Abstract
Abdominal obesity is prevalent and often accompanied by an array of metabolic perturbations including elevated blood pressure, dyslipidemia, impaired glucose tolerance or insulin resistance, a prothrombotic state, and a proinflammatory state, together referred to as the metabolic syndrome. The metabolic syndrome greatly increases coronary heart disease (CHD) risk. Social stress also increases CHD although the mechanisms through which this occurs are not completely understood. Chronic stress may result in sustained glucocorticoid production, which is thought to promote visceral obesity. Thus, one hypothesis is that social stress may cause visceral fat deposition and the metabolic syndrome, which, in turn increases CHD. CHD is caused by coronary artery atherosclerosis (CAA) and its sequelae. Cynomolgus monkeys (Macaca fascicularis) are a well-established models of CAA. Social subordination may be stressful to cynomolgus monkeys and result in hypercortisolemia and exacerbated CAA in females. Herein is reviewed a body of literature which suggests that social stress increases visceral fat deposition in cynomolgus monkeys, that subordinate females are more likely than dominants to have visceral obesity, that females with visceral obesity have behavioral and physiological characteristics consistent with a stressed state, and that females with high ratios of visceral to subcutaneous abdominal fat develop more CAA. While these relationships have been most extensively studied in cynomolgus macaques, obesity-related metabolic disturbances are also observed in other primate species. Taken together, these observations support the view that the current obesity epidemic is the result of a primate adaptation involving the coevolution with encephalization of elaborate physiological systems to protect against starvation and defend stored body fat in order to feed a large and metabolically demanding brain. Social stress may be engaging these same physiological systems, increasing the visceral deposition of fat and its sequelae, which increase CHD risk.
Keywords: coronary artery atherosclerosis, visceral obesity, metabolic syndrome, social stress, cortisol
INTRODUCTION
Visceral Obesity and Public Health
Recent epidemiological data suggest that the epidemic of obesity is beginning to plateau. However, the rate of severe obesity in adults and the prevalence of overweight in children continue to grow, suggesting that the burden of obesity-related illnesses will continue to increase [Bessesen, 2008]. It is thought that fat deposited in the abdominal region, and particularly the viscera, results in greater systemic pathologic effects than other patterns of fat deposition. Visceral obesity is often accompanied by an array of metabolic perturbations including elevated blood pressure, dyslipidemia, impaired glucose tolerance or insulin resistance, a prothrombotic state, and a proinflammatory state, together referred to as the metabolic syndrome. Each of these characteristics of the metabolic syndrome is known to exacerbate coronary artery atherosclerosis (CAA). Since coronary heart disease (CHD) is caused by CAA and its sequelae, increased visceral obesity results in increased CHD, the leading cause of death [see Després, 2007 for a review]. Metabolic syndrome also increases the risk of other diseases and disorders including type 2 diabetes mellitus [Després & Lemieux, 2006]. It is estimated that over 50 million Americans have the metabolic syndrome, and its prevalence is increasing.
Evolutionary Origins of Visceral Obesity and the Metabolic Syndrome
Ultimately, the current obesity epidemic is the result of the interaction of biology and culture over the course of human evolution. Bellisari [2008] has provided an in depth review of this subject; some of these ideas are briefly summarized here. Primates have the capacity to store body fat when opportunities to consume excess energy arise. During most of primate evolution such opportunities were rare. More common historically was food scarcity accompanied by high levels of physical activity. A large brain is metabolically demanding. Thus, it is thought that the evolution of encephalization in primates was accompanied by the coevolution of elaborate physiological systems to protect against starvation and defend stored body fat to provide for the metabolic needs of a large brain. This concept is an elaboration of the “thrifty genotype” described by James Neel more than 40 years ago [Neel, 1962, 1982]. Visceral fat is especially well constructed to resist lipolysis (discussed in greater detail below). With these biological mechanisms to maximize fuel efficiency in place, human primates went on to devise technological aids for increasing energy consumption and reducing physical effort. In the last century, industrialization provided access to great quantities of mass-produced, high-calorie foods and many labor-saving and transportation devices, virtually abolishing starvation and heavy manual work in much of human society. In this modern obesogenic environment, individuals with the thriftiest combination of ancestral energy-conserving genes are at greatest risk for obesity and associated chronic diseases [Bellisari, 2008]. Thus, obesity is the result of the complex interaction of genetic and environmental factors. This review will focus on social environmental factors that promote visceral obesity and the metabolic syndrome.
Social Stress and Visceral Obesity
One explanation of the obesity epidemic in Western society is that chronic, mild, social stress is characteristic of the lives of many, and occurs in a permissive environment of over-nutrition [Adam & Epel, 2007]. The mechanistic pathways through which stress may promote visceral obesity and associated metabolic perturbations have been reviewed in detail [Black, 2006; Kyrou & Tsigos, 2007]. Briefly, adipose depot size is a result of the balance between actions which promote lipid accumulation vs. those that promote lipolysis. Unremitting stress may result in chronic hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis, and sustained glucocorticoid production. The long-term effects of glucocorticoids on adipocyte metabolic processes are thought to promote visceral obesity. Visceral fat has some unique properties which distinguish it from subcutaneous fat and make it more sensitive to glucocorticoids [Black, 2006]. Visceral fat is highly innervated by blood vessels and nerves and has a high concentration of glucocorticoid receptors. When bound, the glucocorticoid-receptor complex promotes lipoprotein lipase (LPL) gene expression and activates LPL on the cell surface. LPL promotes fatty acid uptake and storage as triglycerides in fat, promoting visceral fat accumulation. The stromal cells in visceral fat contain relatively high levels of the enzyme 11β-hydroxysteroid dehydrogenase type 1, which converts cortisone to the active form of cortisol [Lundgren et al., 2008]. Thus, the downstream effects of glucocorticoids may be enhanced, again promoting visceral fat accumulation. More recently, obesity, particularly visceral obesity, has been recognized as a systemic low-grade inflammatory state. The proinflammatory cytokines secreted from visceral fat promote a hypersecretory HPA axis, which in turn promotes visceral fat accumulation. Thus, the relationship between stress and visceral obesity may be bidirectional and self-sustaining [Beasley et al., 2009; Black, 2006; Kyrou & Tsigos, 2007].
Autonomic nervous system responses to stress also play an important role. Visceral fat has an abundance of β-adrenergic receptors, is relatively less responsive to α-adrenergic agonists, and is less responsive to the antilipolytic effects of insulin [Bolinder et al., 1983]. Under stress-induced sympathetic arousal, these characteristics promote lipolysis. Visceral adipocyte lipolysis results in increased delivery of free fatty acids to the portal vein which may promote glucose intolerance, hyperinsulinemia, and dyslipidemia by influencing hepatic function [Arner, 1997]. Recently, Kuo et al. [2007] observed in rats that chronic stress, combined with a high-fat/high-sugar diet, increased release of sympathetic neurotransmitter neuropeptide Y and the expression of neuropeptide Y-Y2 receptors in visceral fat, promoting preadipocyte proliferation, differentiation, and lipid-filling, thus increasing visceral obesity and the metabolic syndrome. Thus, several characteristics of visceral fat may contribute to the tendency for it to accumulate in response to stress [Kyrou & Tsigos, 2007].
