened cells with long cytoplasmic processes arranged in layers parallel with the corneal surface (i.e., “keratocyte network”) (Assouline et al., 1992; Nishida et al., 1988; Poole et al., 1993). As such, the corneal stroma presents a unique challenge for infiltrating cells migrating from the limbal vasculature.
Electron micrographs of injured mouse corneas show migrating PMNs make extensive surface contact not only with collagen fibrils, but also keratocytes. Mice deficient in leukocyte β2 (CD18) integrin, or its ligand ICAM-1 (CD54), show reduced contact between PMNs and keratocytes with no change in PMN contact with collagen (Burns et al., 2005; Gagen et al., 2010; Petrescu et al., 2007). Based on these studies, it was suggested that PMNs migrate preferentially upon the keratocyte network, mediated, at least partially, by β2 integrin on the PMN and ICAM-1 on the keratocyte. However, β1 (CD29) and β3 (CD61) integrin families are also expressed on extravascular migrating PMNs and may be involved in interstitial migration (Carter, 2009; Gonzalez et al., 2007; Lindbom and Werr, 2002). In vitro studies suggest PMN locomotion over 2-D surfaces is dependent on integrin binding while migration within 3-D matrices can be integrin-independent (Friedl and Brocker, 2000; Friedl et al., 1998; Khandoga et al., 2009; Koenderman et al.; Lammermann et al., 2008; Lindbom and Werr, 2002; Mandeville et al., 1997). Interest in the involvement of integrins in corneal inflammation has prompted research and development of integrin blocking agents for use as anti-inflammatory therapies (Chen et al., 2007; Dietrich et al., 2007; Ecoiffier et al., 2008). However, the role of integrin binding during in vivo leukocyte migration within the corneal stroma has yet to be clearly defined.
The purpose of the present study was to investigate the role of integrin binding in facilitating PMN motility within the corneal interstitium using in vivo confocal microscopy. Time lapse image sequences obtained using the Heidelberg Retina Tomographer III with Rostock Corneal Module (HRT-RCM) provided the means to quantify cell motility while observing PMN interaction with keratocytes and other stromal components in the living eye. The relative contribution of β1, β2 and β3 integrins to PMN locomotion in the inflamed mouse cornea was investigated using blocking antibodies against the respective integrins.
2. Materials and methods
2.1 Animals
Female C57BL/6 mice between the ages of 8–16 weeks were bred and housed at the University of Houston, College of Optometry (UHCO) and were handled according to the guidelines described in the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Vision and Ophthalmic Research and UHCO animal handling guidelines.
2.2 Corneal inflammation induced by epithelial debridement
Animals were anesthetized with an intraperitoneal (IP) injection of ketamine (75mg/Kg body weight) and xylazine (7.5mg/Kg body weight). With the aid of a stereo dissecting microscope, eyelashes were trimmed to prevent interference with later imaging. The corneal epithelium was removed in a single vertical stripe approximately 0.5mm wide and extending to within 0.5mm of the inferior and superior vascular limbus using an AlgerbrushII with a 0.5mm burr (Alger Equipment Co., Inc., Lago Vista, TX) held tangentially to the corneal surface so that the direction of burr rotation was downward at the advancing edge. The wound was initiated in the upper cornea (superior or inferior, depending on the orientation of the mouse) moving toward the lower limbus. The mouse was then rotated 180° and the Algerbrush again applied moving from upper to lower cornea. This method provided the most consistent results with well-defined wound edges. The vertical stripe injury elicited an acute inflammatory response initiated at the peripheral vascular limbus. Within the wound area, keratocyte death was observed. However, the wound was small enough that ample parawound area was preserved for imaging PMN migration in the uninjured portion of the stroma where keratocytes remained viable. Mice were kept on an isothermal heating pad while under anesthesia and then placed in an isolation cage for the duration of the 8 hours prior to imaging using the Heidelberg Retinal Tomographer III with Rostock Cornea module (400µm size lens) (HRT-RCM).
2.3 Antibodies
Blocking antibodies were used to assess the contribution of integrins to PMN migration within the stroma. Specific antibody clones were selected based on previously published reports documenting their ability to selectively block β1, β2 or β3 integrin-mediated adhesion. The following antibodies were diluted in normal saline and applied topically immediately post wounding (25µg/ml or 250µg/ml working concentrations): anti-β1 (anti-CD29; BioLegend, clone HMβ1-1); anti-β2 (anti-CD18; BD Pharmingen, clone GAME-46); anti-β3 (anti-CD61; BioLegend, clone HMβ3-1). The specificity of these antibodies for their specific integrin targets is well described in the literature [anti-β1 (clone HMβ1-1) (Sangaletti et al., 2008; Werr et al., 1998); anti-β2 (clone GAME-46) (Bowden et al., 2002; Ridger et al., 2001); anti-β3 (clone HMβ3-1) (Piali et al., 1995)].
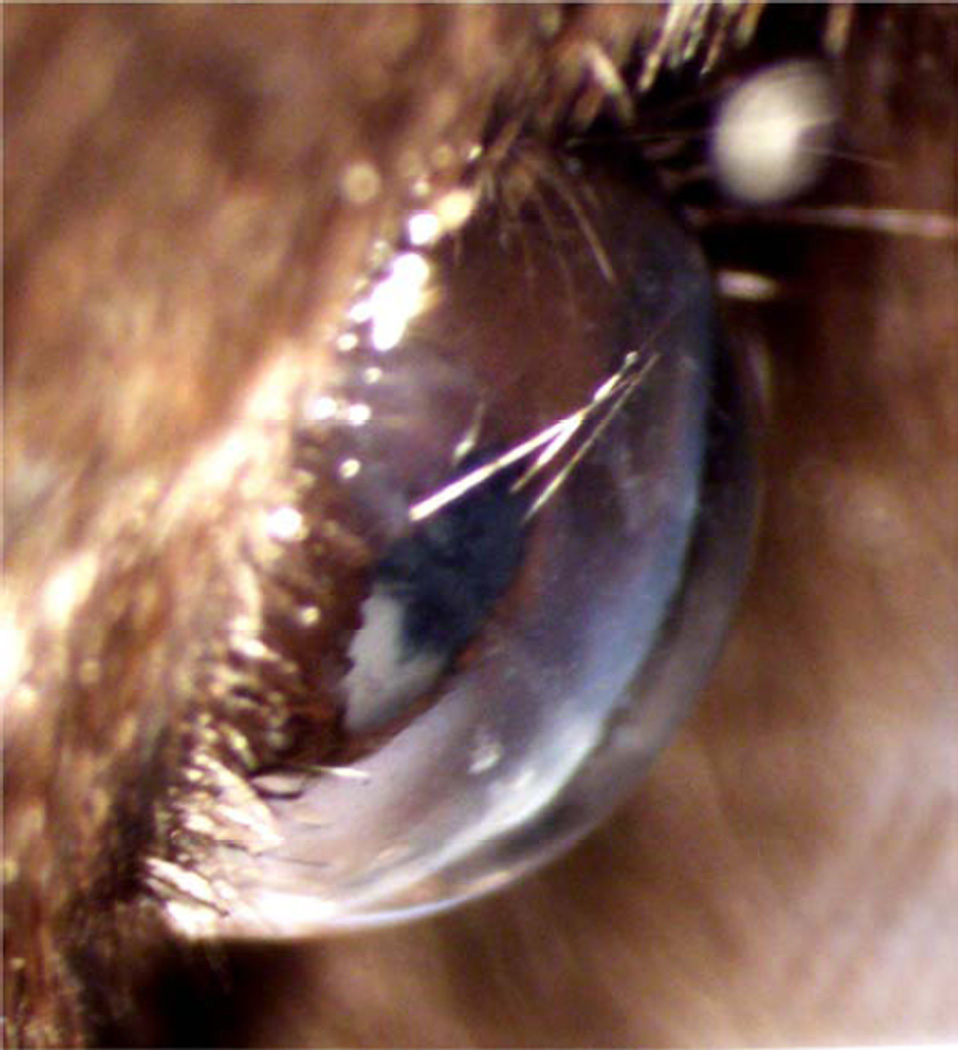
A combination of all three antibodies (same individual concentrations) was used to test for additive or compensatory effects. Isotype-matched non-immune IgG antibodies were used to control for non-specific antibody effects. Topically applied antibodies (5µl) were allowed to penetrate the wounded cornea for 5 minutes. The excess solution was then wicked off the cornea along with any cellular debris and a second application of the antibody solution was applied and allowed to penetrate until the animal recovered from anesthesia (typically 20–30 minutes without blinking). Left undisturbed, the droplet of solution could still be seen after 30 minutes and covered the entire cornea (Figure 1).
