Abstract
Objective
To examine depressive behavior and early coronary artery atherogenesis in 36 socially housed female cynomolgus monkeys, an established model of atherogenesis and depression. Coronary heart disease (CHD) is caused by coronary artery atherosclerosis (CAA) and its sequelae which develop over a period of decades. Thus, in prospective studies of depression and CHD, CAA was likely present at baseline in most subjects who experienced cardiac events. Little is known about the relationship between depression and CAA.
Methods
The monkeys were free of atherosclerosis before being fed a diet containing moderate amounts of fat and cholesterol for 52 months. Depressed behavior and activity levels recorded in weekly 15-minute focal samples, telemetered 24-hour heart rate, plasma total (TPC) and high-density lipoprotein cholesterol (HDLC), luteal phase serum progesterone concentrations, basal cortisol, cortisol response to corticotrophin-releasing hormone (CRH), and CAA extent were assessed.
Results
Time spent in depressed behavior over 4 years was significantly associated with early CAA (r = 73, p = .001), as were activity level, 24-hour heart rate, TPC, HDLC, cortisol response to CRH, and mean peak progesterone (all p ≤ 0.05). Depressed females had four times the CAA compared with nondepressed females.
Conclusions
Depression in primates is associated with perturbations in multiple CHD risk factors and accelerated early atherogenesis. These data are consistent with the hypotheses that depression and CAA both stem from a common mechanism and that depression may cause CAA.
Keywords: depression, CHD, coronary artery atherosclerosis, heart rate, ovarian function, women’s health, cholesterol
INTRODUCTION
Depressive disorders are twice as likely in women as men (1). Excluding suicide, major depression is associated with increased mortality, perhaps in part due to a high rate of comorbidities (2–5). The comorbidity of depression and coronary heart disease (CHD) is particularly marked; because CHD is the leading cause of death of women (6), depression may be particularly important to the cardiovascular health of women.
A history of major depression or current depression predicts CHD morbidity and mortality in cohorts with no clinical evidence of CHD when depression was initially assessed, an effect that remains after controlling for confounding factors such as smoking (7,8). Meta-analyses suggest that having depression is associated with an elevated relative risk of 1.64 for onset of coronary disease (9,10). Thus, the majority of the published literature supports an etiologic role for depression in CHD. Furthermore, several studies demonstrated a graded relative risk of CHD with depression severity, suggesting that milder forms of depression in addition to major depressive disorder may be clinically relevant to CHD risk (8,9,11).
CHD is caused by coronary artery atherosclerosis (CAA) and its sequelae, and coronary artery atherogenesis begins very early in life and proceeds over a period of decades (12). Thus, individuals who are free of clinical CHD may still have significant subclinical CAA. In most of the available prospective studies of depression and CHD, CAA was likely present at the baseline evaluation in subjects who experienced cardiac events during the follow-up periods. This observation suggests that studies in which depression at baseline predict subsequent cardiac events may be describing the coincidence of depression with CAA, rather than a causal role for depression in the pathogenesis of CHD. In support of this hypothesis, a relationship between a history of depression and coronary and aortic calcification has been observed in middle-aged women (13).
A growing body of literature identifies perturbations in underlying mechanisms that may be causally related to both depression and CHD. Some of the more commonly cited work includes arrhythmias, platelet reactivity, and proinflammatory processes (8). Perturbations of stress responsive systems, such as the autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis, may also link these two diseases. Depression is often accompanied by increased heart rate, which is associated with increased CHD risk in people and increased CAA in monkeys (14,15). Excessive sympathetic arousal, manifested as high heart rate or blood pressure, has long been thought to contribute to endothelial damage, leading to inflammation and procoagulatory events (12). Likewise, perturbations of the stress-reactive HPA axis are common in depression (16), and recent observations suggested that cortisol may promote endothelial dysfunction (17). The literature is mixed as to whether lipid metabolism is perturbed in depression. Notably, the results of two of the largest studies, both evaluations of several thousand adults, suggested inverse relationships between high-density lipoprotein cholesterol (HDLC) and depressive symptomology in women, and this effect seems to be sex-specific (18,19). Poor ovarian function increases the risk of CHD in women (20), and decreased ovarian hormone concentrations exacerbate CAA in nonhu- man primates (Macaca fascicularis) (21). We have observed suppressed ovarian function in cynomolgus monkeys (M. fascicularis) exhibiting depressive behavior (22). Thus, one mechanism through which depression might increase CHD risk in women is hypothalamic-pituitary-gonadal (HPG) axis suppression.
Thorough characterization of the relationship between depression and CAA and identification of potential common mediators that might be perturbed in both diseases is difficult to accomplish in clinical studies. An appropriate animal model would be helpful in sorting out temporal relationships between depressive disorder and atherogenesis. Adult female cynomolgus monkeys have been used effectively as a model to study both atherogenesis and depressive behavior and to understand risk factors for atherogenesis. This model was the first to demonstrate experimentally that poor ovarian function results in increased CAA (21), social factors exacerbate atherosclerosis (21), and the timing of estrogen therapy in menopause determines whether it has beneficial effects on CAA (23,24). All of these observations have since been confirmed in women (20,25–29); hence, this has been a useful translational model of CAA in female primates. Some adult female cynomolgus monkeys exhibit depressive behavior, defined as including three components: 1) a slumped or collapsed body posture; 2) a relative lack of responsiveness to environmental stimuli to which other monkeys are attending; and 3) open eyes to distinguish this behavior from resting (30). Many behavioral and biological characteristics of monkeys that exhibit this depressive behavior are similar to those of depressed human beings including reduced body fat, low levels of activity, suppressed ovarian function, low insulin-like growth factor-1 (IGF-1), dyslipidemia, high heart rate, HPA axis disturbances, and reduced neural serotonin receptor 1a binding potential determined pre-mortem by positron emission tomography (22,31–33). Also, like human beings, low social status increases the likelihood of depressive symptomology in monkeys (30,34). Taken together, adult female cynomolgus monkeys seem to be a valid model of depression and CHD risk.
The purpose of this study was to examine the relationship between coronary artery atherogenesis and depressive behavior in female cynomolgus monkeys. This study is especially valuable in determining the temporal relationship between atherogenesis and depressive behavior because, unlike human beings, these monkeys have little or no atherosclerosis at the start of the study. Atherogenesis is induced by the consumption of a Western-like experimental diet containing fat and cholesterol.