Social Stress and Visceral Obesity: A Mechanism Through Which Social Stress Increases CHD Risk?
CHD risk is also increased by psychological stress, although the mechanisms are not well understood [Brotman et al., 2007; European Guidelines, 2007; Executive Summary of the NHLBI Working Group, 2004]. One way chronic social stress could promote CHD is by increasing visceral obesity and the metabolic syndrome, which promote coronary artery atherogenesis, leading to CHD [Després, 2007]. However, the relationships among social stress, visceral obesity, and CAA/CHD are difficult to study in human beings.
A Primate Model of Social Stress Effects on Health
An appropriate animal model is helpful to understanding the complex relationships among stress, obesity, and CAA. Cynomolgus monkeys (Macaca fascicularis) are a widely used model of diet-induced atherogenesis, obesity, and type 2 diabetes mellitus. The deleterious effects of social stressors, including social instability, isolation, challenge, and subordination, on CAA have been well documented in this species [Adams et al., 1985; Kaplan et al., 1983, 2009; Shively et al., 1989; Strawn et al., 1991]. Social subordination stress is particularly likely to result in pathology in female cynomolgus macaques of reproductive age.
When placed in social groups female cynomolgus monkeys quickly organize themselves into linear social status hierarchies, which are stable for extended periods of time [Shively & Kaplan, 1991]. In our studies, social status is measured as the outcomes of aggressive interactions between all pairs of monkeys in a social group. The monkey to which all other monkeys in the group direct submissive behaviors is considered the most dominant. The monkey to which all monkeys, except the most dominant monkey, direct submissive behaviors is considered the second ranking monkey, and so on. In the studies reported here, the monkeys lived in groups of 3–5 animals and consumed a diet that modeled the typical American diet containing moderate amounts of fat and cholesterol. Those that were, on average, first or second ranking were considered dominant in statistical analyses, and all others were considered subordinate [Shively, 1998]. Compared to dominant monkeys, subordinates received more aggression, were groomed less, were more vigilant, and spent more time alone [Shively, 1998]. Thus, subordinates experienced more hostility and had less positive social experiences to buffer that hostility than dominants. Subordinate monkeys responded to a standardized stressor with higher heart rates than dominants. Subordinate monkeys had higher basal cortisol levels, secreted more cortisol in response to an adrenocorticotropin challenge, and had heavier adrenal glands than dominants [Shively, 1998; Shively & Kaplan, 1984]. Thus, subordinate animals appear behaviorally and physiologically to be socially stressed [Shively, 1998]. Subordinate female cynomolgus monkeys are at increased risk of various pathologies including exacerbated CAA [see Kaplan et al., 2009]. Here we focus on visceral obesity and review the results of several studies describing a relationship between social subordination, fat distribution, associated metabolic characteristics, and their relation to CAA in adult female cynomolgus monkeys.
