Abstract
Myocardial infarction (MI) is a leading cause of death worldwide. Permanent ligation of the left anterior descending coronary artery (LAD) is a commonly used surgical model to study post-MI effects in mice. LAD occlusion induces a robust wound healing response that includes extracellular matrix (ECM) remodeling. This chapter provides a detailed guide on the surgical procedure to permanently ligate the LAD. Additionally, we describe a prototype method to enrich cardiac tissue for ECM, which allows one to focus on ECM remodeling in the left ventricle following surgically induced MI in mice.
Keywords: Myocardial infarction, Cardiac wound healing, Mice, Extracellular matrix, Matrix metalloproteinases, Inflammation, Decellularization
1 Introduction
Over the last 40 years, successful cardiovascular research has led to increases in 30-day post-myocardial infarction (MI) survival rates of 60 % in the 1970s to current rates of >90 %. Based on this achievement, the current therapeutic challenge has shifted from immediate survival issues to ways to improve the long-term survival of patients undergoing cardiac infarct healing [1, 2]. Cardiac infarct healing in the left ventricle (LV) is referred to as LV remodeling and includes changes in LV size, shape, and function [3-5].
The extent of LV remodeling is dependent on the severity of the following component events that occur after the infarct: (1) myocyte death, (2) inflammatory response, (3) granulation tissue deposition to form the scar, and (4) granulation tissue remodeling [6, 7]. Throughout LV remodeling, the critical balance between extracellular matrix (ECM) degradation and deposition influences ventricular function and patient survival [5, 8]. Excessive ECM degradation predisposes the LV to aneurysm formation and ventricular rupture, while excessive ECM deposition may result in arrhythmias and congestive heart failure [8]. In this chapter, we describe an established murine MI model followed by a protocol to enrich the ECM content during the LV remodeling process post MI.
The surgically induced MI model presented here consists of permanent occlusion of the LAD at the site where it emerges from under the left atrium. This chronic infarct model evokes an anterolateral, apical LV infarct with an average MI size that equals 44 ± 2 % (n = 25) of the total LV muscle mass by day 7 post MI in C57BL/6 wild-type mice of both genders [9-12]. The mortality associated with the MI procedure ranges from 37 to 50 % over the first 7 days and is primarily due to ventricular rupture, sudden cardiac death, or acute heart failure, with mortality being greater for male mice [13]. This model is suitable for cardiac pathophysiology studies of MI responses, as indicated by the significantly decreased ejection fraction at day 7 post MI (64 ± 2 % for controls versus 18 ± 2 % post MI, p < 0.05) [11]. In addition to inducing progressive wall thinning, the permanent LAD occlusion model induces an increase in LV dilation. Further, this model is a convenient tool to investigate biochemical, cellular, and molecular responses to MI.
The advantage of using the mouse permanent occlusion MI model is that the availability of genetically modified mice makes this a practical model to investigate the role of proteins of interest during the LV remodeling process. A constraint of using this animal model is that the mouse infarct and non-infarct LV tissue sizes provide limited amounts of material. Therefore, the experimental design and execution must be carefully planned in order to completely test the hypothesis under investigation. It is important to account for the mortality rate when determining the number of animals required for the study, in order to have an appropriate sample size.
The quality control for infarct confirmation in our permanent occlusion MI model is based on visual inspection of the LV for blanching, electrocardiogram (EKG) assessment for ST segment elevation, and imaging by two-dimensional echocardiography. For every mouse, baseline echocardiograms of the long and short axes should be recorded and analyzed before surgery to assure that the animals have normal LV function. During the surgery, an EKG is used to continuously monitor the animal. Post surgery, LV function is assessed by echocardiography after 3 h and serial echocardiograms are recorded for up to 4 weeks post MI. At the time of sacrifice, the LV is dissected, sectioned into three slices (apex, mid-cavity, and base), and stained using 1 % 2,3,5-triphenyltetrazolium chloride (TTC). Viable myocardium stains red, which facilitates infarct sizing [14].