Figure 1.
A 5µl drop of anti-integrin antibody solution covered the entire cornea and remained intact during the anesthesia recovery time (30–40 min).
2.4 HRT Imaging
In vivo leukocyte cell motility was evaluated 8 hours after epithelial abrasion. At that time, each mouse was anesthetized with a slightly higher dose of IP ketamine (100mg/Kg body weight) and xylazine (10mg/Kg body weight) to ensure adequate anesthesia during the imaging session. For imaging, the mouse was placed in a heated holding device consisting of a 50ml centrifuge tube (VWR, Houston TX) with the bottom cut out to allow the mouse head to protrude for imaging and a triangular piece remaining to support the head. The tube was wrapped with a rheostat-controlled heating cable (Zoo Med, Inc., San Luis Obispo, CA) and insulating foam. Body temperature was monitored by rectal probe and maintained between 36.0 and 37.0°C (Microtherma 2, ETI Ltd. UK). As we observed in pilot experiments, and as others have reported (Miller et al., 2002), cell migration speed is influenced by body temperature and declines as body temperature falls below normal. Corneal temperature was initially measured before and after imaging using an infrared thermistor (Vario-Therm 6000L, Everest Interscience, Inc., Tucson AZ) and found to be nearly constant (33–34°C) as long as the body temperature was maintained within the specified range. The 8 hour time point was selected for imaging and represents a time point that precedes the peak PMN influx (Li et al., 2006). At this time there was minimal congestion of cell traffic that might affect individual cell speed but enough PMNs were present that at least 12 cells could be typically observed in a single plane within the 400×400µm image frame of the HRT-RCM.
The cornea was applanated with just enough force to maintain a stable image. Previous experiments established that even maximum (with the head free to move) force had no effect on cell speed (data not shown). Six 100-second scan sequences at 1 frame per second were obtained in the parawound area. Depth of the scans was 15–20 µm beneath the epithelial basement membrane where keratocytes were clearly visible but still within the anterior stroma where the vast majority of migrating PMNs are found (Petrescu et al., 2007). Motility parameters were found not to vary significantly for various depths within the anterior stroma (data not shown). Immediately after scanning, the animals were sacrificed by CO2 asphyxiation and cervical dislocation. Harvested corneas were fixed and processed for immunofluorescence microscopy and imaged as corneal whole mounts (see section 2.6).
2.5 Data analysis
To assess inflammatory cell motility, the six individual image sequences (100 seconds each) were combined into a single 10 minute sequence and post-stabilized using a custom MatLab program (MathWorks, Natick MA). Cell tracking was accomplished using a second custom MatLab program which semi-automatically tracked 12 randomly selected cells from each stabilized movie sequence. Since automatic cell trackers frequently need manual corrections, we chose to manually mark the cell location by selecting the centroid of the cell’s visible area. Following convention, cells that were non-motile (displaced <10 µm in 10 minutes) were not included (Smith, 2000; Werr et al., 1998). The number of non-motile cells was minimal and similar between experimental groups. Likewise, motile cells that were so close to the edge of the frame and moved out of view during the image sequence were not included. Twelve was the typical minimum number of cells per imaging field although on rare occasions 12 cells were not visible (the fewest was 10 cells, in only 2 corneas). The X,Y coordinates for the centroid of each cell were marked every 20 frames (seconds) and the distance and direction of movement recorded at each time interval.
Three motility parameters were considered in analyzing the data. Cell speed (CS) is a commonly reported parameter used to describe cell motility (Beltman et al., 2009) and is simply determined by dividing the total distance a cell traveled (path length) by the amount of time it took to travel that distance. Cell velocity (CV), on the other hand, is the straight-line displacement of a cell from the initial location to the final location divided by the measured time period. Average values for CS and CV were determined for corneas within each experimental group.
CS and CV describe motility for an “average” cell. However, when studying effects on cell migration, it is also meaningful to consider parameters which describe the population of cells. One such parameter we call the migration angle (MA) describes the resultant direction of displacement for a group of cells. It was determined by averaging individual cell vector displacements using the average total × displacement (right + or left −) and average total y displacement (up + or down −). MA was compared to the direction of the wound for each group of cells within a given cornea. The group movement was considered toward the wound if the MA was within ±45° of horizontal in the direction toward the wound (positive) or away from the wound (negative) if MA was within ±45° of horizontal in the opposite direction from the wound. If the MA was within ±45° of vertical it was considered ambiguous.
To determine measurement reliability, five representative movies (10 minute image sequences) were analyzed on two separate occasions by the same observer after randomly selecting 12 cells. Cell tracking data were collected by the same observer for all experimental groups.
2.6 Efficacy of integrin blockade
The ability of a blocking antibody to inhibit integrin function is influenced by, among other factors, its rate of diffusion within the corneal stroma. In order to ascertain that sufficient topically-applied antibody diffused into the region of interest for cell tracking, indirect immunofluorescence antibody labeling was used. Immediately after the mice (which had received primary blocking antibodies) were sacrificed, whole eyes were removed and placed in 2% paraformaldehyde fixative for 10 minutes at room temperature. At that time the posterior scleral shell and crystalline lens were removed from each eye and the remaining cornea with scleral rim returned to fixative for an additional 50 minutes. Corneas were then thoroughly rinsed with PBS and placed in blocking buffer (PBS + 2% BSA) overnight at 4°C, washed in blocking buffer containing 0.1% Triton-X for 30 minutes at 4°C and then labeled with fluorophore-conjugated secondary antibody (10µg/ml diluted in permeabilizing/blocking buffer) and DAPI (labels cell nuclei) and left over night in the dark at 4°C. The corneas were then rinsed in PBS and mounted on microscope slides using ProLong Gold (Invitrogen, Grand Island NY). The concentration of blocking antibody used for motility quantification was 2.5 times the typical concentration for immunolabeling (10µg/ml) and comparable to or higher than other concentrations reported in integrin blocking experiments (Bienvenu et al., 1994; Egles et al., 2010; Forsyth et al., 2002; Rainger et al., 1999). To assure antibody saturation the experiments were repeated with 250µg/ml (10× the original concentration). The degree of antibody saturation was assessed not only by comparing the effect on cell motility between the two concentrations, but also by comparing PMN staining between corneas cut in half with one half being incubated overnight in additional primary antibody.
2.7 Confirmation of PMN identity
To confirm the identity of infiltrating PMNs, labeling was accomplished using a primary conjugated Ly-6G antibody (BD Pharmingen, clone 1A8). Immediately after imaging with HRT-RCM, animals were euthanized the eyes removed and placed in fixative. Corneal wholemounts were imaged on a DeltaVision Core Spectris microscope (Applied Precision, Issaquah, WA). HRT-RCM and DeltaVision images were adjusted to same magnification and, using distinguishing landmarks, cell locations were compared between images.
2.8 Statistical Analysis
Cell motility data were determined to be normally distributed using the D’Agostino-Pearson omnibus normality test (Prism 6 for Windows, GraphPad software, Inc.). Data are expressed as mean ± standard error and statistical significance (p < 0.05) was established using ordinary ANOVA (parametric) and Dunnett’s multiple comparisons test. Repeatability of observer cell tracking analysis was assessed by paired t-test.