MATERIALS AND METHODS
Subjects
Details of the study have been published previously (22,33). Thirty-six female cynomolgus monkeys were obtained directly from Indonesia (Institut Pertanian Bogor, Bogor, Indonesia). Selection criteria were 1) good health as determined by a clinical veterinarian, and 2) adult age as indicated by dentition. After a 3-month quarantine, the monkeys were trained for 4 months to willingly participate in awake blood sampling and vaginal swabbing. All procedures involving primates were conducted in compliance with institutional, state, and federal law for the use of primates in laboratory settings. During the study, nine animals died, resulting in 27 animals completing the protocol. Behavioral depression was associated with an increased mortality rate (correlation between days lived and depression: r = −.41, p ≤ .02). Causes of death followed no discernible pattern; for example, two monkeys died of trauma inflicted by cage mates, two did not recover from sedation, and one became ketotic and died. Depression in humans is also associated with increased (nonsuicide) mortality rates (2).
Diet
The animals consumed an imprudent diet, designed to approximate that consumed by some Americans, which contained 0.28 mg of cholesterol/Cal (approximately equal to a human consumption of 500 mg/day) and 42% of calories as fat for 53 months, from 3 months after arrival until necropsy.
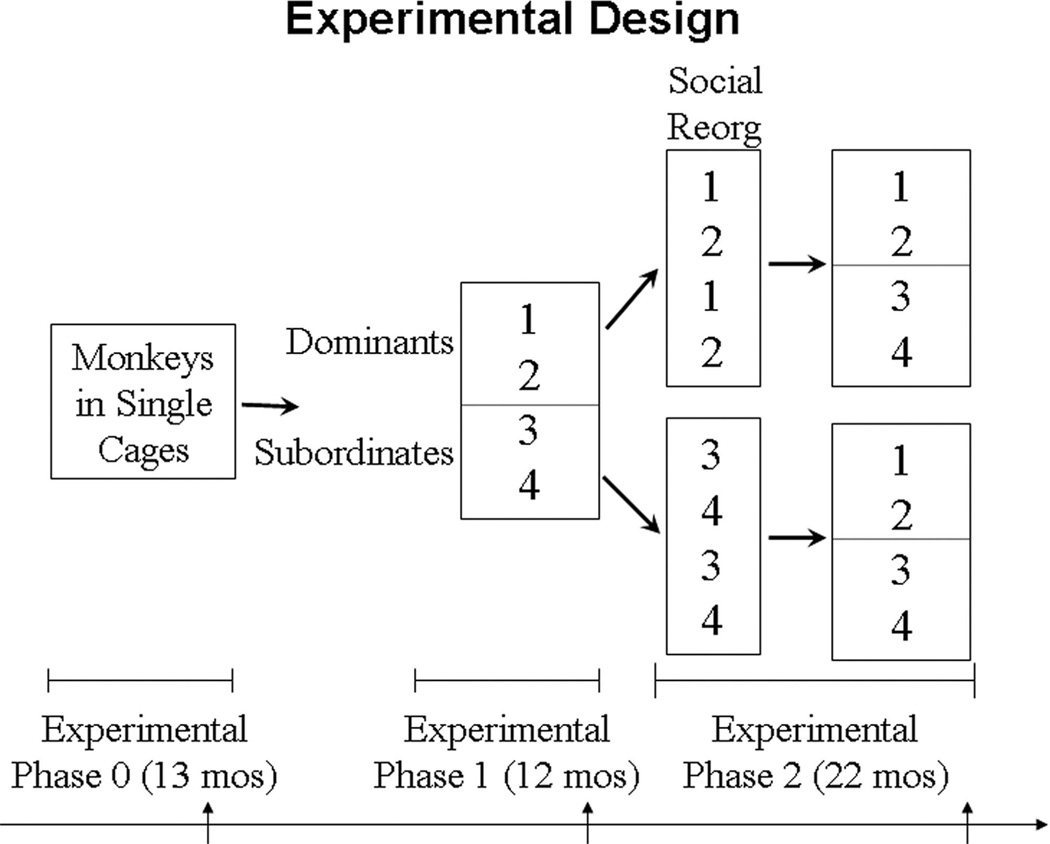
Experimental Design
The experiment was divided into three phases (Figure 1). During Phase 0, the animals were housed in single cages for 13 months. They had visual, auditory, and olfactory but not tactile contact with other monkeys. In Phase 1, the monkeys were randomly assigned to small social groups of four animals each and housed in 3.05 m × 3.05 m × 3.05 m indoor enclosures with visual access to the outside for 12 months. Social status hierarchies formed. At the onset of Phase 2, social groups were systematically reorganized once. Animals that were subordinate in Phase 1 (third or fourth ranking) were housed together in social groups of four; likewise, animals that were dominant (first or second ranking) in Phase 1 were housed together in social groups of four. Linear social status hierarchies reformed. Thus, half of the animals that were previously subordinate became dominant, and half of the previously dominant animals became subordinate, presumably changing the amount of social stress monkeys experienced. The monkeys lived in their second social groups for 22 months. This design allowed us to determine whether there were characteristics of individuals in nonsocial settings that predicted subsequent disease, and it permitted us to evaluate whether changing social status changed disease risk, which was reported in the work of Shively and associates (22).
Figure 1.
Experimental design. Social groups were reorganized once at the onset of Phase 2 (Social Reorg). Behavior and ovarian function were assessed throughout the study, and other clinical evaluations were made at the end of each phase as indicated by the arrows.
Behavior and menstrual cycles were documented throughout the experiment. At the end of each phase, the following measurements were made: HPA axis function (dexamethasone suppression test, adrenocorticotropic hormone (ACTH) challenge test, corticotrophin-releasing hormone (CRH) challenge test), heart rate, total plasma cholesterol (TPC), HDLC, body weight and body mass index (body weight/body length [suprasternal notch to pubic symphysis]2). Body weight and body mass index were significantly inversely correlated with depression in Phase 1 only (22), and not significantly correlated with CAA extent; therefore, they are not considered further in this report.
Social Status
After the formation of social groups, social status was determined monthly by recording the outcomes of aggressive interactions between cage mates. The animal in each social group to which all members submitted was designated the first ranking monkey. The animal to which all but the first ranking monkey submitted was designated the second ranking monkey, and so forth. As observed in previous experiments, the resulting social status hierarchies for each social group were stable over time (35). Social status was used as a continuous variable in statistical analysis. The number of monkeys to which an animal was dominant was divided by social group size minus 1, resulting in dominance scores that ranged from 0 to 1. Average monthly social status within Phase 1 and Phase 2 were used for analysis.