The decellularization protocol that follows the permanent LAD occlusion surgery is a perfusion-free version of the procedure described for rats [15]. In addition to decellularizing the heart to remove cellular constituents, the glycosaminoglycans, collagen type I, collagen type III, laminin, and fibronectin that form the scaffold are preserved [15]. The ECM enrichment process described below yields a fully functional three-dimensional scaffold that facilitates investigation of ECM responses post MI [15].
2 Materials
2.1 Mouse Intubation and Coronary Artery Ligation
All animal procedures should be conducted according to the Guide for the Care and Use of Laboratory Animals and need to be reviewed and approved by the appropriate institutional animal care and use committee.
Paper towels and examination gloves.
Glass bead sterilizer.
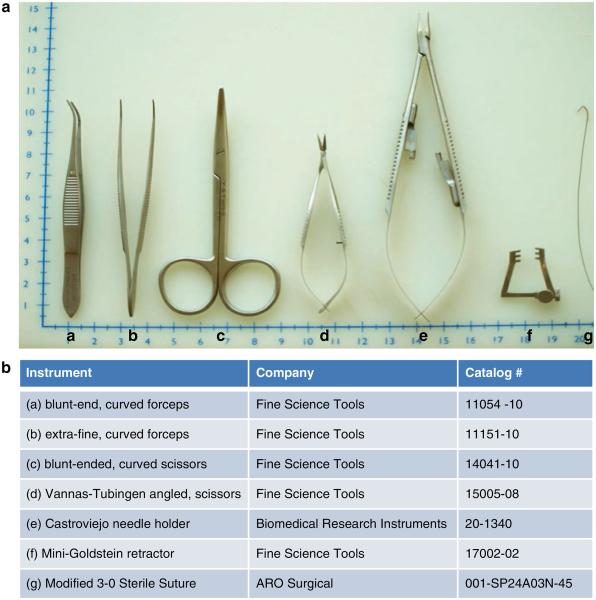
Surgical instruments (Fig. 1).
Oxygen cylinder equipped with regulator.
Isoflurane.
Induction chamber for anesthesia.
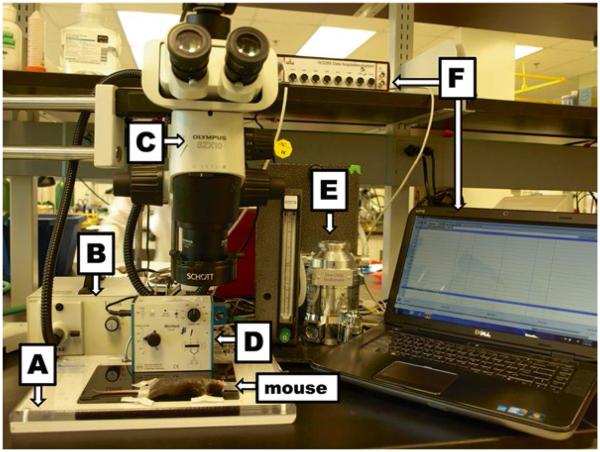
Mouse Surgery Board (Fig. 2A).
Mouse ventilator equipped with a mouse nose cone (Fig. 2D).
Anesthetic vaporizer (Fig. 2E).
Surgical tape.
Hair remover lotion (Nair®).
Cotton-tipped applicators.
EtOH (70 % [v/v] solution in H2O).
Povidone-Iodine Prep Solution USP (Betadine).
EKG recording system (iWorx Software) and computer (Fig. 2F).
IV catheter 20 ga × 1.
- Sterile sutures.
- 3–0 AROSurgical™ (Black Polyamide Monofilament) sterile suture.
- 6–0 Ethicon Prolene (Polypropylene) sterile suture.
- 8–0 AROSurgical™ (Black Polyamide Monofilament) sterile suture.
Gauze sponges (100 % cotton, 4 in. × 4 in., 8-ply).