3. Results
3.1 HRT imaging and the visualization of inflammatory cells within the injured cornea
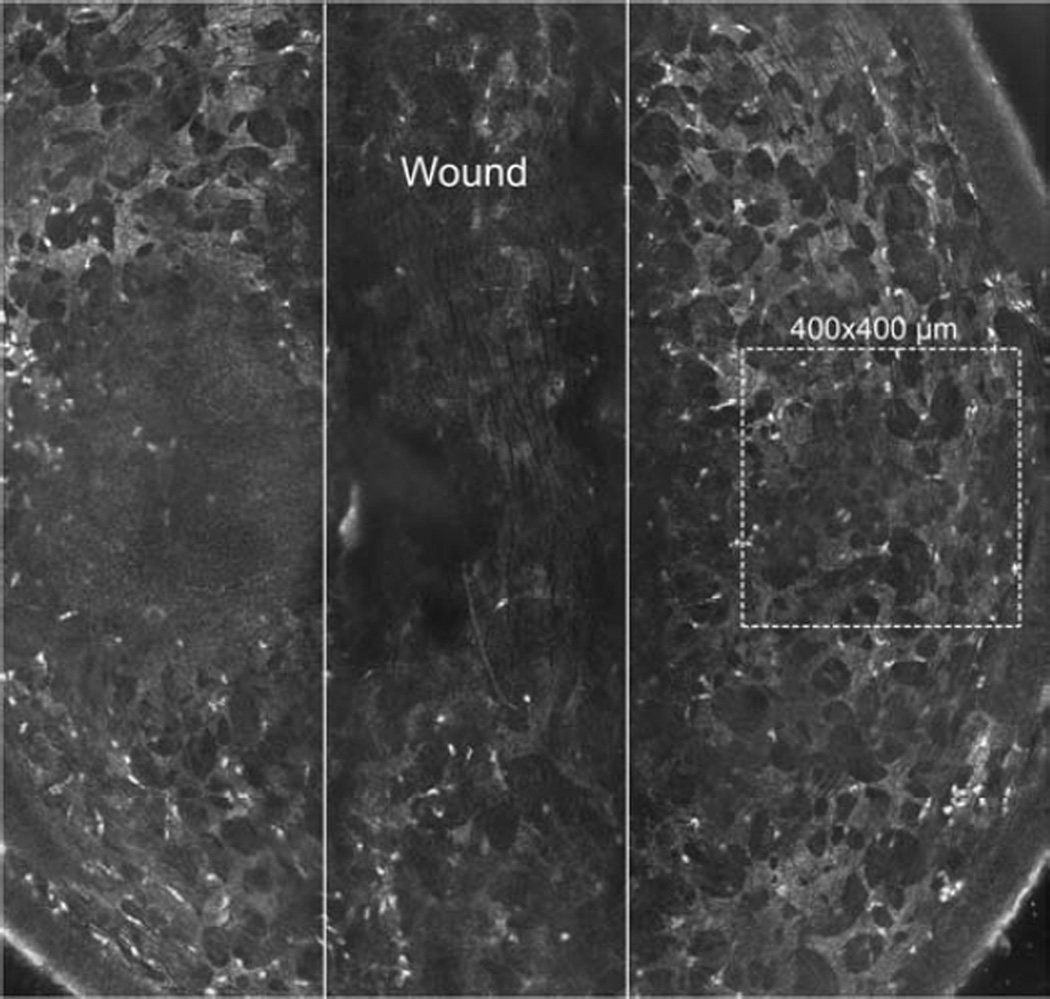
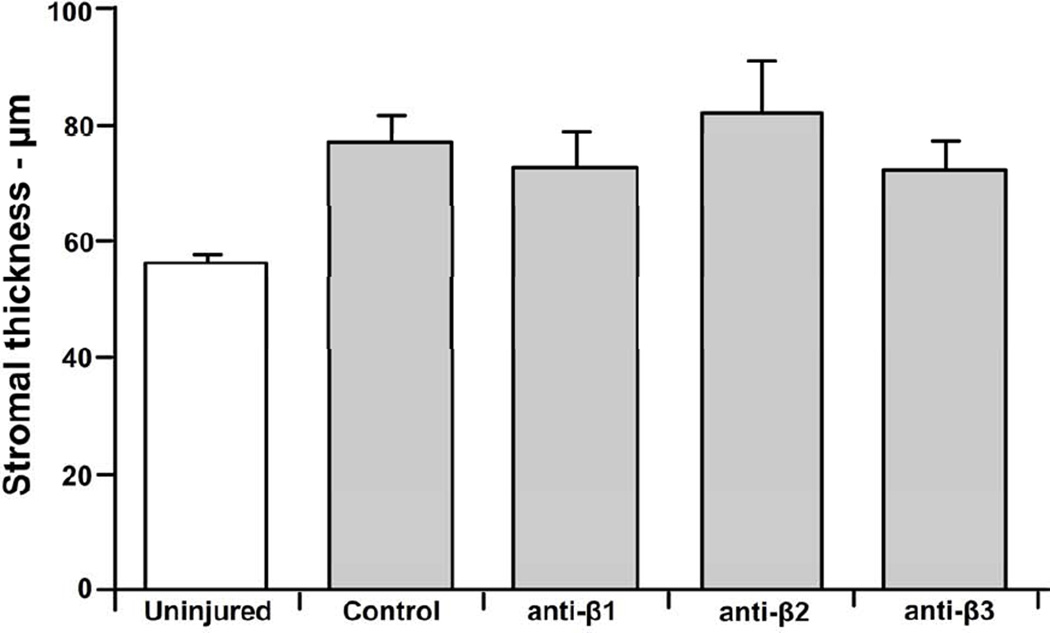
The Alger brush provided an efficient means to remove the corneal epithelium in a vertical stripe that was approximately 0.5mm in width. As others have reported (Labbe et al., 2006), scanning with the HRT-RCM reveals a well-defined keratocyte network in the mouse cornea which was easily visualized in the parawound region in our study. Associated with this network, in the anterior stroma, we observed numerous, highly reflective bodies, often elongated in shape with minor and major axes of ~ 3–4µm and 6–10µm, respectively. These bodies were not seen in uninjured corneas and their size was consistent with that of infiltrating inflammatory PMNs (Figure 2). At the time of imaging, 8 hours after wounding, the epithelial wound remained open and the stroma was edematous having increased in thickness by approximately 36% (Figure 3; p ≤ 0.05) compared to the unwounded cornea as measured using HRT through-focus. The addition of anti-integrin or control antibodies did not affect stromal thickness.
Figure 2.
Montage of multiple HRT images showing the vertical wound (between solid lines). Dotted 400×400 µm square is representative area scanned on either side of wound. The area shown is representative of the extent of the mouse cornea typically able to be imaged. To the left of the center some basal epithelial cells are seen due to the curvature of the cornea. Note the presence of small highly reflective bodies (PMNs) in the parawound region.
Figure 3.
Corneal stroma thickness increased 8 hours after epithelial abrasion. The degree of corneal swelling (20–25%) adjacent to the wound was similar when corneas were treated with non-immune IgG control antibodies (Control) or blocking antibodies directed against β1, β2, or β3 integrins.
3.2 Infiltrating inflammatory cells are PMNs
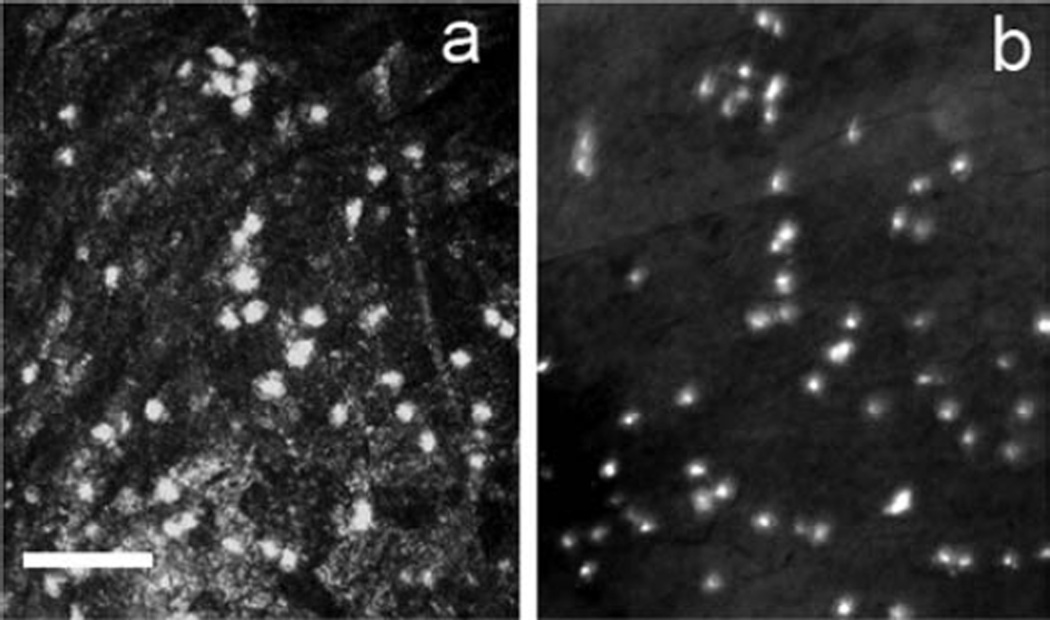
Eight hours after wounding harvested corneas were immunostained for Ly-6G, a specific PMN marker. At this time point, the pattern of infiltrating cells showed the greatest density in the peripheral cornea, gradually tapering toward the center of the cornea (Figure 2). The timing of central corneal infiltration was consistent with previous reports for PMNs (Li et al., 2006) as was the size and speed of locomotion (as seen with time-lapse imaging). Fifty out of fifty-one hyper-reflective cells imaged with the HRT-RCM stained positively for Ly-6G, confirming their identity as PMNs (Figure 4). The one hyper-reflective object that did not stain with Ly-6G was likely not motile (therefore not included in analysis) but even if it were, the Ly-6G (+) cells accounted for 98% of the HRT-RCM cells presumed to be PMNs.