Behavioral Depression and Locomotion
The definition of depressive behavior includes three components: 1) a slumped or collapsed body posture; 2) a relative lack of responsiveness to environmental stimuli to which other monkeys are attending; and 3) open eyes to distinguish this behavior from resting (30). An example is depicted in Figure 2. Locomotion was defined as traversing space equivalent to a minimum of one body length. Time spent in the depressed posture and in locomotion was recorded for 15 minutes weekly (an average of 50 hours/monkey total), counterbalanced for time of day, throughout the experiment using a focal animal technique described previously (22,36). These behaviors are easily recognizable; interrater reliability, determined biannually, was ≥0.92 throughout the experiment. The average time spent depressed each month was calculated from these observations. Previously, we demonstrated that the number of months in which a monkey was observed in the depressed posture was highly correlated with the average time spent in the depressed posture throughout the experimental phase (r = .84, p < .001) (22). Thus, time spent in the depressed posture was averaged during each phase and over the entire experiment for analysis. Time spent locomoting was averaged during each phase and over the entire experiment for analysis.
Figure 2.
Monkey displaying depressed (left) or alert (right) behavior.
Reproductive System Function
The monkeys were trained to present themselves for vaginal swabbing to detect menses and for femoral venipuncture to collect blood for progesterone assay three times a week throughout the experiment. Luteal-phase progesterone concentration was used as an indicator of the quality of a menstrual cycle. Previous studies suggested that high progesterone concentrations (>4 ng/mL) indicate that ovulation had occurred; low progesterone concentrations (<2 ng/mL) indicate an anovulatory cycle; and concentrations between 2 and 4 ng/ml suggest luteal phase impairment (30,37). The highest progesterone value found during the luteal phase was used to represent that menstrual cycle, and these peak progesterone concentrations were averaged for each phase and over the duration of the experiment.
Heart Rate
Physiological responsivity was assessed by recording telemetered heart rate for 24 hours. After capture and sedation with ketamine hydrochloride (10 mg/kg), each monkey was outfitted with a nylon mesh protective jacket over a portable electrocardiogram telemetry unit. After overnight recovery from sedation, heart rate recording began the next morning (approximately 7 AM) and continued for 24 hours. These measurements were averaged over the 24-hour period for analysis for each phase and over all three phases (32).
HPA Function
Dexamethasone suppression tests were performed (22). Blood samples were obtained within 5 minutes of capture and sedation with ketamine hydrochloride (10 mg/kg). The difference between the first and second morning cortisol levels (percentage suppression) was calculated as an indicator of sensitivity to negative feedback. Per cent suppression was not significantly associated with either depressive behavior or atherosclerosis extent. Baseline cortisol level (first morning sample) and percentage suppression were used as dependent variables. The cortisol response to ACTH challenge was determined (22). Area under the cortisol curve was used as the dependent variable. It was not significantly associated with either depressive behavior or atherosclerosis. The response to CRH challenge was assessed as previously described (22). Circulating ACTH and cortisol levels were measured 15, 30, 45, and 60 minutes after CRH injection. The areas under the cortisol and ACTH curves within each phase, and averaged over all three phases, were used as dependent variables. The area under the ACTH curve was not significantly associated with either depressive behavior or atherosclerosis extent.
Assays
TPC and HDLC concentrations were measured, using enzymatic methods on the COBAS FARA II analyzer (Roche Diagnostics Inc., Montclair, New Jersey), with protocols and reagents supplied by Boehringer Mannheim (Mannheim, Germany). HDLC concentrations were measured, using the heparin-manganese precipitation procedure (38) and as described in the Manual of Laboratory Operations of the Lipid Research Clinics Program (39). Progesterone, cortisol, and ACTH were determined, using commercially available radioimmunoassays (Diagnostic Products Corporation, Los Angeles, California). Interassay coefficients of variation for all clinical chemistry values were <7%.
CAA Evaluations
Twenty-seven monkeys completed the 53-month protocol and were euthanized in accordance with guidelines established by the American Veterinary Medical Association. The animals were sedated with ketamine hydrochloride (15 mg/kg body weight, IM) and administered sodium pentobarbital to attain surgical anesthesia (approximately 13 mg/kg, IV). An infusion of lactated Ringer’s solution was initiated via the left ventricle, an incision was made in the abdominal vena cava for drainage of blood from the cardiovascular system, and euthanasia was affected with an overdose of sodium pentobarbital (approximately 80 mg/kg, IV). The heart was removed, the coronary arteries were perfused via the aorta for 1 hour at 100-mm Hg pressure using 10% neutral buffered formalin, and then preserved by immersion of the heart in 10% neutral buffered formalin. Five tissue blocks (each 3 mm in length) were cut perpendicular to the long axis of the left anterior descending, left circumflex, and right coronary arteries. The blocks were embedded in paraffin and one 5 µm section from each block was stained with Verhoeff-van Gieson’s stain. Artery size (i.e., cross-sectional area inside the internal elastic lamina) and lumen area were determined using computer-assisted morphometry (Image Pro Plus, Media Cybernetics, Bethesda, MD). Atherosclerotic plaque area (cross-sectional area of the intima) was calculated as the total area between the internal elastic lamina and the lumen (40). Measurements were made blind by an experienced technician. The mean plaque area of these 15 sections was used in the analysis.
Statistical Analysis
The data were analyzed using correlations and t tests. Nonparametric Spearman rho correlations gave essentially the same results as Pearson r correlations; thus, Pearson r correlations are reported here. The criteria for significance was set at p = .05. All reported p values are the result of two-sided tests. The data were analyzed from two perspectives: 1) values for all end points were averaged over the course of the study and examined in relationship to CAA extent; and 2) values for all end points for each phase (Phase 0, single cage; Phase 1, first social group; Phase 2, second social group) were examined in relationship to CAA extent to assess the temporal relationship between depressive behavior and CAA extent.
RESULTS
Characteristics of CAA
After 4 years of consumption of a moderately atherogenic diet, CAA lesions ranged from fatty streaks, which usually have cross-sectional areas of ≤0.2 mm2, to small fatty and fibrous plaques, which usually have cross-sectional areas of >0.2 mm2. Mean ± standard error of the mean plaque size was 0.245 ± 0.06 mm2.