0.9 % Sodium Chloride Irrigation Solution USP.
Buprenorphine hydrochloride.
Fig. 1.
(a) Instruments recommended for the surgical procedure. (b) Instrument names and catalogue numbers
Fig. 2.
Surgical equipment and setup include (A) surgical board, (B) microscope light source, (C) microscope, (D) rodent ventilator, (E) vaporizer, and (F) EKG system with computer
2.2 Extracellular Matrix Decellularization and Proteomic Analysis
1 % Sodium dodecyl sulfate [w/v] in distilled, deionized water.
Distilled, deionized water.
1× Complete Mini Protease Inhibitor cocktail with 1 mM EDTA.
Protein extraction reagent type 4.
5 mL Round-bottom tubes.
1.5 mL tube.
Gyro Twister™ 3D Shaker.
Homogenizer.
3 Methods
3.1 Mouse Intubation and Coronary Artery Ligation
Pre-surgical Preparation
Sterilize surgical instruments using the glass bead sterilizer for 12–15 s per instrument.
Place the mouse in the induction chamber.
Adjust vaporizer to instill a 2 % isoflurane in 100 % oxygen mix into induction chamber.
Induce anesthesia: 30-s to 1-min exposure or until mouse is unconscious.
Move mouse to a warm surgical board and place anesthetic nose cone over the face of the mouse.
Administer inhalant anesthetic to maintain an unconscious state, i.e., 2 % isoflurane and 100 % oxygen mix via ventilator. Adjust stroke volume and ventilation rate according to the manufacturer’s recommendations.
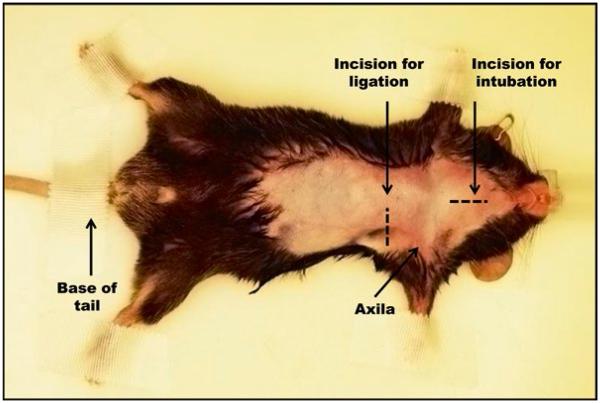
Place the mouse in a supine position; tape its paws and base of the tail to the surgical board (Fig. 3).
Using a cotton-tipped applicator, gently apply Nair® to the thorax and neck area to remove hair.
Sanitize the neck and thorax with a gauze containing 70 % EtOH followed by a gauze containing betadine.
Fig. 3.
Pre-surgical preparation and immobilization of mouse. The mouse is in the supine position and immobilized at paws and base of tail, under anesthesia delivered through a mouse nose cone. Area to be shaved and surgical incisions are indicated by the broken lines.
Intubation Procedure
Insert needle electrodes into the subcutaneous space of the forelimbs and hindlimbs to monitor the animal’s EKG. Record baseline EKG.
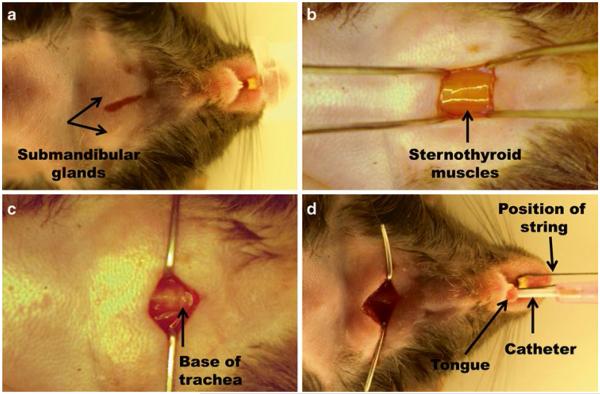
Make a 1 cm longitudinal incision on the ventral midline of the neck (Fig. 4a) exposing the submandibular glands; use blunt dissection to expose the sternothyroid muscles (see Fig. 4b and Note 1).