Figure 4.
HRT-RCM highly reflective small cells stained positively for Ly-6G, a PMN marker. (a) HRT image of infiltrating highly reflective bodies (light spots) 8 hours after epithelial injury. Immediately after HRT imaging the eye was removed and labeled with a primary antibody against Ly-6G conjugated with FITC. (b) Fluorescence microscopy of the same corneal region as (a). Comparison of the two images confirms the identity of the cells imaged with HRT-RCM to be Ly-6G+ PMNs. There is a very slight shift in cell positions due to the whole-mount process but out of 51 cells identified in (a), only one could not be correspondingly identified in (b).
3.3 Tracking PMN migration
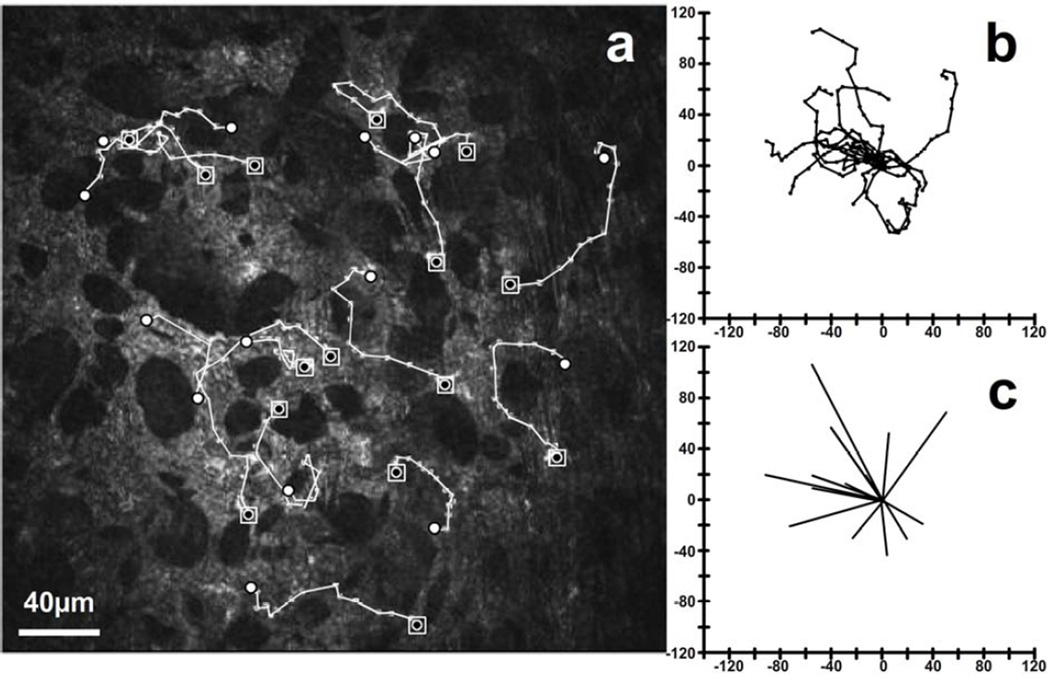
Routine observation of time-lapse sequences showed migrating PMNs were closely associated with the keratocyte network. During the course of tracking each cell by manual location of the cell’s centroid, it was very clear PMNs migrate along paths that keep them in contact with keratocytes. Although there were rare instances where this observation was ambiguous, there were no clear examples of PMNs not following the keratocytes. Superimposing PMN paths on the HRT-RCM images, such as seen in Figure 5, illustrates the preferential migration of PMNs along the keratocyte network.
Figure 5.
PMNs migrate preferentially along the keratocyte network. (a) Cell tracks (white lines; ◙ = starting point; ○ = end point) are coincident with the keratocyte network (lighter structures against dark background) from an extended 23 minute sequence. The area of wounding was outside the field, to the left. Individual cell paths (b) and vectors (c) show predominant movement toward the wound.
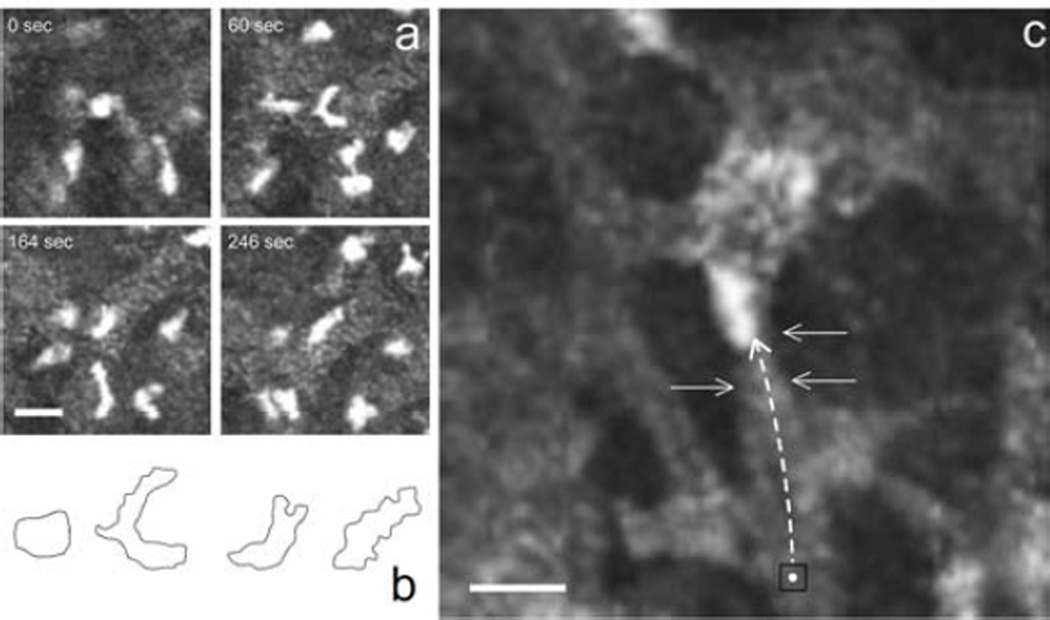
Inflammatory cell movement was readily observed, for the first time within the living cornea, using the HRT-RCM to capture 10 minute time-lapse sequences in wounded corneas. Typically the cells followed a somewhat circuitous path, but with a distinct directional preference for the keratocyte network (Supplemental movie 1). Individual cells displayed amoeboid locomotion as they underwent extension and compression. In some instances, cells made a complete loop or re-traced portions of their path, but remained associated with the keratocyte network. While most cells showed persistence of motion, some exhibited momentary pauses. Although there was variability in how fast the cells were moving, there did not appear to be subsets of high/low motility (data not shown). At times the migrating cells were seen momentarily hesitating at a point where the keratocyte network bifurcated, extending dual leading edges along the two competing paths until one leading edge collapsed and the cell was drawn along the “winning” path (Figure 6a & 6b, Supplemental movie 2). These observations are consistent with in vitro experiments in which PMNs were confronted with symmetric bifurcations in migrating channels with equal chemoattractant gradients (Ambravaneswaran et al., 2010). In those instances, PMNs extended two leading edges with the eventual collapse of one and this bi-directional choice was determined to be random. We also observed numerous instances of cells moving in single file across a narrow cytoplasmic “bridge” between neighboring keratocytes (Figure 6c, Supplemental movie 3).
Figure 6.