Characteristics of Depressive Behavior
The pattern of expression of behavioral depression is described by Shively and colleagues (22). Over the course of the experiment, monthly averages for time spent in the depressed posture varied from 0% to 52% (2.4% ± 0.17) (Figure 2). Over the 47 months in which behavior was observed, all but one monkey displayed this behavior at some point. However, there was much intra- and intermonkey variability. The percent time spent in behavioral depression was highest during the Phase 1 social groups and lowest during the Phase 2 social groups, probably due to early mortality of the most depressed monkeys.
Associations Between Depressive Behavior, Cardiovascular Risk Factors, and CAA Extent
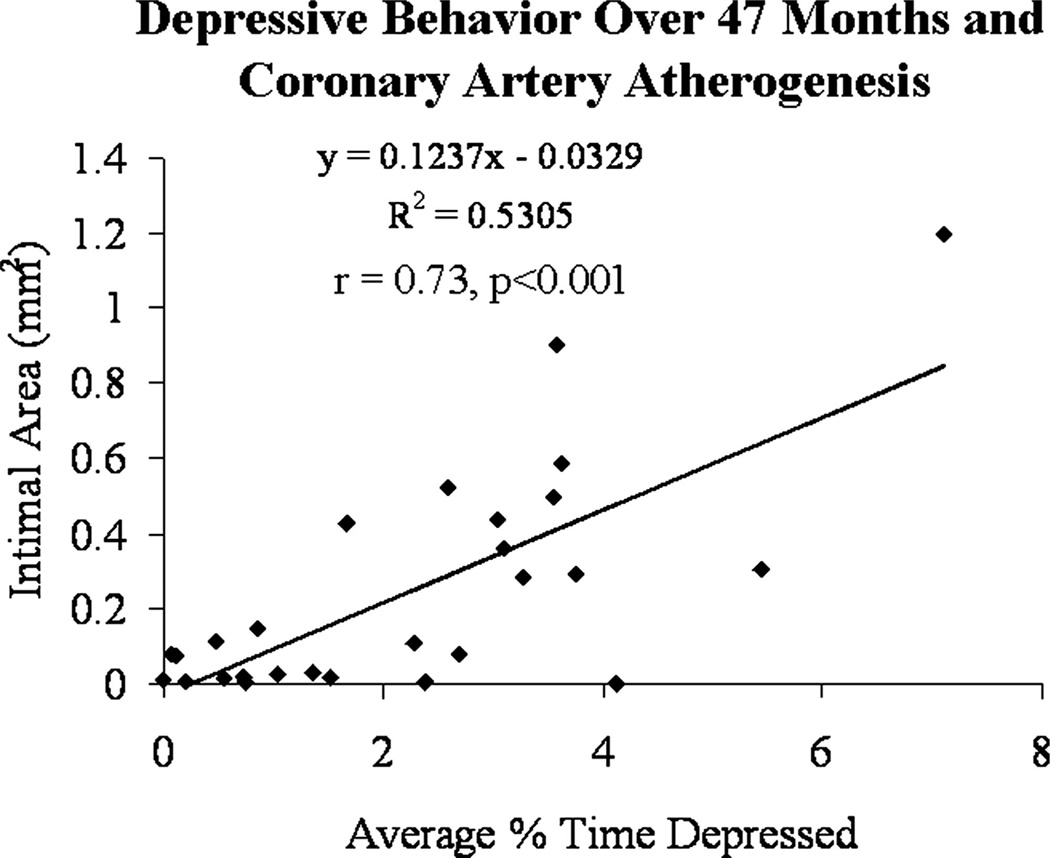
Initially, associations between risk factors averaged over all phases of the experiment and CAA extent were determined (Table 1). Significant correlations with atherosclerosis extent were observed with the percent time spent in the depressed posture, percent time spent locomoting, TPC, HDLC, mean 24-hour heart rate, mean peak luteal phase progesterone concentrations, basal cortisol, and the cortisol response to CRH challenge measured as area under the curve (AUC) (Table 1). Correlations between atherosclerosis extent and all other variables measured did not reach significance. Percent time spent locomoting, TPC and HDLC concentrations and 24-hour heart rate were also significantly associated with the average percent time spent in the depressed posture. The average percent time spent depressed, observed over 47 months, was closely associated with coronary artery atherogenesis (r = .73, p < .001) (Figure 3).
TABLE 1.
Pearson r Correlations Between Risk Factors, Depression, and Coronary Artery Atherosclerosis
| Variables Averaged Over the Entire Experiment |
% Time Spent Depressed |
Coronary Artery Atherosclerosis Extent (mm2) |
|---|---|---|
| % Time spent depressed | 0.73***3 | |
| % Time locomoting | −0.61*** | −0.53** |
| TPC | 0.57*** | 0.863 |
| HDLC | −0.40* | −0.66*** |
| 24-Hour heart rate | 0.57** | 0.67*** |
| Mean peak progesterone (ng/ml) | −0.35 | −0.52** |
| Basal cortisol (µg/ml) | −0.23 | −0.45* |
| Cortisol AUC in CRH test | −0.32 | −0.40* |
AUC = area under the curve; CRH = corticotrophin-releasing hormone.
p ≤ .05;
p ≤ .01;
p ≤ .001.
Figure 3.
Linear relationship between a history of depressive behavior and coronary artery atherosclerosis extent.