Whole Body and Regional Fat Distribution in a Colony Consuming an Atherogenic Diet
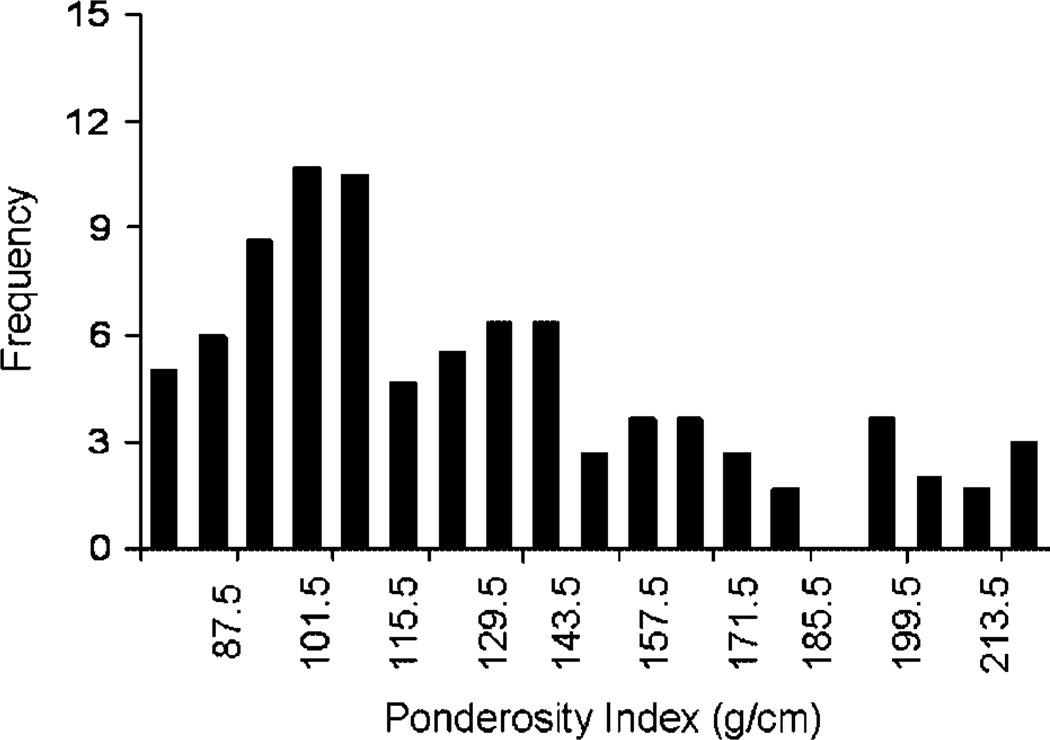
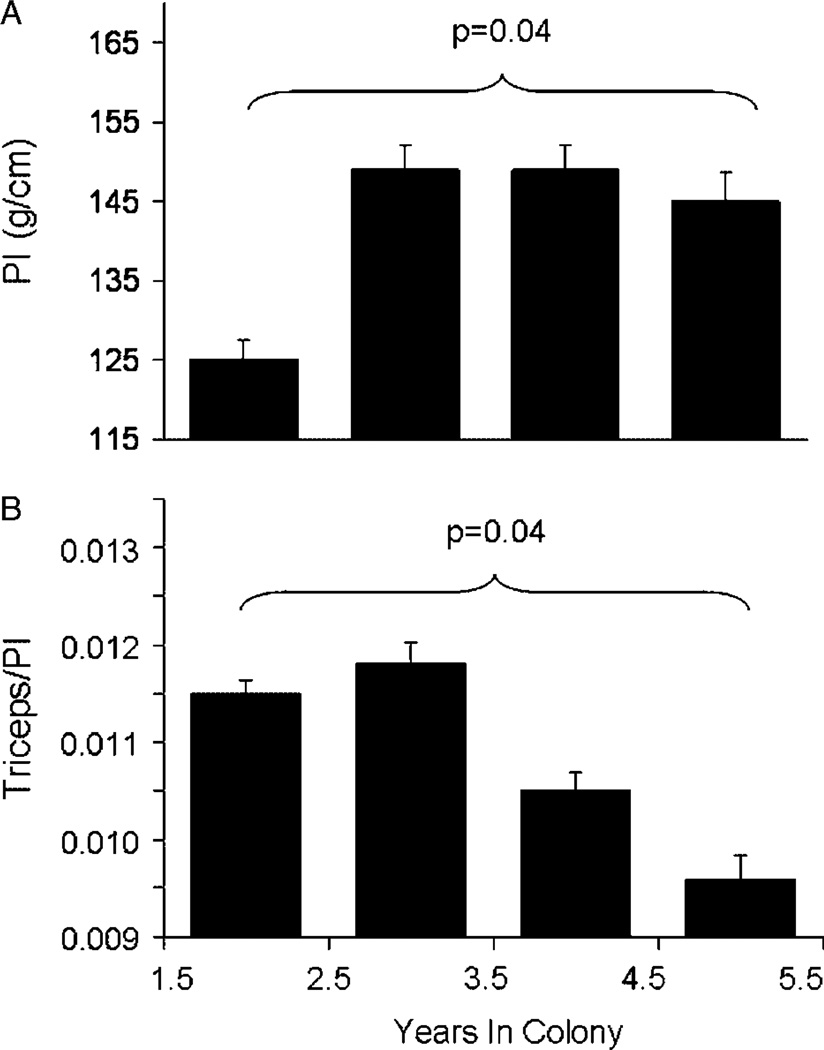
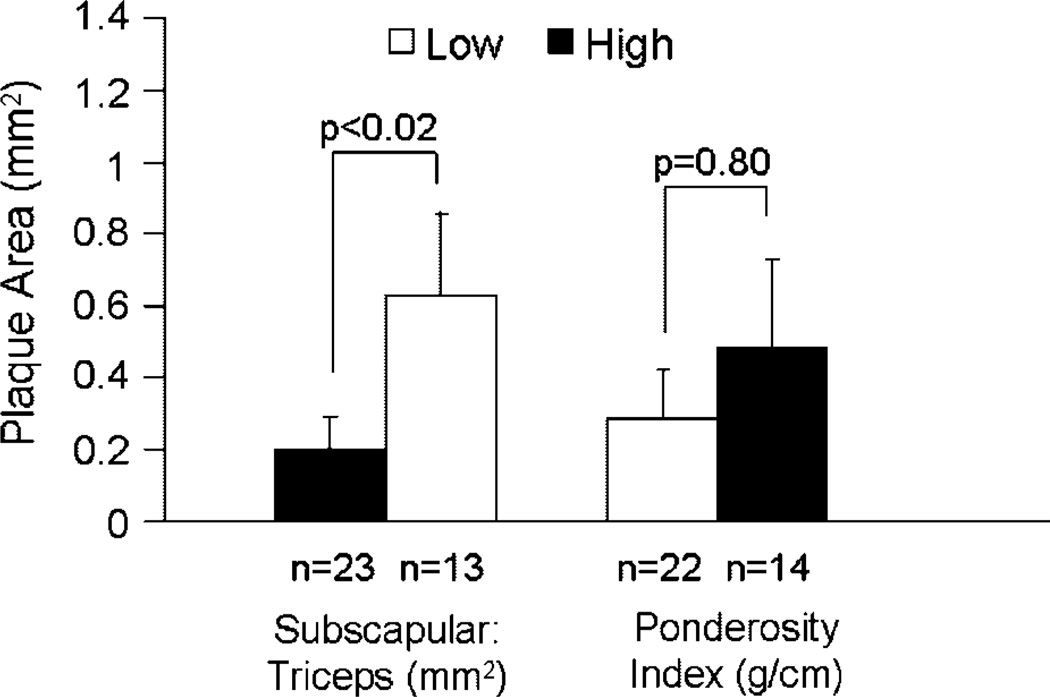
In our earliest investigations into potential relationships between obesity and CAA, we investigated whole body and regional fat deposition, quantitated anthropometrically, in a colony of 82 adult female cynomolgus monkeys consuming an atherogenic diet containing 0.39mg cholesterol/calorie with 38% of calories as fat. Whole body obesity was estimated using a body weight (BW) to body length ratio (g/cm, termed the Ponderosity Index [PI]). The range of BW:length was broad and skewed, similar to human populations (Fig. 1). Among those for which anthropometric data were available for 5 years or more (n = 15), the BW:length increased with time spent in the colony consuming the atherogenic diet (Fig. 2A). Although whole body obesity increased with time in the colony, fat deposited in the periphery (estimated by triceps skinfold thickness) decreased, suggesting that increasing body fat was preferentially deposited in central depots (Fig. 2B) [Shively et al., 1987]. A representative example of a normal weight and an overweight female cynomolgus monkey with a central fat deposition pattern is shown in Figure 3. Coronary arteries were available from 36 of the 82 subjects, and atherosclerosis extent in the coronary arteries was quantified as the mean cross-sectional plaque area of nine coronary artery sections. The distribution of the ratio of BW:length (Ponderosity Index [PI]) in this group was divided at the mean, and CAA was compared in the resulting high and low fat groups. There was no difference in CAA between these two groups (Fig. 4). At this time in human studies, subscapular skinfold thickness was being used as a measure of central fat deposition, and the distribution of the ratio of subscapular:triceps (SS:TRI) skinfold thickness was being used to estimate the relative deposition of fat centrally. The distribution of the ratio of SS:TRI skinfold thickness in this group was divided at the mean, and CAA was compared in the resulting high and low central obesity groups. Females with high central obesity had more CAA than those with relatively low central obesity (Fig. 4) [Shively et al., 1987]. Later, these findings were replicated and extended to carotid artery atherosclerosis extent [Shively et al., 1990]. These were the first data to suggest that central fat deposition results in exacerbated atherosclerosis. These observations suggested that female cynomolgus monkeys might provide a primate model of the health consequences of regional fat distribution.
Fig. 1.
Frequency distribution of whole body obesity of all adult female members (n = 82) of a social group living cynomolgus macaque breeding colony. Whole body obesity was estimated by a ponderosity index (PI): BW (g): body length (pubic symphysis to suprasternal notch [cm]). [Adapted from Shively et al., 1987.]
Fig. 2.