Retract the sternothyroid muscles laterally to expose the ventral aspect of the trachea (Fig. 4c).
Before intubation, isoflurane percentage may be increased for a brief period in order to increase the level of anesthesia and facilitate intubation.
The nose cone is removed; a 10–15 cm piece of string is placed around the upper incisors and gentle traction is applied cranially so that the airway is open and straight.
To intubate, lift the tongue with forceps and guide 20 ga IV catheter into trachea with IV catheter needle as support while monitoring with microscope to ensure clear passage. Advance the catheter until the tip is visible within the trachea through the neck incision (Fig. 4d). The tip of the catheter can be colored with a black marker to facilitate visualization within the trachea.
Remove the support needle and connect the anesthesia tubing from the nose cone to the IV catheter. Once intubated, isoflurane percentage should be adjusted to 2 % and the stroke volume and stroke/min adjusted based on the size of the animal.
Fig. 4.
Intubation procedure. (a) The incision for intubation is made using the submandibular glands as a landmark. (b) Beneath submandibular glands the sternothyroid muscles cover the trachea. (c) Retraction of the sternothyroid muscles allows for visualization of the base of trachea. (d) The catheter is properly guided using the incision located in the neck area
Ligation Procedure
Cut a 1 cm × 1 cm strip of gauze and place in saline.
Make a 1–1.5 cm transverse incision at mid-thorax starting left of ventral midline and extending laterally, parallel to the ribs and approximately 2 cm below the left axilla.
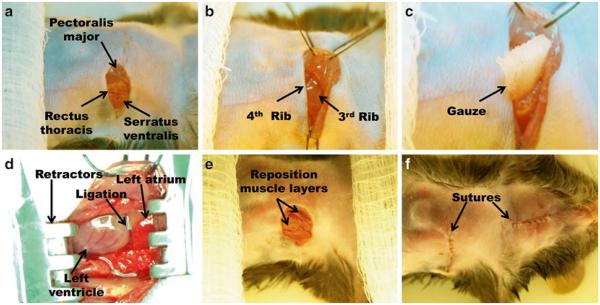
Use blunt dissection to expose the left pectoralis major muscles. Place a 3–0 retention suture around the muscles and retract toward the right shoulder of the mouse in order to expose the rib cage (Fig. 5a, b).
Place additional 3–0 retention suture around the left rectus thoracis and serratus ventralis muscles. Retract these muscles toward the left side of the mouse and affix the retention suture with tape to the surgical board as close to the body of the mouse as possible (Fig. 5a, b).
Bluntly dissect the muscle striations at the intercostal space between the third and fourth ribs to make a 0.25 cm incision that penetrates into the thoracic cavity (Fig. 5b).
Place the strip of gauze into the thoracic incision and gently push down toward lungs (Fig. 5c). It is important to avoid damage to the lungs.
Slowly enlarge the thoracic incision to approximately 1 cm long using blunt dissection technique applied medially and laterally while using the gauze to protect the lungs.
Apply the Mini-Goldstein retractor to the incision and gently spread ribs in order to clearly visualize the left atrium and left ventricle (Fig. 5d).
Use fine forceps and blunt-ended curved scissors to remove pericardium and provide access to the left ventricular free wall for identification of the left anterior descending coronary artery (LAD). Identifying the artery is required for successful occlusion. For references on the murine coronary artery anatomy, please see refs. 9, 16.
Using a Castroviejo needle holder, guide an 8–0 suture under the LAD 1 mm distal to the left atrium and ligate securely with a square knot.
Confirm a discrete blanched region on the LV under microscope and ST segment elevation on the EKG.
Remove the rib retractor.
Place one 6–0 suture to encircle the outer edges of the separated ribs, remove the gauze, and secure the knot so that the ribs are repositioned in the chest and the incision is closed. Remember to double-check that the gauze was removed prior to closing the chest.