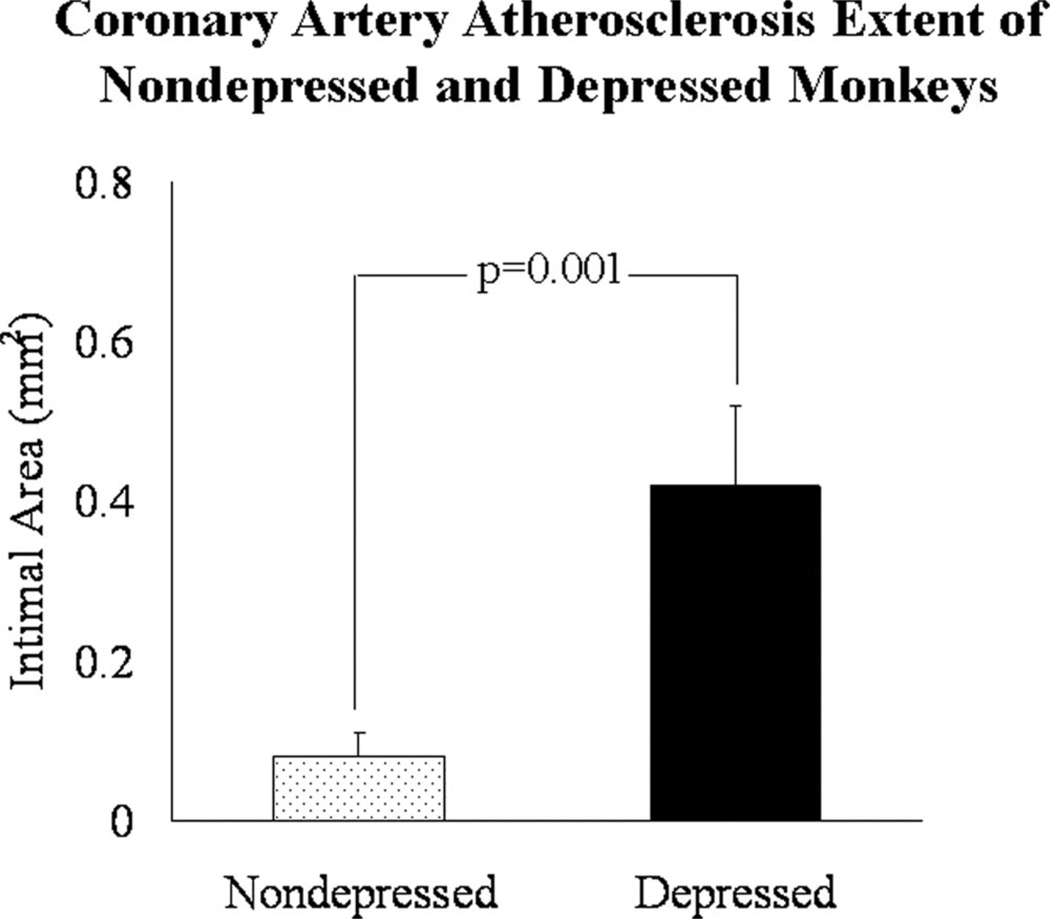
Graphical representation of the magnitude of the differences in atherosclerosis extent in monkeys who fell above (designated “more depressed”) versus those that fell below (designated “less depressed”) the mean in percent time spent depressed are depicted in Figure 4. Monkeys in the more depressed group developed plaques that were five times larger than those from monkeys in the less depressed group. Representative examples of atherosclerotic lesion from cross sections of the left anterior descending artery of a more depressed and less depressed monkey are shown in Figure 5. Lesion area of the less depressed monkey was minimal and consisted of eccentric fatty streak and small fibro-fatty plaque. The lesion of the more depressed monkey was much more extensive, consisting of a moderately advanced concentric fibro-fatty to fibro-muscular plaque with a small focus of calcium. Multifocal destruction of the internal elastic lamina (IEL) and media and lipid infiltration into the media were apparent.
Figure 4.
Magnitude of the differences in coronary artery atherosclerosis extent of less depressed (n = 17) and more depressed (n = 10) females. A t test was used to generate the p value.
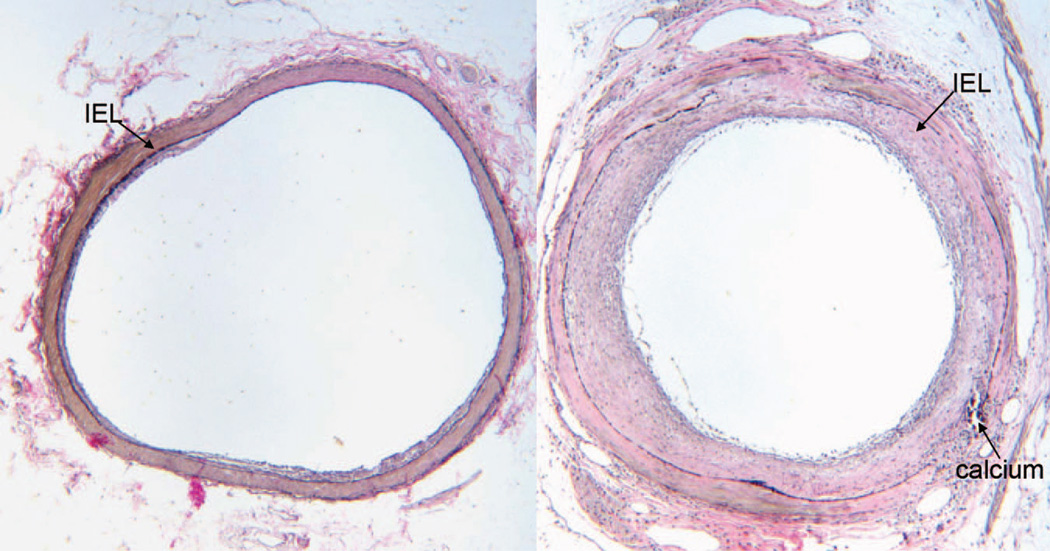
Figure 5.
Representative examples of atherosclerotic lesion from cross sections of the left anterior descending artery of a nondepressed (left) and depressed (right) monkey. Atherosclerotic lesion was quantified as the area of the intima (IA), i.e., the area internal to the internal elastic lamina (IEL). Sections were stained with Verhoeff van Gieson stain. Lesion area of the nondepressed monkey was minimal (IA = 0.08 mm2), consisting of eccentric fatty streak and small fibro-fatty plaque. The lesion of the depressed monkey was much more extensive (IA = 0.52 mm2), consisting of a moderately advanced concentric fibro-fatty to fibro-muscular plaque with a small focus of calcium. Multifocal destruction of the IEL and media and lipid infiltration into the media were apparent.
The degree of association between risk factors and CAA extent was examined by experimental phase. Table 2 depicts the correlations of risk factors within each phase and CAA extent measured at the end of the experiment. Depressive behavior in single cages and the Phase 1 (first) social groups predicted CAA. Depressive behavior in the Phase 2 (second) social groups did not predict CAA extent, perhaps because of the early deaths of some of the most depressed monkeys. Plasma cholesterol concentrations (TPC and HDLC) and quality of ovarian function (measured as mean peak luteal phase progesterone concentrations) were consistently highly correlated with CAA extent throughout the experiment. The 24-hour heart rate and CAA were closely associated while animals lived in social groups but not single cages. As observed in previous studies, social status in Phase 1 social groups was inversely related to CAA extent, but the association did not reach significance in this relatively small experiment. Risk factors were significantly correlated with depression, particularly in Phase 1, when the monkeys were socially housed and before most of the differential mortality of the depressed animals.