PMNs move in amoeboid fashion as they move across the keratocyte network. (a) PMN confronted with competing paths temporally extends two leading edges. (b) A schematic representation of the cell shape in the 4 panels above. (c) A cytoplasmic “bridge” between keratocytes is clearly the preferred route (black square marks the initial PMN location; arrows indicate cytoplasmic bridge. (scale bars = 10µm)
3.4 A role for integrins in PMN migration within the injured cornea
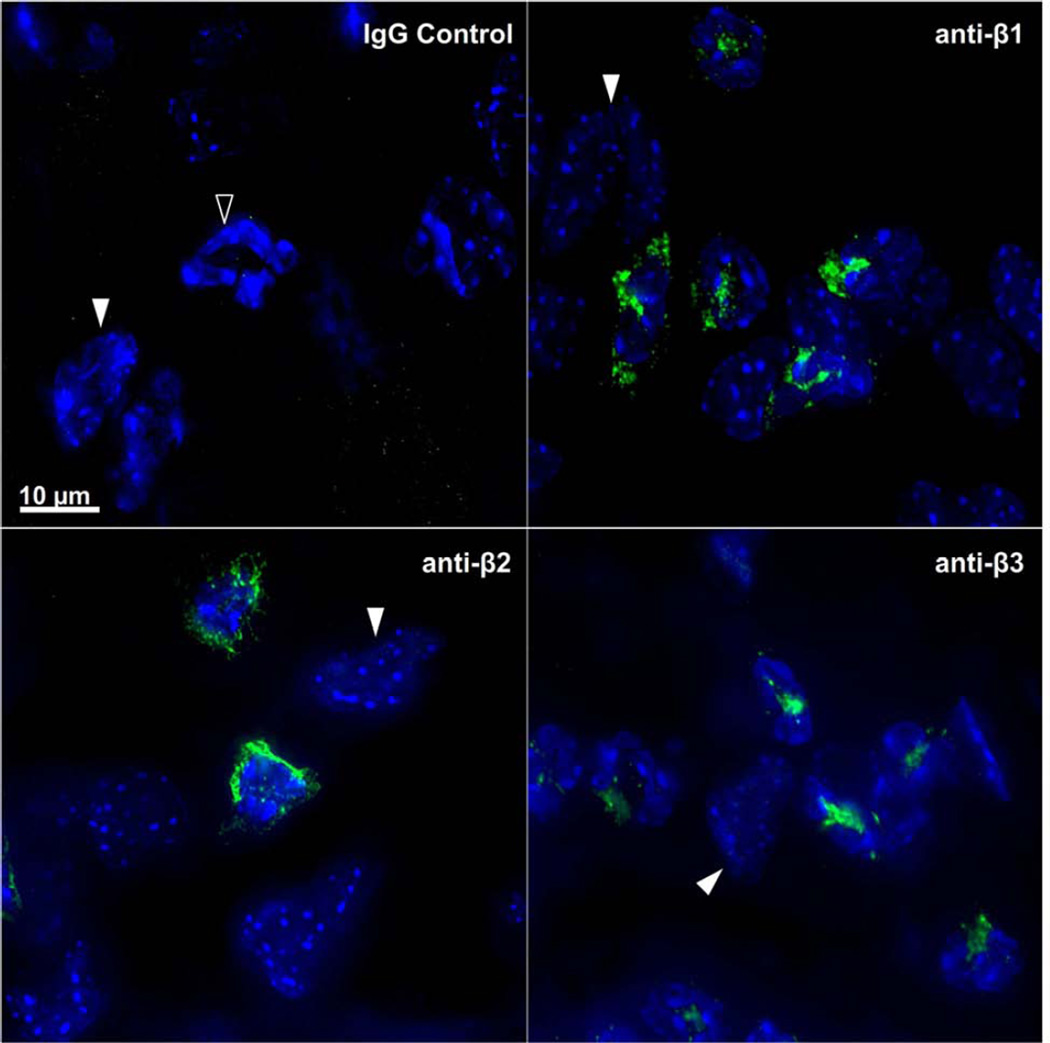
Blocking antibodies against the β1, β2 and β3 integrin families were topically applied at the time of wounding and imaged 8 hours later with the HRT-RCM in order to assess the relative contribution of each integrin to PMN locomotion. Penetration and diffusion of the blocking primary antibody within the region of PMN tracking was evaluated by immunofluorescence microscopy after incubating the excised corneas in FITC-conjugated secondary antibody. Uniform labeling of PMNs was observed not only in the parawound region, but in all regions of the cornea. Figure 7 shows representative images of labeled PMNs obtained from the parawound area (25 µg/ml primary antibody) used for motility analysis. No significant increase in staining intensity was observed following additional overnight incubation in primary antibody (data not shown) suggesting antibody concentrations of 25µg/ml in vivo are saturating. No labeling was observed when the primary anti-integrin antibody was substituted with isotype matched non-immune IgG.
Figure 7.
Immunolabeling of PMNs in the parawound region of the cornea. Eight hours after anti-integrin antibody blockade, excised corneas were immunolabeled with FITC (green) conjugated secondary antibody. PMNs fluoresced green and their polymorphic nuclei fluoresced blue (DAPI). Topical application of non-immune IgG control antibody did not bind to the PMNs as evidenced by a lack of green staining (upper left panel; open arrowhead). Keratocytes remained unlabeled by secondary antibody but their oval-shaped nuclei were visible after DAPI staining (white arrowheads).
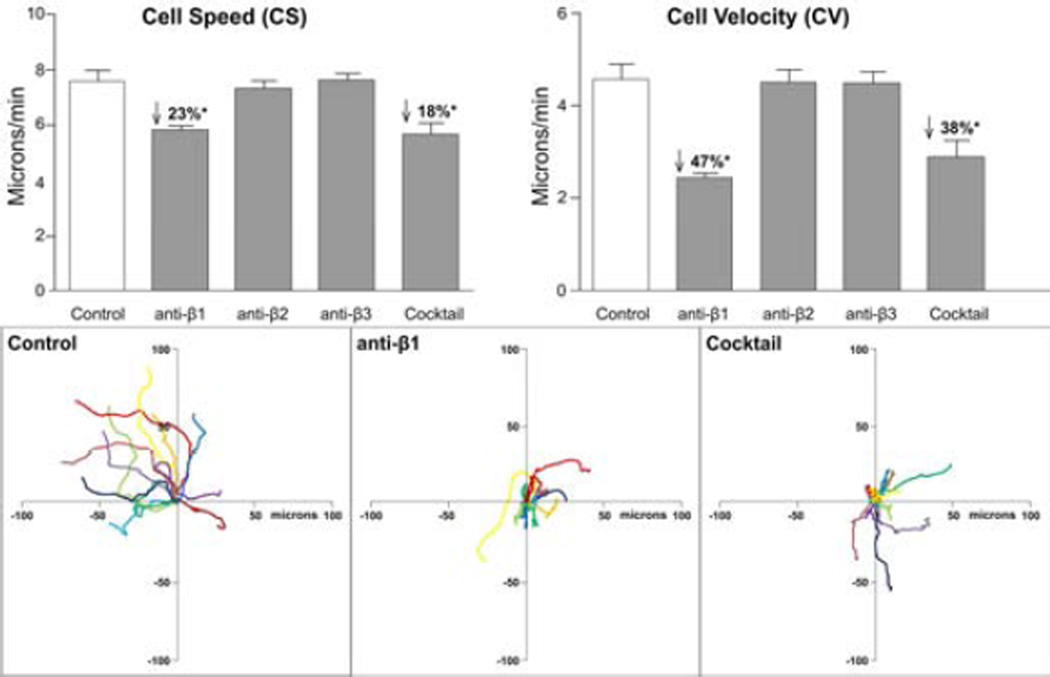
The effect of integrin blockade on the average cell speed (CS) and cell velocity (CV) was determined and the results are shown in Figure 8. Intraobserver error associated with these measured parameters was negligible when the same observer repeated the analysis on 12 random cells from each of five corneas (data not shown). When comparing the two antibody concentrations (25µg/ml and 250µg/ml) no significant difference was found in motility parameters, suggesting antibody binding was saturated, even at 25 µg/ml. Hence, data for the two antibody concentrations were pooled. PMN cell speed and velocity were significantly reduced after β1 blockade but blocking β2 or β3 integrin had no effect. When all three blocking antibodies (anti- β1, anti-β2 and anti-β3) were combined into a single topical “cocktail” the CS and CV were not significantly different compared to the effect of β1 blockade alone. These data suggest β1 integrin does play a role in PMN motility within the injured cornea.
Figure 8.