Remove the retention sutures and reposition the retracted muscle layers of the chest (Fig. 5e).
Close the thoracic and neck incisions using 6–0 suture (see Fig. 5f and Note 2).
Administer Buprenex (0.1 mg/kg IP).
Remove all the tape, and turn off isoflurane but allow the oxygen to continue to flow.
Move the mouse to the prone position and extubate. After extubation, spontaneous breathing should begin immediately.
Remove the EKG needle electrodes.
Place animal in a 37 °C incubator to recover (see Notes 3 and 4).
Fig. 5.
Ligation procedure. (a) Incision and identification of the muscle layers covering the rib cage. (b) Retraction of muscle layers and location of the third intercostal space. (c) Position of the gauze to protect the lungs and assist with the intercostal incision. (d) Position of LAD ligation with respect to left atrium. (e) Relocation of muscle layers and (f) suturing the skin incisions
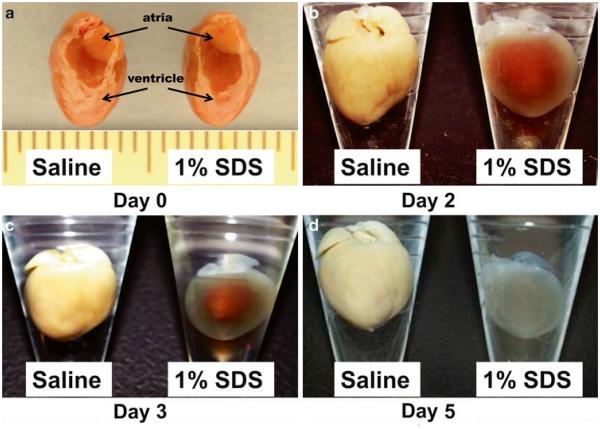
Day 0 (Fig. 6a)
Fig. 6.
Decellularization process of the whole heart. (a) Representative day 0 images of freshly dissected heart ventricle and atria in saline (left ) and 1 % SDS (right ). (b–d) Representative images of (b) day 2, (c) day 3, and (d) day 5 whole heart in saline (left ) and 1 % SDS (right )
Dissect the LV and separate remote from infarct tissue, based on TTC staining.
Record infarct and remote wet tissue weight separately.
Incubate remote and infarct tissue separately in a round-bottom tube containing 4mL of distilled H2O with 1× protease inhibitor for 30 min with gentle shaking.
Decant the solution and replace with 1 % SDS with 1× protease inhibitor cocktail at room temperature for 24 h with gentle shaking.
Day 1
Collect 1 mL of the supernatant, discard the remaining solution, and replace with fresh 1 % SDS with 1× protease inhibitor cocktail for 24 h with gentle shaking.
Day 2 (Fig. 6b), Day 3 (Fig. 6c), and Day 4
Each day, discard the solution and replace with fresh 1 % SDS with 1× protease inhibitor cocktail at room temperature for 24 h with gentle shaking.
Day 5 (Fig. 6d)
Discard the solution and wash decellularized tissue with 2 mL of distilled H2O with 1× protease inhibitor for 5 min with gentle shaking (see Note 5).
Discard the solution and wash with 4 mL of distilled H2O with 1× protease inhibitor for 24 h with gentle shaking.
Discard the solution and transfer decellularized tissue to 1.5 mL tube and add Sigma reagent 4 with 1× protease inhibitor cocktail at 5 μL for every 1 mg of wet tissue weight.
Homogenize the tissue for 5 s, four times with 1-min intervals.
Sonicate the homogenate for 5 s, four times. Incubate the sample at 30 °C for 1 h.
Store at −80 °C.
Rewarm the sample to 30 °C for 1 h before determining protein concentration assay.