Changes in whole body obesity and fat distribution with time spent in the breeding colony (n = 15 adult females). A: Whole body obesity was estimated by the Ponderosity Index (PI): BW (g): body length (pubic symphysis to suprasternal notch [cm]). B: Triceps skinfold (mm) divided by the PI. [Adapted from Shively et al., 1987.]
Fig. 3.
Example of a female cynomolgus monkey with excessive accumulation of abdominal fat and a normal weight female.
Fig. 4.
Obesity and CAA. CAA was measured as the average cross-sectional plaque area of nine sections of coronary artery: three from the left anterior descending, three from the left circumflex, and three from the right coronary artery. Central obesity was estimated by the subscapular:triceps skinfold thickness. Whole body obesity was estimated by the Ponderosity Index (PI): BW (g): body length (pubic symphysis to suprasternal notch [cm]). [Adapted from Shively et al., 1987.]
Metabolic Characteristics of Females with Central Fat Deposition
A central fat distribution pattern itself is not a health hazard; however, it may signal perturbations in metabolism that are health damaging. Thus, next we determined whether metabolic characteristics were associated with regional fat deposition. One-hour intravenous glucose tolerance tests were done in 37 socially housed, adult female cynomolgus monkeys consuming an atherogenic diet (0.39mg cholesterol/Cal, 38% of calories as fat), after an overnight fast, with a glucose dose based on BW. BW, body length, and subscapular and triceps skinfold thicknesses were measured at the same time. As in the previous study, the distribution of BW:length and the distribution of SS:TRI were divided at the mean into two groups, high and low. The area under the glucose curve was greater in the high (n = 13) than the low (n = 24) BW:length group. The area under the glucose curve was greater in the high (n = 17) than the low (n = 20) SS:TRI skinfold thickness group. Clearance rates (k) were not different between the high and low BW:length or SS:TRI groups. Thus, hyperglycemia was associated with both whole body and central obesity. The correlation between BW:length and SS:TRI was higher in this group than in either the last study or the next study discussed; thus it was not possible in this sample to tease apart independent contributions of regional vs. whole body obesity to carbohydrate metabolism [Shively & Clarkson, 1988]. These observations suggest that whole body and central obesity are associated with carbohydrate metabolism in cynomolgus monkeys.
We next studied fat distribution in 73 adult female cynomolgus monkeys housed in social groups of 4–5 animals each and fed a moderately atherogenic diet (0.39mg cholesterol/Cal, 38% of calories as fat) for 31 months. Anthropometric measures of obesity were made every 6 months, plasma lipids were measured bimonthly, and blood pressure was measured quarterly [Shively et al., 1990]. Social status was determined bimonthly by recording the outcomes of aggressive interactions. For each of these variables the mean of all measures was calculated for analysis. As in the previous study, both the distribution of BW:length and the distribution of SS:TRI were divided at the mean into two groups, high and low. Females in the high BW:length group had significantly higher systolic, and mathematically estimated mean arterial blood pressure ([SBP−DBP/3]+DBP) than those in the low BW:length group. Females in the high SS:TRI group tended to have higher systolic (P = 0.06), and mathematically estimated mean arterial blood pressure (P = 0.09) than those in the low SS:TRI group. There were no differences between high and low BW:length groups in total plasma cholesterol (TPC) or high density lipoprotein cholesterol (HDLC). Females with high SS:TRI skinfold ratios had higher TPC and lower HDLC concentrations than females with low SS: TRI skinfold ratios [Shively & Clarkson, 1988]. Taken together, these observations suggest that whole body and central obesity are associated with metabolic perturbations similar to those observed in human beings with abdominal or truncal obesity.
Social Subordination and Central Obesity
The hypercortisolemia associated with Cushing’s syndrome had been shown to be associated with the abdominal obesity characteristic of that disorder. We reasoned that social stress-induced hypercortisolemia might influence patterns of fat deposition in primates. Thus, we examined BW:length and SS:TRI skinfold ratios with respect to social status in these 73 monkeys. The BW:length ratio and SS:TRI ratio of the lowest ranking female in social groups of four and the two lowest ranking females in groups of five (n = 25) were compared with all other females (n = 48). There were no differences between subordinates and dominants in the frequency with which they fell in the high vs. low BW:length group; however, subordinates were more likely to fall in the high vs. the low SS:TRI skinfold ratio group than dominants (Table I). Thus, low social status was associated with a more centralized pattern of fat deposition [Shively & Clarkson, 1988]. Nine years later, Brunner et al. [1997] published their seminal paper describing similar associations between central obesity/metabolic syndrome and low socioeconomic status in the Whitehall study of British civil servants. Therefore, this nonhuman primate model appears to predict the human condition.
TABLE I.
Social Status and Abdominal Obesity in Monkeys*
| Dominant (n = 48) |
Subordinate (n = 25) |
|
|---|---|---|
| Subordinate females have greater central fat deposition than dominantsa | ||
| SS/TRIb | ||
| High | 40% | 68% |
| Low | 60% | 32% |
Adapted from Shively and Clarkson [1988].
χ2 = 5.38, P = 0.025.
SS/TRI: The ratio of the subscapular:triceps skinfold thickness (mm).
Social Stress Results in Visceral Fat Accumulation
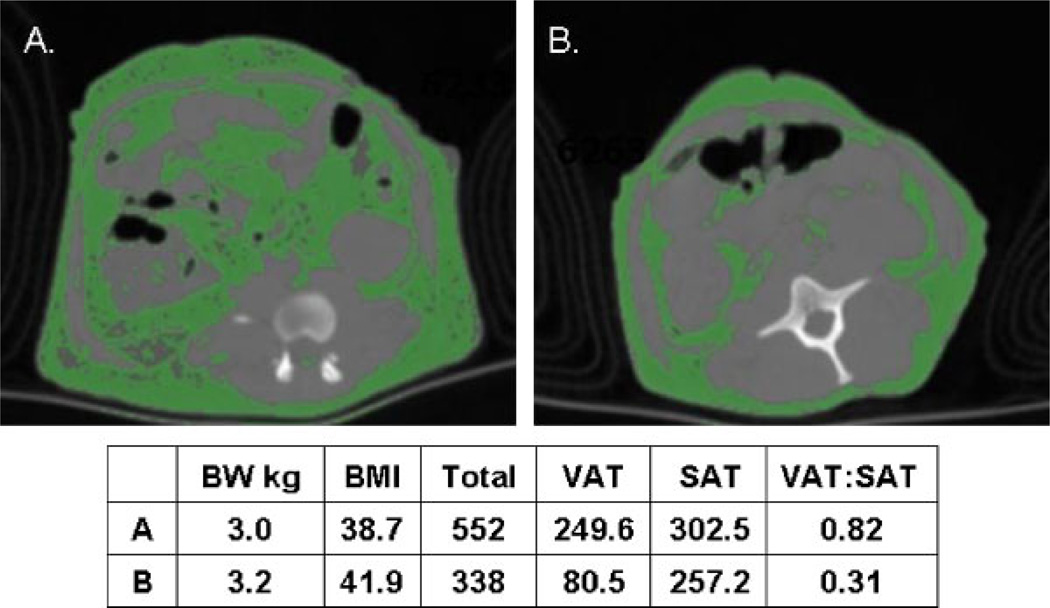
The association of a socially stressful condition with central fat deposition raised the novel question of whether social stress might actually cause abdominal fat deposition. At this point in time, growing evidence suggested that fat accumulation in the intra-abdominal depot might be more closely related to metabolic perturbations and CHD than subcutaneous abdominal depots [Björntorp, 1990]. Thus, we validated the use of computed tomography (CT) for the determination of visceral and subcutaneous abdominal fat mass in cynomolgus monkeys [Laber-Laird et al., 1991]. There is considerable variation in the distribution of adipose tissue between the subcutaneous abdominal and visceral depots in cynomolgus monkeys. Figure 5 depicts cross-sectional computed tomographs with fat identified in green in two cynomolgus monkeys, matched for BW and body mass index (BMI; estimated as the ratio of BW:kg to the square of trunk length (m) from the suprasternal notch to the pubic symphysis) that differ dramatically in the amount of fat deposited in the visceral depot.