TABLE 2.
Pearson r Correlations Between Risk Factors and Depression and Coronary Artery Atherosclerosis Within Social Condition
| Variables Averaged During Each Phase | % Time Depressed Phase 0 |
% Time Depressed Phase 1 |
% Time Depressed Phase 2 |
Coronary Artery Atherosclerosis |
|---|---|---|---|---|
| % Time depressed Phase 0 | 1.00 | 0.20 | 0.11 | 0.48** |
| % Time depressed Phase 1 | 0.20 | 1.00 | 0.28 | 0.67*** |
| % Time depressed Phase 2 | 0.11 | 0.28 | 1.00 | 0.09 |
| Social status Phase 1 | −0.04 | −0.07 | 0.01 | −0.31 |
| Social status Phase 2 | 0.03 | −0.14 | −0.39* | −0.06 |
| % Time locomoting Phase 0 | −0.24 | −0.37* | −0.19 | −0.40* |
| % Time locomoting Phase 1 | −0.21 | −0.60*** | −0.22 | −0.44* |
| % Time locomoting Phase 2 | −0.23 | −0.41* | −0.00 | −0.39* |
| Basal cortisol Phase 0 | −0.07 | −0.33* | −0.05 | −0.37 |
| Basal cortisol Phase 1 | 0.01 | −0.37* | −0.03 | −0.19 |
| Basal cortisol Phase 2 | 0.12 | −0.20 | 0.30 | −0.37 |
| Cortisol AUC CRH Phase 0 | 0.07 | −0.35* | −0.29 | −0.36 |
| Cortisol AUC CRH Phase 1 | 0.06 | −0.41* | −0.15 | −0.25 |
| Cortisol AUC CRH Phase 2 | −0.11 | −0.27 | 0.15 | −0.39* |
| TPC Phase 0 | 0.15 | 0.38* | −0.13 | 0.78*** |
| HDLC Phase 0 | −0.03 | −0.24 | 0.00 | −0.55** |
| TPC Phase 1 | 0.10 | 0.45** | −0.02 | 0.84*** |
| HDLC Phase 1 | −0.07 | −0.37* | −0.15 | −0.66*** |
| TPC Phase 2 | 0.11 | 0.76*** | 0.09 | 0.83*** |
| HDLC Phase 2 | 0.01 | −0.47** | −0.07 | −0.45* |
| Mean 24-hour HR Phase 0 | 0.33 | 0.15 | 0.46* | 0.21 |
| Mean 24-hour HR Phase 1 | 0.32 | 0.45* | 0.08 | 0.78*** |
| Mean 24-hour HR Phase 2 | 0.31 | 0.18 | 0.04 | 0.48** |
| Mean peak progest Phase 0 | −0.41* | −0.25 | −0.16 | −0.54** |
| Mean peak progest Phase 1 | −0.38* | −0.21 | −0.15 | −0.44* |
| Mean peak progest Phase 2 | −0.25 | −0.19 | −0.19 | −0.44* |
AUC = area under the curve; CRH = corticotrophin-releasing hormone; HDLC = high-density lipoprotein cholesterol; TPC = total plasma cholesterol; HR = heart rate; progest = progesterone.
p ≤ .05;
p ≤ .01;
p ≤ .001.
DISCUSSION
The relationship between depression and CHD could be one of three natures: CHD may cause depression; depression may cause CHD; or both diseases may be the product of perturbations of common underlying mechanisms. Clinical studies cannot easily discriminate among these three possibilities; the present study is unique in this regard. Atherosclerosis and its sequelae cause atherothrombotic CHD. Wild monkeys have little or no atherosclerosis; atherogenesis is driven by the consumption of a Western-type diet containing cholesterol and saturated fat (41), allowing the examination of the relationship between depressive behavior and early atherogenesis in the current study. Because little or no atherosclerosis was present at the initiation of the study, it is unlikely that atherosclerosis caused depressive behavior.
The average percent time spent in depressed behavior over 4 years was a strong predictor of atherosclerosis extent at the end of the study. Furthermore, depressive behavior in the first year predicted CAA extent 4 years later. These observations suggest that depressive behavior predicts early coronary artery atherogenesis in female primates. Clinical observations suggest that depression predicts CHD up to perhaps a decade before a clinical event. Atherogenesis has been underway for several decades by that time, and atherosclerosis is well advanced. The observations reported here push back the temporal relationship between depression and atherosclerosis to the earliest stages of atherogenesis.
Depressive behavior was apparent in the earliest behavior observations. After the onset of consumption of a Western-type diet, atherosclerosis develops continuously over long periods of time. Depression may become measurable before atherosclerosis. Therefore, from a temporal perspective, the two pathologies seemed to co-occur. Thus, a common mechanism could promote both disease processes—inflammation, for instance. On the other hand, depression could promote atherosclerosis because it was present from the earliest moments, and we know from decades of prior study of the pathogenesis of atherosclerosis that it progressed throughout the time period in which the animals were exposed to the atherogenic diet. The fact that depressive behavior predicts early atherogenesis is consistent with the hypothesis that depression and CAA both stem from a common mechanism, and with the hypothesis that depression and associated sequelae, may cause CAA. These hypotheses are not mutually exclusive; both may be true.
Depressive behavior measured during the first 2 years of the study (Phase 0 and Phase 1) was a better predictor of atherosclerosis extent than depressive behavior measured later in the experiment (Phase 2). The lack of association between depressive behavior late in the experiment and atherosclerosis extent may be due to the differential mortality of depressed monkeys. Most of the premature deaths of depressed monkeys occurred near the end of Phase 1, and included monkeys with the highest depression scores, thus truncating the distribution of depression scores in Phase 2. This reduction in the number of severely depressed monkeys may have eliminated a significant association between depressive behavior and atherosclerosis extent.
Depression and CHD have several pathophysiological risk factors in common, which could promote both diseases or through which depression could promote CHD. The most commonly cited are arrhythmias, platelet reactivity, and proinflammatory processes (9). Inflammation is of particular interest here because an atherogenic diet may promote inflammatory processes (42). Depression may be viewed as a chronic state of alarm also characterized by heightened inflammatory processes (43), and inflammation promotes atherogenesis. It could be that certain individuals are particularly likely to respond to distress with increased inflammation; these individuals may be at heightened risk for atherosclerotic heart disease and perhaps depression as well. From this perspective, an atherogenic diet plus social stress may have a synergistic proinflammatory effect that increases the risk of depression and CHD.