β1 blockade produced a significant reduction in CS, and CV. Compared to the IgG control group, the β1 and Cocktail groups (upper panels) had significantly reduced cell speed and an even greater reduction in velocity. The lower panels show representative polar plots of PMN paths for 10 minute periods. The left panel shows a group of cells from an IgG control cornea where the majority of the cells are moving in a similar direction. Representative plots after β1 integrin blockade and after combined β1, β2, and β3 (Cocktail) integrin blockade graphically illustrate the reduction in CS (evidenced by a reduction in total path length) and CV (evidenced by a reduction in net displacement).
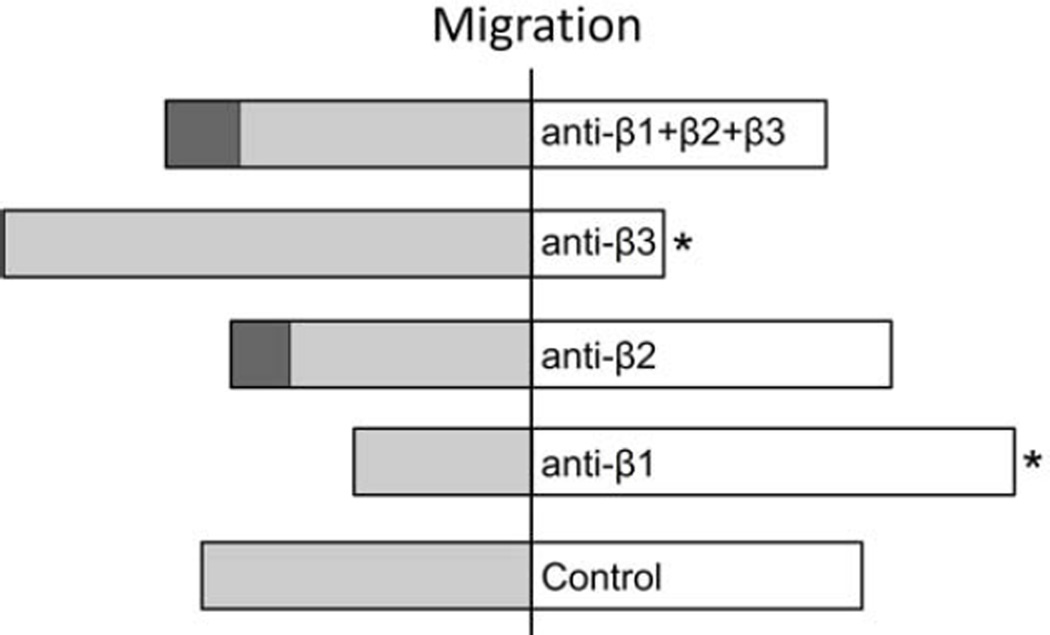
Comparing the experimental groups, β1 integrin blockade showed more PMN groups (12 PMN/group/cornea) with resultant population migration angles (MAs) directed toward the wound whereas β3 integrin blockade resulted in fewer groups with MAs directed toward the wound (Figure 9). In the combined presence of all three blocking antibodies, the number of PMN groups with MAs directed toward the wound was not different from control. None of the blocking antibodies or the combination of the three antibodies affected the observed preference for PMN migration to follow the keratocyte network (data not shown).
Figure 9.
The effect of integrin blockade on MA. The number of corneas where the group of cells showed a resultant migration toward the wound was less for the β3 blockade group (* = p≤0.05), higher for the β1 blockade group (** = p≤0.05), while the combination of all three blocking antibodies was similar to the control value. (dark gray = negative MA; light gray = ambiguous MA; white = positive MA).
Collectively, these results show the stromal concentration of topically-applied blocking antibodies was sufficient to saturate the binding sites on migrating PMNs and β1 antibody blockade alone reduced cell speed and velocity. This reduction in motility was only partial and did not affect the strong preference for PMN migration paths to coincide with the keratocyte network. In addition to a role for β1integrin in individual cell motility, β1 and β3 integrins, although in opposition, contribute to the directional response for a population of migrating PMNs.
4. Discussion
The cornea is deceivingly simplistic in its macroscopic appearance. However its strength and transparency are the result of a unique and complex ultrastructure. Using in vivo confocal microscopy (IVCM) it is now possible to obtain images in the living cornea without perturbation of the tissue. In the current study PMN stromal motility during acute corneal inflammation was quantified using time-lapse sequences of HRT-RCM scanning and the potential role of integrins was investigated using topical blocking antibodies for specific integrins known to be present on PMNs. HRT-RCM time-lapse imaging documents, for the first time, PMN motility within the injured cornea and illustrates a role for β1 integrin while showing a predilection for migration to follow the keratocyte network.
Studies in a variety of tissues have reported PMNs moving in amoeboid fashion at average speeds ranging from 7 µm/min to as high as 30 µm/min (Bienvenu et al., 1994; Friedl and Weigelin, 2008; Li et al., 2008; Planck et al., 2008; Werr et al., 1998) making them among the fastest moving cells in mammals (Friedl and Brocker, 2000). In the present study, the average PMN speed in the stroma adjacent to the wound (7.57±0.41 µm/min) is on the lower end of this range likely due to the compact nature of the corneal stroma, not encountered in most other tissues. Leukocyte locomotion in 2-D in vitro models has been described as integrin-dependent (Ambravaneswaran et al.; Friedl and Weigelin, 2008; Khandoga et al., 2009; Planck et al., 2008) while 3-D in vitro studies report PMN motility may be integrin-independent and operate by contact guidance, following paths of least resistance and involve the use of mechanical force to squeeze through the matrix (Friedl and Weigelin, 2008; Koenderman et al.; Lammermann et al., 2008; Lindbom and Werr, 2002; Mandeville et al., 1997). These 2-D and 3-D studies have provided much information however, PMNs in vivo are in a complex environment that can only be partially replicated in vitro and this environmental difference may have profound effects on cell migration behavior. Whether PMN locomotion within the corneal stroma is dependent on integrin binding has not been previously studied in the living cornea.
Petrescu and colleagues (2007) described an extravascular role for PMN β2 integrins in mediating close surface contacts with corneal keratocytes (Petrescu et al., 2007). In additionGagen et al. (2010) showed that ICAM-1, a β2-ligand is expressed on keratocytes and is also required to maintain close contacts between PMNs and keratocytes (Gagen et al., 2010). Based on the observation that PMNs make β2-dependent close contacts with keratocytes during corneal stromal migration, it was proposed that the keratocytes act as a β2 integrin-dependent contact guidance mechanism or “cellular highway” for PMN migration (Petrescu et al., 2007). However, our present findings using antibody blockade suggest β2 integrins are not required for PMN motility within the corneal stroma. This observation is consistent with an earlier (immunohistological) study in β2-integrin-deficient mice showing that although PMN extravasation is delayed in response to a central corneal epithelial abrasion, once extravasated, β2 integrin-deficient PMNs migrate and accumulate at the wound center (Li et al., 2006). This is also in agreement with earlier findings in other tissues showing β2 integrins do not have a dominant role in extravascular leukocyte migration in vivo (Bienvenu et al., 1994; Lindbom and Werr, 2002; Werr et al., 1998). Whether PMN β2 integrin binding to keratocyte ICAM-1 initiates signaling events within the PMN and/or keratocyte (Lammermann et al., 2008; Muether et al., 2007; Ridger et al., 2001) remains to be determined.
β1 integrins are expressed on numerous cell types, including PMNs, and show high affinity interactions with proteins of the extracellular matrix (Lindbom and Werr, 2002) and are upregulated on activated PMNs (Werr et al., 1998). PMNs have been shown to express α4β1, α5β1, and α9β1 integrins which can play a role in their adhesion and interstitial migration (Gonzalez et al., 2007; Ridger et al., 2001; van den Berg et al., 2001). In addition, α2β1, α4β1, α5β1, and α6β1 integrins have been reported to be present on stromal keratocytes. Their main ligands are laminin, fibronectin, and collagen (Andresen et al., 2000; Stepp, 2006). Our results showing that antibody blockade of β1 integrins significantly reduced the average PMN locomotion (23% reduction in speed) suggest PMNs possibly use integrin(s) from this family to form adhesive “foot holds” that facilitate movement. However, the fact that cell speed was only reduced by 23% shows other mechanisms for movement also exist. With optimal adhesion, cells are more likely to achieve their peak speed, being limited by other factors such as physical constraints and/or chemoattractant forces. Werr et al. (Werr et al., 2000) found a marked reduction (~70%) in PMN speed in rat mesentery in vivo using an anti-β1 blocking mAb while Friedl et al. (Friedl et al., 1998) reported that simultaneous blocking of β1, β2, β3, and αV integrins had no effect on T cell speed. Apparently not all leukocytes use the same mechanisms for migration and the requirement for integrins may be tissue specific.