Fig. 5.
Abdominal computed tomograph with fat in green of A. A monkey with a high ratio of visceral to subcutaneous abdominal fat [VAT:SAT]); and B. A BW and BMI matched monkey with a low VAT:SAT ratio. Total=SAT1VAT. [Adapted from Shively et al., 2009.] Color figures can be viewed in the online issue, which is available at www.interscience.Wiley.com.
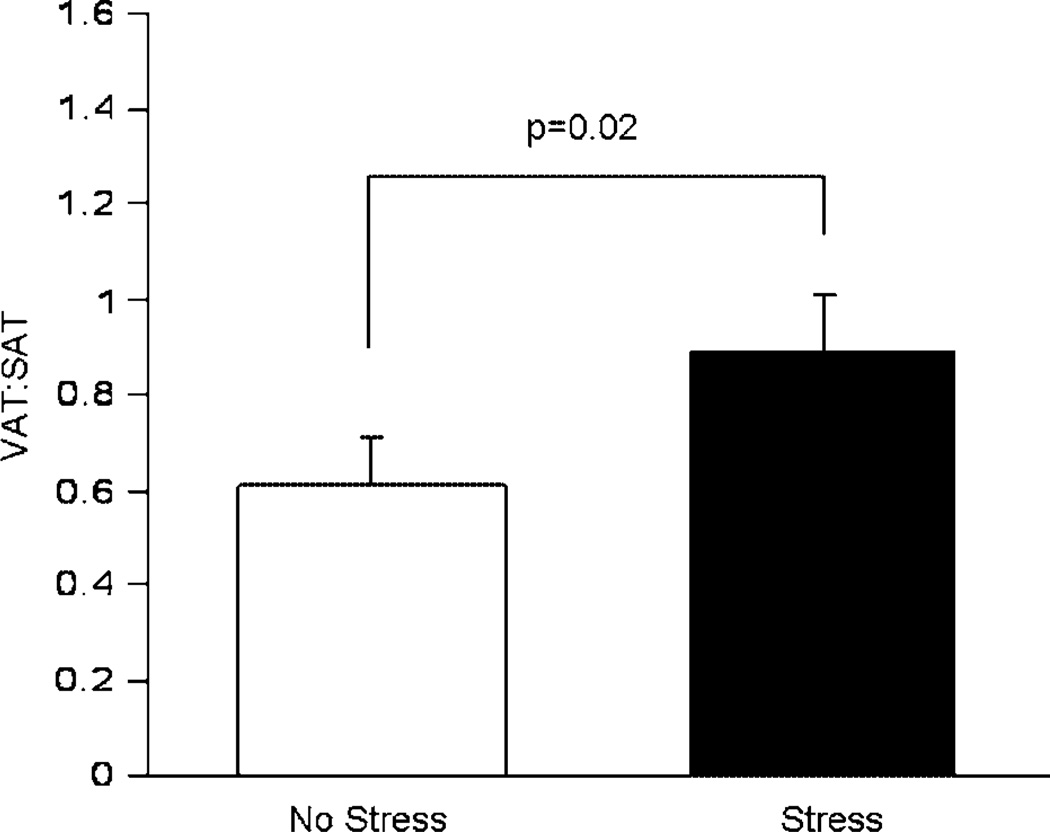
Previously, Manuck et al. [1983] demonstrated that repeated social reorganization of male cynomolgus monkeys exacerbates CAA. We used CT to measure visceral (VAT) and subcutaneous abdominal adipose tissue (SAT) mass in a study of 40 adult male cynomolgus monkeys that consumed a diet containing 188mg cholesterol per day with 43% of calories as saturated fat. Half the animals lived in stable social groups (of about five animals each) and half lived in groups the constituency of which was changed monthly for 34 months [Jayo et al., 1993]. Repeated social reorganization affected patterns of fat deposition. Monkeys in the repeatedly reorganized groups had greater VAT mass, and a higher ratio of VAT:SAT than those in stable social groups. There were no differences between monkeys in stable or reorganized social groups in BW or BMI (Fig. 6). This was the first experimental evidence that chronic long-term social stress caused visceral fat deposition [Jayo et al., 1993]. This observation suggested that one reason socially subordinate females were more likely to have central fat deposition may be the stress of subordination. Furthermore, the finding that social reorganization stress results in greater visceral obesity, in addition to exacerbated atherosclerosis, raised the question of whether visceral obesity might promote atherogenesis, a question that is addressed in greater detail below.
Fig. 6.
Effects of social reorganization stress on visceral:subcutaneous abdominal (VAT:SAT) fat mass in 40 adult male cynomolgus monkeys. [Adapted from Jayo et al., 1993.]
Anthropometry, CT, and the Health Effects of Depot-Specific Fat Accumulation
Although a strong case had been made based on adipocyte physiology and anatomic location that visceral fat had the greatest adverse health effects, interest in other fat distribution patterns and their relation to disease has continued. In particular, upper body subcutaneous fat has been repeatedly observed to increase disease risk [Grunfeld et al., 2007; Haffner et al., 1988; Vague, 1956]. It was thought that anthropometric measures of subcutaneous fat on the trunk, whether upper body fat measured by skinfold thickness, waist circumference, or saggital abdominal diameter, were markers of visceral fat deposition. However, the correlation of anthropometric measures of abdominal fat distribution and direct measures of visceral fat mass using imaging techniques were not always highly correlated in monkey or clinical studies [Jayo et al., 1993; Kekes-Szabo et al., 1996]. Furthermore, it may be that some fat depots are actually protective against disease. Narumi et al. [2009] recently observed that subcutaneous abdominal fat accumulation, measured by CT, protected against subclinical atherosclerosis. Thus, the quest to understand the effects of depot-specific fat accumulation on health continues.