There are several other pathophysiological characteristics that may be common to both diseases, through which depression could promote CHD. For example, depression is often accompanied by increased heart rate, which is associated with increased CHD in people and CAA in monkeys (14,15,31). Here, we show that depressed females have high 24- hour heart rates; and previously, we demonstrated that depressed females also have high night time (midnight to 4 AM) or resting heart rates. Because of the tonic inhibitory control of heart rate by the parasympathetic nervous system, resting heart rate is an index of vagal function and autonomic balance (44). High resting heart rate may denote relatively reduced parasympathetic modulation of cardiac function, which is associated with arrhythmias (8). An autonomic balance shift in favor of sympathetic input can induce inflammatory reactions and reduce anti-inflammatory reactions, resulting in cytokine release and monocyte cell activation which in turn may exacerbate atherogenesis (45). These observations suggest the possibility that perturbed central nervous system control of autonomic function in depression may increase damage to the endothelium and contribute to atherogenesis.
Likewise, HPA axis function is often perturbed in depressed people and associated with endothelial dysfunction (17). Perturbed HPA function is also characteristic of depressed monkeys (22,30). Manifestations of changes in HPA function in depressed human beings are variable. HPA axis secretions and responses to stimulation change over time, are different in individuals with a history of depression depending on whether they are currently depressed, and may vary with prior experience (46). For example, Heim et al. (47) compared women with and without a history of child abuse who did or did not have major depressive disorder. These four groups each had distinctive ACTH and cortisol secretion patterns. Women with a history of childhood abuse and major depressive disorder had lower cortisol levels in response to CRH, a pattern similar to that displayed by the depressive monkeys in this study (22). However, basal cortisol, an easier measure to acquire, was as good a predictor of CAA as the cortisol response to CRH challenge. In this experiment, low circulating cortisol predicted greater time spent in depressive behavior and more CAA. Lower cortisol levels may indicate a relatively exhausted adrenal gland.
There is a linear dose-response relationship between physical activity and CHD in human beings (48). Likewise, physical activity is associated with reduced depression (49). Here, time spent locomoting was inversely associated with both depressive behavior and CAA extent. Because there is no reason to believe that early atherogenesis reduces physical activity, it seems likely that reduced physical activity is due to the depressive state; thus, low physical activity is one potential mechanism through which depression could promote CAA. Although exercise training has been studied in animal models of atherogenesis, this is the first observation, of which we are aware, of a relationship between atherogenesis and naturally occurring physical activity in an experimental model. This observation suggests that further study of the effects of naturally occurring physical activity on mechanisms of atherogenesis in this model are warranted.
Poor ovarian function increases CAA in monkeys and CHD in women (20,21), and depressed monkeys have relatively poor ovarian function (22). Thus, it is not surprising that poor ovarian function predicted CAA in this experiment. The function of the HPG axis in depressed women has received little attention. Grambsch et al. (50) reported alterations in luteinizing hormone pulsatility in women with major depressive disorder. Although not clinically obvious, these changes are enough to disrupt ovulation and result in lower sex steroid levels throughout the menstrual cycle. Thus, one mechanism through which depression may increase CHD risk in women is HPG suppression. Further clinical research of ovarian function in depression is needed.
The literature is mixed as to whether lipid metabolism is perturbed in depression. Notably, the results of two of the largest studies, both evaluations of several thousand adults, suggested inverse relationships between HDLC and depressive symptomology in women, and this effect seems to be sex-specific (18,19). HDLC was low in depressed monkeys also. Low HDLC is characteristic of a hypogonadal state, consistent with the impaired ovarian function observed in these monkeys. Depressed monkeys also had lower body weight and body mass index, suggesting that their atherogenic plasma lipid profile was not due to greater consumption of the atherogenic diet than their relatively nondepressed counter-parts. Thus, lipid metabolism perturbations were associated with depressive behavior in these monkeys.
We studied CAA and depressive behavior in a previous experiment and did not observe a statistically significant relationship (32). The previous experiment differed from the present study in several ways. The monkeys in the previous study consumed an atherogenic diet for 32 months, whereas the monkeys in this study consumed the atherogenic diet for 53 months. These differences in diet exposure resulted in differences in atherosclerosis extent. CAA extent was less extensive in the previous study (0.16 ± 0.03 mm2) than this study (0.24 ± 0.06 mm2). The monkeys in the previous study, on average, had higher TPC and lower HDLC concentrations. Variability in TPC and HDLC was also lower in the previous study compared with this study, and there was no relationship between cholesterol concentrations and depression as observed here. The lack of relationships between plasma lipids and depression may have been due to more atherogenic and less variable plasma lipids. The lack of relationship between atherosclerosis extent and depression in the previous experiment may be due to less extensive atherogenesis.
Social status predicts cardiovascular disease in human and nonhuman primate females. In female monkeys, the correlation between social status and CAA is about half the magnitude of the correlation between depression and CAA. This lower degree of association suggests some variability in CAA extent among subordinates. Because depression is more common among subordinates than dominants, depressive behavior may more specifically identify the subset of subordinates that will develop significantly worsened atherosclerosis.
In summary, depressive behavior was associated with adverse perturbations in multiple CHD risk factors and predicted early CAA development in primates. These are the first experimental data to demonstrate associations of depressive behavior with early coronary artery atherogenesis. These data are consistent with the hypothesis that depression and CAA both stem from a common mechanism, and with the hypothesis that depression and its sequelae may cause CAA. These hypotheses are not mutually exclusive; both may be true. More research is needed to understand the mechanisms underlying these observations and to determine whether treatment of depression prevents the exacerbation of coronary artery atherogenesis.
Acknowledgments
This work was supported by Grant MH56881 from the National Institute of Mental Health (C.A.S.) and the John D. and Catherine T. MacArthur Foundation (C.A.S.).