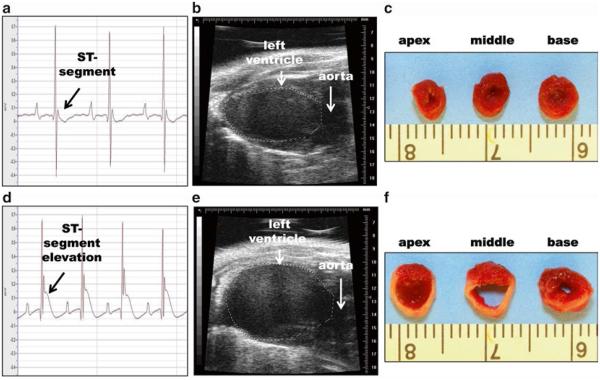
Fig. 7.
Control (a–c) and post-MI (d–f) EKGs, echocardiograms, and TTC-stained images. (a) Murine EKG, (b) long-axis echocardiogram, and (c) TTC-stained left ventricle in control mice. (d) 15-min post-MI EKG, (e) day 7 post-MI long-axis echocardiogram, and (f) matching day 7 TTC-stained left ventricle
Acknowledgements
We acknowledge funding from AHA (09POST2150178) to RZ, NCCAM (1K99AT006704-01) to GH, and NHLBI HHSN 268201000036C (N01-HV-00244) for the UTHSCSA Cardiovascular Proteomics Center and R01 HL-075360, the Max and Minnie Tomerlin Voelcker Fund, and the Veteran’s Administration (Merit) to MLL.
Footnotes
4 Notes
Blunt dissection is the primary technique used in this surgical procedure. The only steps that require the use of surgical scissors are step 2 during intubation and steps 2, 7, and 9 during the ligation procedure.
When performed correctly, no bleeding should occur throughout the entire procedure. However, if blood loss occurs, saline can be injected intraperitoneally.
Average time for the entire procedure is 25–30 min.
Representative baseline and post-MI EKGs, echocardiograms, and TTC-stained images are shown in Fig. 7.
Decellularized tissue appears translucent in color. If the tissue still appears yellow in the center after 5 days, add fresh 1 % SDS with 1× protease inhibitor cocktail and continue to incubate at room temperature for an additional 24 h.
References
- 1.Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the united states: risk-standardized mortality rates from 1995–2006. JAMA. 2009;302:767–773. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Pfeffer JM. Ventricular enlargement and reduced survival after myocardial infarction. Circulation. 1987;75:IV93–IV97. [PubMed] [Google Scholar]
- 4.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 5.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: patho-physiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 6.Blankesteijn WM, Creemers E, Lutgens E, Cleutjens JP, Daemen MJ, Smits JF. Dynamics of cardiac wound healing following myocardial infarction: observations in genetically altered mice. Acta Physiol Scand. 2001;173:75–82. doi: 10.1046/j.1365-201X.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther. 2012;30(1):31–41. doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. J Mol Cell Cardiol. 2010;48:558–563. doi: 10.1016/j.yjmcc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salto-Tellez M, Yung Lim S, El-Oakley RM, Tang TP, ZA AL, Lim SK. Myocardial infarction in the c57bl/6j mouse: a quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 2004;13:91–97. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Bo H, Meng X, Wu Y, Bao Y, Li Y. A simple and fast experimental model of myocardial infarction in the mouse. Tex Heart Inst J. 2006;33:290–293. [PMC free article] [PubMed] [Google Scholar]
- 11.Zamilpa R, Kanakia R, Jt C, Dai Q, Escobar GP, Martinez H, Jimenez F, Ahuja SS, Lindsey ML. Cc chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300:H1418–H1426. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar D, Hacker TA, Buck J, Whitesell LF, Kaji EH, Douglas PS, Kamp TJ. Distinct mouse coronary anatomy and myocardial infarction consequent to ligation. Coron Artery Dis. 2005;16:41–44. doi: 10.1097/00019501-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Klocke R, Tian W, Kuhlmann MT, Nikol S. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res. 2007;74:29–38. doi: 10.1016/j.cardiores.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- 15.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 16.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]