Social Subordination Stress, Visceral Obesity, and CAA
For these reasons it seemed important to extend our observations of an association between social stress and central obesity to visceral obesity, and to determine whether visceral obesity predicts CAA as well as central obesity determined by anthropometry. We studied 41 socially housed females that consumed an atherogenic diet for 32 months. Social behavior and ovarian function were recorded for 26 months. Dexamethasone suppression tests, heart rate telemetered overnight while in their social groups, BMI, and VAT and SAT quantified with CT, were measured during the last six months.
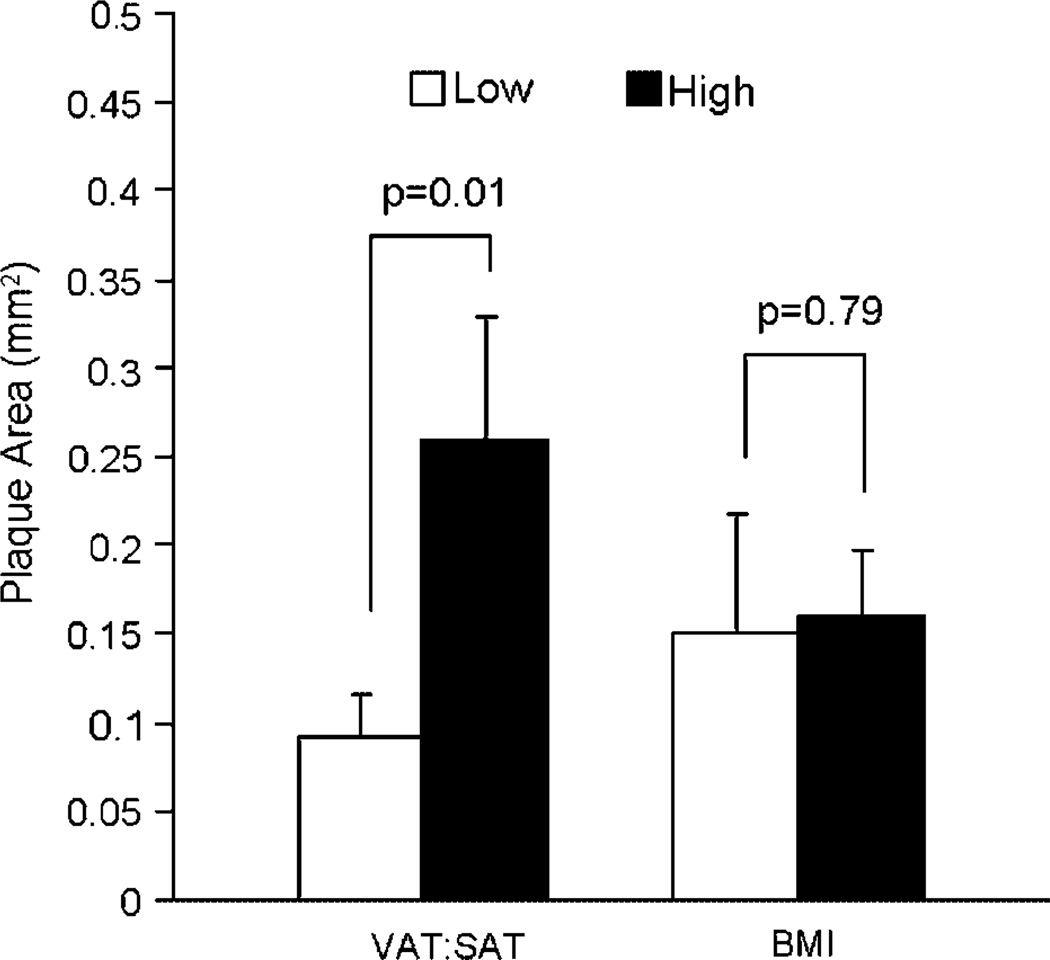
Females with high (above the mean) ratios of VAT:SAT were of lower social status, received more aggression, were groomed less, and spent more time alone than females with low ratios of VAT:SAT (Table II). Furthermore, high VAT:SAT females were relatively insensitive to glucocorticoid negative feedback in a dexamethasone suppression test (Table II), suggesting downregulation of glucocorticoid receptors in central areas, which modulate the HPA response to peripheral negative feedback. High VAT:SAT females also had relatively fewer ovulatory menstrual cycles, suggesting suppressed ovarian function. These behavioral and physiological characteristics are consistent with a stressed state. Furthermore, high VAT:SAT females had higher heart rates in the late afternoon and early evening (time × group interaction, P<0.000001, data not shown) than low VAT:SAT females. Heart rates are normally higher during the day and lower at night, in part due to an endogenous circadian rhythm and in part due to the high levels of activity in the monkey buildings during the day. The autonomic reactivity to daily activities in the monkey building did not recover as quickly in the late afternoon and evening in high VAT:SAT females relative to their low VAT:SAT counterparts. This observation may indicate that the stressed behavioral and physiological profile of these animals is due to sustained stress responses that do not recover as quickly in females with high VAT:SAT ratios relative to those with low VAT:SAT ratios. Finally, high VAT:SAT females had more CAA than low VAT:SAT females (Fig. 7). High (above the mean) and low (below the mean) BMI females were not different on any of these measures except heart rate. High BMI females maintained higher heart rates throughout the 24-hr day than low BMI females (P<0.05) [Shively et al., 2009].
TABLE II.
Behavioral and Physiological Indicators of Stress*
| BMI | VAT:SAT | |||||
|---|---|---|---|---|---|---|
| Low | High | P≤ | Low | High | P≤ | |
| Social statusa | 0.48 (0.1) | 0.55 (0.1) | 0.60 | 0.6 (0.1) | 0.3 (0.1) | 0.002 |
| Rate of aggressionb | 4.3 (0.8) | 5.6 (1.0) | 0.34 | 6.0 (0.7) | 2.8 (0.9) | 0.01 |
| Rate of receiving aggressionb | 5.0 (1.0) | 4.4 (1.0) | 0.65 | 3.2 (0.6) | 7.5 (1.4) | 0.003 |
| % time being groomed | 10.7 (1.3) | 10.6 (1.7) | 0.95 | 12 (1.3) | 8 (1.5) | 0.07 |
| % time grooming | 10.6 (1.6) | 11.6 (2.1) | 0.71 | 13 (1.8) | 7.5 (0.8) | 0.03 |
| % time alone | 49 (2.8) | 47 (3.7) | 0.66 | 45 (2.6) | 54 (3.4) | 0.06 |
| % suppression of cortisolc | 68.6 (4.4) | 72.5 (8.4) | 0.64 | 82.4 (2.0) | 56.3 (7.7) | 0.008 |
| % cycles ovulatory | 82.1 (4.7) | 82.5 (5.7) | 0.63 | 89.5 (0.2) | 74.7 (6.8) | 0.01 |
Adapted from Shively et al. [2009].