β1 integrin binding between PMNs and the ECM (contact guidance) allows the migration path to follow a more direct route while still remaining on the keratocyte network, whereas without the benefit of β1 binding, PMNs are less likely to move from one keratocyte to another, which results in route retracing or circuitous paths. This was reflected in our data where the reduction in CV was greater than the reduction in CS, graphically illustrated in Figure 8. When PMN β1integrin/ECM adhesion is blocked, the number of sample populations with a resultant migration angle (MA) directed toward the wound increases while PMN locomotion slows. β2 binding between the PMN and keratocyte (Petrescu et al., 2007) may make it more difficult for PMNs to move from keratocyte to keratocyte. When all three integrins are blocked the PMNs are guided by the tactic stimulus but still preferentially follow the keratocyte network.
The dense nature of the collagen lamellae presents a formidable obstacle for infiltrating PMNs whereas the interlamellar space, in which the keratocytes reside, is strongly favored by migrating PMNs (Burns et al., 2005; Lammermann et al., 2008; Petrescu et al., 2007). The ability of PMNs to deform and “squeeze” through confined spaces without the need for integrin engagement is well documented (Friedl and Weigelin, 2008; Friedl et al., 1998; Khandoga et al., 2009; Koenderman et al.; Lammermann et al., 2008; Lindbom and Werr, 2002; Mandeville et al., 1997). Integrin-independent squeezing locomotion is consistent with PMN migration along the keratocyte network because the interlamellar space exists and is defined by the keratocytes and, to the migrating PMNs, this represents the path of least resistance within the corneal stroma. β2 and β3 integrins do not appear to compensate for the lack of β1 integrin binding since PMN migration behavior was similar when all three integrins were simultaneously blocked. According to Saltzman and colleagues, PMN migration through collagen gels is β2 integrin-dependent but only under conditions of high hydration, suggesting adhesion is only important when fiber density is relatively low such as occurs with edema (Saltzman et al., 1999). While we did not observe β1 integrin staining on keratocytes in this study, integrin staining on keratocytes has been reported by others (reviewed by Carter) (Carter, 2009). Since β1 integrins bind various components of the extracellular matrix (collagen in particular), it seemed plausible that its blockade may increase the tendency for keratocytes bearing β1 integrins to “let go” of the extracellular matrix, thereby enhancing tissue expansion (edema). If this were the case, it might be expected that antibody-induced edema would indirectly affect PMN motility. However, our results showed no difference in the amount of stromal swelling post-wounding in any of the antibody-blocked corneas as compared to control corneas (Figure 3).
The third family of integrins investigated in this study, β3 is essentially represented by only one member, αvβ3, found on both PMNs and stromal keratocytes (Rainger et al., 1999; Stepp, 2006). Its ligands include fibronectin, vitronectin, fibrinogen, and tenascin-C which have been reported to be present in inflamed corneal stroma (Carter, 2009). In contrast to β1 integrin blockade, β3 integrin blockade produced a significant reduction in the tendency for populations of PMNs to migrate toward the wound. The effect of β3 integrin blockade on migration angle (MA) was an unexpected result. Since neither CS nor CV was affected it would suggest that β3 integrin binding may function through cell signaling to contribute to guidance of PMNs to a site of inflammation. This is consistent with reported findings showing that blocking αvβ3 in some cells inhibits their ability to migrate along a chemotactic gradient (Gonzalez et al., 2007; Liaw et al., 1995). The lack of effect on cell speed when blocking β2 or β3 does not appear to be the result of a compensatory shift to dependence on one of the other integrins as evidenced by the finding that when the three integrin blocking antibodies were combined the results were not different than β1 blockade alone. Without integrin binding, migrating PMNs may be forced to rely on non-adhesive mechanisms such as physical squeezing (Lammermann et al., 2008) between interstitial elements through paths of least resistance, facilitated by “non-specific” biophysical interactions with the 3-D tissue as suggested by Friedel and Brocker (Friedl and Brocker, 2000) for T cell migration in 3-D collagen matrices following integrin blockade.
While immunofluorescent staining of β1 and β3 integrin expression on PMNs was clearly observed across the corneal stroma, it did not confirm the presence of β1 and β3 integrins on keratocytes. To date, there are no published studies documenting integrin expression on mouse keratocytes. Previous reports of β1 and β3 integrin expression on keratocytes were based on in vitro studies relevant to species other than mouse (Doane and Birk, 1994; Jester et al., 1994; Lauweryns et al., 1991; Masur et al., 1993). If these integrins are expressed on mouse keratocytes, they appear to be at a level too low for detection by immunofluorescence microscopy. Alternatively, there may be a lack of free binding sites on the keratocyte surface because of engagement with the ECM. Hence, the reduction in PMN motility resulting from β1 integrin blockade appears to be a specific PMN response and not due to neutralization of keratocyte β1 integrin activity. This conclusion is further supported by the lack of any functional effect on stromal swelling when β1 integrin blockade was used. Whether PMNs use β1 integrins to adhere to keratocytes, extracellular matrix, or both, remains to be determined.
In summary, in vivo imaging provided functional evidence of preferential migration of PMNs along the keratocyte network. Three integrin families (β1, β2 and β3) were investigated for their potential role in PMN migration, with only β1 integrin antibody blockade producing a significant, but partial, reduction in PMN migration speed. Hence, the dominant mechanism for PMN motility within the corneal stroma appears to be integrin-independent as does the restriction of PMN migration paths to the keratocyte network. We provide some evidence that β1and β3 integrins may play a role in modulating the tactic response. Whether surface contacts between PMNs and keratocytes, mediated by PMN β2 integrins and keratocyte ICAM-1, serve some other extravascular function (e.g., cell signaling) remains to be determined.
Supplementary Material
Supplemental movie 1: Time-lapse PMN motility within the living cornea captured with the HRT-RCM. Individual cells are seen with amoeboid locomotion extending and retracting cytoplasmic processes with a distinct directional preference for the keratocyte network.
Supplemental movie 2: PMN seen momentarily hesitating at a point where the keratocyte network bifurcated. Dual leading edges extended along the two competing paths until one leading edge collapsed and the PMN was drawn along the “winning” path.
Supplemental movie 3: PMN moving in single file across a narrow cytoplasmic “bridge” connecting neighboring keratocytes.
Highlights.