Glossary
- CHD
coronary heart disease
- CAA
coronary artery atherosclerosis
- HPA
hypothalamic-pituitary-adrenal
- HDLC
high-density lipoprotein cholesterol
- ACTH
adrenocorticotropic hormone
- CRH
corticotrophin-releasing hormone
- TPC
total plasma cholesterol
- SEM
standard error of the mean
- AUC
areas under the curve
- HPG
hypothalamic-pituitary-gonadal
REFERENCES
- 1.Kessler RC, McGongle KA, Zhao S, Nelson CB, Hughes M, Eshlelman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Rovner BW, German PS, Brant LJ, Clark R, Burton L, Folstein MF. Depression and mortality in nursing homes. JAMA. 1991;265:993–996. doi: 10.1001/jama.265.8.993. [DOI] [PubMed] [Google Scholar]
- 3.Kouzis A, Eaton WW, Leaf PJ. Psychopathology and mortality in the general population. Soc Psychiatry Psychiatr Epidemiol. 1995;30:165–170. doi: 10.1007/BF00790655. [DOI] [PubMed] [Google Scholar]
- 4.von Ammon, Cavanaugh S, Furlanetto LM, Creech SD, Powell LH. Medical illness, past depression, and present depression: a predictive triad for in-hospital mortality. Am J Psychiatry. 2001;158:43–48. doi: 10.1176/appi.ajp.158.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Bradvik L, Berglund M. Late mortality in severe depression. Acta Psychiatr Scand. 2001;103:111–116. doi: 10.1034/j.1600-0447.2001.00212.x. [DOI] [PubMed] [Google Scholar]
- 6.Cardiovascular Health Branch, Division of Chronic Disease Control and Community Intervention, National Center for Chronic Disease Prevention and Health Promotion, CDC. Trends in ischemic heart disease mortality—United States, 1980–1988. Morb Mortal Week Rep. 1992;41:548–556. [PubMed] [Google Scholar]
- 7.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 8.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–240. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 9.Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 10.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge T, Reis SE, Olson MB, Owens J, Kelsey SF, Pepine CJ, Mankad S, Rogers WJ, Merz CN, Sopko G, Cornell CE, Sharaf B, Matthews KA, Vaccarino V. Depression symptom severity and reported treatment history in the prediction of cardiac risk in women with suspected myocardial ischemia: the NHLBI-sponsored WISE study. Arch Gen Psychiatry. 2006;63:874–880. doi: 10.1001/archpsyc.63.8.874. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 13.Agatisa PK, Matthews KA, Bromberger JT, Edmundowicz D, Chang YF, Sutton-Tyrrell K. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005;165:1229–1236. doi: 10.1001/archinte.165.11.1229. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan JR, Manuck SB, Clarkson TB. The influence of heart rate on coronary artery atherosclerosis. J Cardiovasc Pharmacol. 1987;10(Suppl 2):S100–S103. [PubMed] [Google Scholar]
- 16.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Broadley AJ, Korszun A, Abdelaal E, Moskvina V, Deanfield J, Jones CJ, Frenneaux MP. Metyrapone improves endothelial dysfunction in patients with treated depression. J Am Coll Cardiol. 2006;48:170–175. doi: 10.1016/j.jacc.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, McKeown RE, Hussey JR, Thompson SJ, Woods JR, Ainsworth BE. Low HDL cholesterol is associated with suicide attempt among young healthy women: the Third National Health and Nutrition Examination Survey. J Afffect Disord. 2005;89:25–33. doi: 10.1016/j.jad.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Chen CC, Lu F-H, Wu J-S, Chang C-J. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2001;102:153–162. doi: 10.1016/s0165-1781(01)00231-1. [DOI] [PubMed] [Google Scholar]
- 20.Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 21.Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. Ovariectomy, social status and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1985;5:192–200. doi: 10.1161/01.atv.5.2.192. [DOI] [PubMed] [Google Scholar]
- 22.Shively CA, Register TC, Friedman D, Morgan T, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 24.Williams JK, Anthony MS, Honore EK, Herrington DM, Morgan TM, Register TC, Clarkson TB. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol. 1995;15:827–836. doi: 10.1161/01.atv.15.7.827. [DOI] [PubMed] [Google Scholar]
- 25.Fleury J, Keller C, Murdaugh C. Social and contextual etiology of coronary heart disease in women. J Womens Health Gend Based Med. 2000;9:967–978. doi: 10.1089/15246090050199991. [DOI] [PubMed] [Google Scholar]
- 26.Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985;313:1044–1049. doi: 10.1056/NEJM198510243131703. [DOI] [PubMed] [Google Scholar]
- 27.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 28.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (HERS) research group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 29.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 30.Shively CA, Laber-Laird K, Anton RF. The behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 31.Volkers AC, Tulen JH, van den Broek WW, Bruijn JA, Passchier J, Pepplinkhuizen L. Motor activity and autonomic cardiac functioning in major depressive disorder. J Affect Disord. 2003;76:23–30. doi: 10.1016/s0165-0327(02)00066-6. [DOI] [PubMed] [Google Scholar]
- 32.Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- 33.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 34.Lorant V, Deliége D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 35.Shively CA, Kaplan JR. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am J Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- 36.Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;48:1–41. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 37.Wilks JW, Hodgen GD, Ross GT. Endocrine characteristics of ovulatory and anovulatory menstrual cycles in the rhesus monkey. In: Hafez ESE, editor. Human Ovulation. Amsterdam: Elsevier/North-Holland Biomedical Press; 1979. [Google Scholar]
- 38.Burstein M, Samaille J. On a rapid determination of the cholesterol bound to the serum alpha- and beta-lipoproteins. Clin Chim Acta. 1960;5:609. doi: 10.1016/0009-8981(60)90075-9. [DOI] [PubMed] [Google Scholar]
- 39.Lipid and Lipoprotein Analysis. Vol. 1. Bethesda, MD: NHLBI, NIH, DHEW; NIH; 1974. Manual of Laboratory Operations: Lipid Research Clinics Program. [Google Scholar]
- 40.Appt SE, Clarkson TB, Lees CJ, Anthony MS. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas. 2006;55:187–194. doi: 10.1016/j.maturitas.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Jokinen MP, Clarkson TB, Prichard RW. Animal models in atherosclerosis research. Exp Mol Pathol. 1985;42:1–28. doi: 10.1016/0014-4800(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 42.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 43.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 47.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 48.Wannamethee SG, Shaper AG. Physical activity and cardiovascular disease. Semin Vasc Med. 2002;2:257–266. doi: 10.1055/s-2002-35400. [DOI] [PubMed] [Google Scholar]
- 49.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 50.Grambsch P, Young EA, Meller WH. Pulsatile luteinizing hormone disruption in depression. Psychoneuroendocrinology. 2004;29:825–829. doi: 10.1016/S0306-4530(03)00146-X. [DOI] [PubMed] [Google Scholar]