Social status is expressed as the average rank of each animal corrected for social group size and varies from most subordinate (score = 0.0) to most dominant (score = 1.0).
Frequency/hour.
The dexamethasone suppression test assesses sensitivity of the hypothalamus and pituitary to negative feedback from circulating levels of cortisol. A morning blood sample is taken for a baseline measure of cortisol. That evening, a low dose (130 µg/kg BW, i.m.) of dexamethasone is administered. The next morning another blood sample is taken for cortisol assay. Percent suppression = (difference between the first and second morning cortisol concentrations/baseline) *100, and is used as an indicator of sensitivity to negative feedback [Shively et al., 1997].
Fig. 7.
Whole body obesity, abdominal fat distribution and CAA. CAA extent was measured as the average cross sectional plaque area of 15 sections of coronary artery: 5 from the left anterior descending, 5 from the left circumflex, and 5 from the right coronary artery. CAA was more extensive in monkeys in the high vs. the low VAT:SAT group. There was no difference in CAA extent in monkeys in the high vs. the low BMI group. BMI: body mass index; VAT: visceral adipose tissue; SAT: subcutaneous abdominal adipose tissue. [Adapted from Shively et al., 2009.]
Thus, females with visceral obesity had more CAA as well as behavioral and physiological indicators of stress. Since we observed previously that social stress results in visceral fat deposition in cynomolgus monkeys, these observations suggest that one way social stress may exacerbate CAA is by increasing visceral obesity [Shively et al., 2009].
DISCUSSION
The pervasiveness of metabolic syndrome in the human population appears to be due to a confluence of events: (1) of the evolution of elaborate physiological systems in primates to protect against starvation and defend stored body fat; (2) the current permissive environment of over-nutrition; and (3) the current pervasiveness of chronic, low intensity social stress. Here we review a series of studies suggesting that, like human beings, cynomolgus monkeys in a permissive nutritional environment, and perhaps particularly those that are socially stressed, may develop visceral obesity and the metabolic syndrome, which results in exacerbated coronary and carotid atherosclerosis.
Stressed, socially subordinate female cynomolgus monkeys have many physiological characteristics, which increase their risk of CAA. They are dyslipidemic, hypercortisolemic, have poor ovarian function, high heart rates, and visceral obesity all of which may exacerbate CAA [Kaplan & Manuck, 2004; Shively, 1998]. The causal pathways linking all of these are not well understood, although the neural changes associated with stress would seem to be primary to most of the other downstream perturbations. From this perspective, HPA and autonomic nervous system perturbations in response to stress are likely to be initiating events. These perturbations in turn lead to a host of alterations including hypercortisolisolemia and sympathetic arousal as evidenced by high heart rate, as well as suppressed hypothalamic-pituitary-gonadal (HPG) axis, and visceral obesity. Each of these may act directly on the artery wall to promote endothelial damage and dysfunction, resulting in exacerbated atherosclerosis, and also promote further pathologic changes that enhance atherogenesis [Broadley et al., 2006; Kaplan et al., 1987; Miller & Duckles, 2008; Sommer et al., 2009].
At this point in the development of metabolic syndrome, determining whether each of these is causally related and the direction of the causal relationship becomes difficult. For example, the stress-induced autonomic system perturbations suggested by high heart rate may promote visceral obesity and act directly on the artery wall to promote endothelial damage [Shigetoh et al., 2009]. On the other hand, metabolic characteristics of visceral obesity (e.g. hyperinsulinemia, leptin, nonesterified fatty acids, proinflammatory cytokines, etc.) may enhance sympathetic drive [Straznicky et al., 2008]. Likewise, visceral obesity has been associated with a relatively androgenic state in women. The androgenic state may promote visceral fat deposition [Björntorp, 1997]; however, adipokines and proinflammatory cytokines secreted by visceral adipose tissue may inhibit the activity of the HPG axis at multiple levels, resulting in ovarian dysfunction and a relatively androgenic state [Kyrou & Tsigos, 2008]. Thus, while the components of the metabolic syndrome are highly interconnected, it is likely that the response to environmental stress may be an early and influential precipitating factor.
Here we demonstrate that female cynomolgus monkeys exhibit individual variability in fat accumulation while eating the same diet, and in the portion of fat deposited in the visceral depot when matched on BMI. Obesity and visceral obesity have a heritable component in human primates and there are likely genetic influences on these factors in cynomolgus macaques [Bouchard, 1997]. These data also demonstrate that social environmental factors can influence whole body and regional obesity. Thus, cynomolgus macaques may be a useful model to study the interactions of genetic and environmental influences, which result in the metabolic syndrome.
However, it is quite likely that these responses to environmental stress and over-nutrition are shared by many primate species. Previous studies have found substantial variation in BW and body composition in adult baboons sharing the same diet and living conditions. The spontaneous development of obesity [Comuzzie et al., 2003; Papio hamadryas], type 2 diabetes [Stokes, 1986; Papio cynocephalus anubis], and metabolic changes similar to human metabolic syndrome [Banks et al., 2003; Papio cynocephalus] have been described in these species. Kaufman et al. [2005, 2007] reported the development of metabolic disturbances accompanied by obesity, measured by BMI, and abdominal obesity, measured by saggital abdominal diameter, in bonnet macaques (Macaca radiata). Furthermore, Kaufman et al. [2007] have demonstrated that the stress–obesity relationship may not be confined to adults. They observed that early-life stress during a critical period of neurodevelopment in young bonnet macaques can result in peripubertal obesity and insulin resistance. Thus, stress may be an important factor in juvenile as well as adult obesity in primates. Kavanagh et al. [2007] have demonstrated in vervets (Cercocebus aethiops) a relationship between waist circumference and glycated hemoglobin, a measure of impaired glucose tolerance, thus demonstrate clusters of obesity-related metabolic perturbations similar to those seen in human beings. Recently, Tardif et al. [2009] described metabolic perturbations associated with obesity in marmosets (Callithrix jacchus). These observations extend obesity-related metabolic perturbations similar to the human metabolic syndrome to several African and Asian Old World monkeys and a New World monkey.