Neutrophil (PMN) migration is preferentially confined to the keratocyte network
Within the corneal stroma, PMNs migrate at an average speed of 7.6 µm/minute
PMN motility within the stroma is facilitated, in part, by β1 integrin
PMN motility within the stroma is not dependent on β2and β3 integrins
Acknowledgements
We thank Evelyn Brown and Margaret Gondo for their excellent help in preparing specimens and Dr. Ronald S. Harwerth for the generous use of his laboratory space and equipment. This research project was supported by NEI grants: EY17120 (ARB), EY007551 and HL116524 (ARB, CWS), EY018239 (CWS), EY001139 (RSH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambravaneswaran V, Wong IY, Aranyosi AJ, Toner M, Irimia D. Directional decisions during neutrophil chemotaxis inside bifurcating channels. Integr Biol (Camb) 2010;2:639–647. doi: 10.1039/c0ib00011f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen JL, Ledet T, Hager H, Josephsen K, Ehlers N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp Eye Res. 2000;71:33–43. doi: 10.1006/exer.2000.0850. [DOI] [PubMed] [Google Scholar]
- Assouline M, Chew SJ, Thompson HW, Beuerman R. Effect of growth factors on collagen lattice contraction by human keratocytes. Invest Ophthalmol Vis Sci. 1992;33:1742–1755. [PubMed] [Google Scholar]
- Beltman JB, Maree AF, de Boer RJ. Analysing immune cell migration. Nat Rev Immunol. 2009;9:789–798. doi: 10.1038/nri2638. [DOI] [PubMed] [Google Scholar]
- Bienvenu K, Harris N, Granger DN. Modulation of leukocyte migration in mesenteric interstitium. Am J Physiol. 1994;267:H1573–H1577. doi: 10.1152/ajpheart.1994.267.4.H1573. [DOI] [PubMed] [Google Scholar]
- Bowden RA, Ding ZM, Donnachie EM, Petersen TK, Michael LH, Ballantyne CM, Burns AR. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circulation research. 2002;90:562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- Burns AR, Li Z, Smith CW. Neutrophil migration in the wounded cornea: the role of the keratocyte. Ocul Surf. 2005;3:S173–S176. doi: 10.1016/s1542-0124(12)70249-5. [DOI] [PubMed] [Google Scholar]
- Carter RT. The role of integrins in corneal wound healing. Vet Ophthalmol. 2009;12(Suppl 1):2–9. doi: 10.1111/j.1463-5224.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Huq S, Gardner H, de Fougerolles AR, Barabino S, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Archives of ophthalmology. 2007;125:783–788. doi: 10.1001/archopht.125.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, Zahn G, Cursiefen C. Inhibition of Inflammatory Lymphangiogenesis by Integrin α5 Blockade. The American Journal of Pathology. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane KJ, Birk DE. Differences in integrin expression during avian corneal stromal development. Invest Ophthalmol Vis Sci. 1994;35:2834–2842. [PubMed] [Google Scholar]
- Ecoiffier T, El Annan J, Rashid S, Schaumberg D, Dana R. Modulation of integrin alpha4beta1 (VLA-4) in dry eye disease. Archives of ophthalmology. 2008;126:1695–1699. doi: 10.1001/archopht.126.12.1695. [DOI] [PubMed] [Google Scholar]
- Egles C, Huet HA, Dogan F, Cho S, Dong S, Smith A, Knight EB, McLachlan KR, Garlick JA. Integrin-blocking antibodies delay keratinocyte re-epithelialization in a human three-dimensional wound healing model. PLoS One. 2010;5:e10528. doi: 10.1371/journal.pone.0010528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis and rheumatism. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- Friedl P, Brocker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Friedl P, Zanker KS, Brocker EB. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998;43:369–378. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Gagen D, Laubinger S, Li Z, Petrescu MS, Brown ES, Smith CW, Burns AR. ICAM-1 mediates surface contact between neutrophils and keratocytes following corneal epithelial abrasion in the mouse. Exp Eye Res. 2010;91:676–684. doi: 10.1016/j.exer.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AL, El-Bjeirami W, West JL, McIntire LV, Smith CW. Transendothelial migration enhances integrin-dependent human neutrophil chemokinesis. J Leukoc Biol. 2007;81:686–695. doi: 10.1189/jlb.0906553. [DOI] [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- Khandoga AG, Khandoga A, Reichel CA, Bihari P, Rehberg M, Krombach F. In vivo imaging and quantitative analysis of leukocyte directional migration and polarization in inflamed tissue. PLoS One. 2009;4:e4693. doi: 10.1371/journal.pone.0004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderman L, van der Linden JA, Honing H, Ulfman LH. Integrins on neutrophils are dispensable for migration into three-dimensional fibrin gels. Thromb Haemost. 2010;104:599–608. doi: 10.1160/TH09-10-0740. [DOI] [PubMed] [Google Scholar]
- Labbe A, Liang H, Martin C, Brignole-Baudouin F, Warnet JM, Baudouin C. Comparative anatomy of laboratory animal corneas with a new-generation high-resolution in vivo confocal microscope. Curr Eye Res. 2006;31:501–509. doi: 10.1080/02713680600701513. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Lauweryns B, van den Oord JJ, Volpes R, Foets B, Missotten L. Distribution of very late activation integrins in the human cornea. An immunohistochemical study using monoclonal antibodies. Invest Ophthalmol Vis Sci. 1991;32:2079–2085. [PubMed] [Google Scholar]
- Li L, Norrelykke SF, Cox EC. Persistent cell motion in the absence of external signals: a search strategy for eukaryotic cells. PLoS One. 2008;3:e2093. doi: 10.1371/journal.pone.0002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Smith CW. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest Ophthalmol Vis Sci. 2006;47:1947–1955. doi: 10.1167/iovs.05-1193. [DOI] [PubMed] [Google Scholar]
- Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM, Giachelli CM. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. The Journal of clinical investigation. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbom L, Werr J. Integrin-dependent neutrophil migration in extravascular tissue. Semin Immunol. 2002;14:115–121. doi: 10.1006/smim.2001.0348. [DOI] [PubMed] [Google Scholar]
- Mandeville JT, Lawson MA, Maxfield FR. Dynamic imaging of neutrophil migration in three dimensions: mechanical interactions between cells and matrix. J Leukoc Biol. 1997;61:188–200. doi: 10.1002/jlb.61.2.188. [DOI] [PubMed] [Google Scholar]
- Masur SK, Cheung JK, Antohi S. Identification of integrins in cultured corneal fibroblasts and in isolated keratocytes. Invest Ophthalmol Vis Sci. 1993;34:2690–2698. [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Muether PS, Dell S, Kociok N, Zahn G, Stragies R, Vossmeyer D, Joussen AM. The role of integrin alpha5beta1 in the regulation of corneal neovascularization. Exp Eye Res. 2007;85:356–365. doi: 10.1016/j.exer.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Nishida T, Yasumoto K, Otori T, Desaki J. The network structure of corneal fibroblasts in the rat as revealed by scanning electron microscopy. Invest Ophthalmol Vis Sci. 1988;29:1887–1890. [PubMed] [Google Scholar]
- Petrescu MS, Larry CL, Bowden RA, Williams GW, Gagen D, Li Z, Smith CW, Burns AR. Neutrophil interactions with keratocytes during corneal epithelial wound healing: a role for CD18 integrins. Invest Ophthalmol Vis Sci. 2007;48:5023–5029. doi: 10.1167/iovs.07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planck SR, Becker MD, Crespo S, Choi D, Galster K, Garman KL, Nobiling R, Rosenbaum JT. Characterizing extravascular neutrophil migration in vivo in the iris. Inflammation. 2008;31:105–111. doi: 10.1007/s10753-007-9055-x. [DOI] [PubMed] [Google Scholar]
- Poole CA, Brookes NH, Clover GM. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci. 1993;106(Pt 2):685–691. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- Rainger GE, Buckley CD, Simmons DL, Nash GB. Neutrophils sense flowgenerated stress and direct their migration through alphaVbeta3-integrin. Am J Physiol. 1999;276:H858–H864. doi: 10.1152/ajpheart.1999.276.3.H858. [DOI] [PubMed] [Google Scholar]
- Ridger VC, Wagner BE, Wallace WA, Hellewell PG. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol. 2001;166:3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- Saltzman WM, Livingston TL, Parkhurst MR. Antibodies to CD18 influence neutrophil migration through extracellular matrix. J Leukoc Biol. 1999;65:356–363. doi: 10.1002/jlb.65.3.356. [DOI] [PubMed] [Google Scholar]
- Sangaletti S, Di Carlo E, Gariboldi S, Miotti S, Cappetti B, Parenza M, Rumio C, Brekken RA, Chiodoni C, Colombo MP. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer research. 2008;68:9050–9059. doi: 10.1158/0008-5472.CAN-08-1327. [DOI] [PubMed] [Google Scholar]
- Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- Smith CW. Possible steps involved in the transition to stationary adhesion of rolling neutrophils: a brief review. Microcirculation. 2000;7:385–394. [PubMed] [Google Scholar]
- Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- van den Berg TK, Puklavec MJ, Barclay AN, Dijkstra CD. Monoclonal antibodies against rat leukocyte surface antigens. Immunol Rev. 2001;184:109–116. doi: 10.1034/j.1600-065x.2001.1840110.x. [DOI] [PubMed] [Google Scholar]
- Werr J, Johansson J, Eriksson EE, Hedqvist P, Ruoslahti E, Lindbom L. Integrin alpha(2)beta(1) (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–1809. [PubMed] [Google Scholar]
- Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. beta1 integrins are critically involved in neutrophil locomotion in extravascular tissue In vivo. The Journal of experimental medicine. 1998;187:2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental movie 1: Time-lapse PMN motility within the living cornea captured with the HRT-RCM. Individual cells are seen with amoeboid locomotion extending and retracting cytoplasmic processes with a distinct directional preference for the keratocyte network.
Supplemental movie 2: PMN seen momentarily hesitating at a point where the keratocyte network bifurcated. Dual leading edges extended along the two competing paths until one leading edge collapsed and the PMN was drawn along the “winning” path.
Supplemental movie 3: PMN moving in single file across a narrow cytoplasmic “bridge” connecting neighboring keratocytes.