In summary, social stress may result in hypercortisolemia. Glucocorticoids promote visceral obesity. Visceral adipose is a primary site of secretion of proinflammatory cytokines, which cross the blood-brain barrier and stimulate the HPA axis and so promote more cortisol secretion. These mechanisms provide a biologically plausible pathway through which stress and visceral obesity may have a bidirectional and self-sustaining relationship. The coevolution with encephalization of elaborate physiological systems to protect against starvation and defend stored body fat, in order to feed a large and metabolically demanding brain, is a primate-specific adaptation that predisposes the organism to fat accumulation in a sustained nutrient-rich environment. Social stress may be engaging these same physiological systems, increasing the visceral deposition of fat and its sequelae, which increase metabolic syndrome and disease risk.
ACKNOWLEDGMENTS
All procedures described in the foregoing research projects involving monkeys were conducted in accordance with state and federal laws, standards of the Department of Health and Human Services, and Institutional Animal Care and Use Committee guidelines.
Contract grant sponsor: Wake Forest University Claude D. Pepper Older Americans Independence Center; Contract grant number: P30-AG21332; Contract grant sponsor: National Heart, Lung, and Blood Institute, National Institutes of Health; Contract grant numbers: HL-39789; HL087103; Contract grant sponsor: John D. and Catherine T. MacArthur Foundation.
REFERENCES
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1985;5:192–200. doi: 10.1161/01.atv.5.2.192. [DOI] [PubMed] [Google Scholar]
- Arner P. Regional adipocity in man. J Endocrinol. 1997;155:191–192. doi: 10.1677/joe.0.1550191. [DOI] [PubMed] [Google Scholar]
- Banks WA, Altmann J, Sapolsky RM, Phillips-Conroy JE, Morley JE. Serum leptin levels as a marker for a syndrome X-like condition in wild baboons. J Clin Endocrinol Metab. 2003;88:1234–1240. doi: 10.1210/jc.2002-021695. [DOI] [PubMed] [Google Scholar]
- Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M, Rubin SM, Goodpaster BH, Harris TB The Health ABC Study. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellisari A. Evolutionary origins of obesity. Obes Rev. 2008;9:165–180. doi: 10.1111/j.1467-789X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027–2034. doi: 10.1210/jc.2008-0520. [DOI] [PubMed] [Google Scholar]
- Björntorp P. Portal adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795–803. doi: 10.1016/s0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bolinder J, Kager L, Ostman J, Arner P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes. 1983;32:117–123. doi: 10.2337/diab.32.2.117. [DOI] [PubMed] [Google Scholar]
- Bouchard C. Genetic determinants of regional fat distribution. Hum Reprod. 1997;12:1–5. doi: 10.1093/humrep/12.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- Broadley AJ, Korszun A, Abdelaal E, Moskvina V, Deanfield J, Jones CJ, Frenneaux MP. Metyrapone improves endothelial dysfunction in patients with treated depression. J Am Coll Cardiol. 2006;48:170–175. doi: 10.1016/j.jacc.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, Juneja M, Alberti KG. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 1997;40:1341–1349. doi: 10.1007/s001250050830. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman model for the study of the genetics of obesity. Obes Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- Després JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–59. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- Executive Summary of the NHLBI Working Group on Cardiovascular Consequences of Chronic Stress. 2004 http://www.nhlbi.nih.gov/meetings/workshops/heart_stress.htm.
- Grunfeld C, Rimland D, Gibert CL, Powderly WG, Sidney S, Shlipak MG, Bacchetti P, Scherzer R, Haffner S, Heymsfield SB. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–290. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM, Fong D, Hazuda HP, Pugh JA, Patterson JK. Hyperinsulinemia, upper body adiposity, and cardiovascular risk factors in non-diabetics. Metabolism. 1988;37:338–345. doi: 10.1016/0026-0495(88)90133-3. [DOI] [PubMed] [Google Scholar]
- Jayo JM, Shively CA, Kaplan JR, Manuck SB. Effects of exercise and stress on body fat distribution in male cynomolgus monkeys. Int J Obes Relat Metab Disord. 1993;17:597–604. [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Miller EW. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–735. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB. The influence of heart rate on coronary artery atherosclerosis. J Cardiovasc Pharmacol. 1987;10:S100–S102. discussion S103. [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta analysis. Am J Primatol. 2009 doi: 10.1002/ajp.20707. In press, this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D, Smith EL, Gohil BC, Banerji M, Coplan JD, Kral JG, Rosenblum LA. Early appearance of the metabolic syndrome in socially reared bonnet macaques. J Clin Endocrinol Metab. 2005;90:404–408. doi: 10.1210/jc.2004-0452. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, Rosenblum LA, Kral JG. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes. 2007;56:1382–1386. doi: 10.2337/db06-1409. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 2007;15:1666–1674. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- Kekes-Szabo T, Hunter GR, Nyikos I, Williams M, Blaudeau T, Snyder S. Anthropometric equations for estimating abdominal adipose tissue distribution in women. Int J Obes Relat Metab Disord. 1996;20:753–758. [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Tsigos C. Stress mechanisms and metabolic complications. Horm Metab Res. 2007;39:430–438. doi: 10.1055/s-2007-981462. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Tsigos C. Chronic stress, visceral obesity and gonadal dysfunction. Hormones. 2008;7:287–293. doi: 10.14310/horm.2002.1209. [DOI] [PubMed] [Google Scholar]
- Laber-Laird K, Shively CA, Karstaedt N, Bullock BC. Assessment of abdominal fat deposition in female cynomolgus monkeys. Int J Obesity. 1991;15:213–220. [PubMed] [Google Scholar]
- Lundgren M, Burén J, Lindgren P, Myrnäs T, Ruge T, Eriksson JW. Sex- and depot-specific lipolysis regulation in human adipocytes: interplay between adrenergic stimulation and glucocorticoids. Horm Metab Res. 2008;40:854–860. doi: 10.1055/s-0028-1087168. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Clarkson TB. Social instability and coronary artery atherosclerosis in cynomolgus monkeys. Neurosci Biobehav Rev. 1983;7:485–491. doi: 10.1016/0149-7634(83)90028-3. [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi H, Yoshida K, Hashimoto N, Umehara I, Funabashi N, Yoshida S, Komuro I. Increased subcutaneous fat accumulation has a protective role against subclinical atherosclerosis in asymptomatic subjects undergoing general health screening. Int J Cardiol. 2008 Jun 30; doi: 10.1016/j.ijcard.2008.03.044. [Epub ahead of print] In press. [DOI] [PubMed] [Google Scholar]
- Neel JV. Diabetes mellitus: a thrifty genotype rendered detrimental by progress. Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- Neel JV. The thrifty genotype revised. In: Koebberling J, Tattersal R, editors. The genetics of diabetes mellitus: Proceedings of the Serono Symposia. London: Academic Press; 1982. pp. 283–293. [Google Scholar]
- Shigetoh Y, Adachi H, Yamagishi S, Enomoto M, Fukami A, Otsuka M, Kumagae S, Furuki K, Nanjo Y, Imaizumi T. Higher heart rate may predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. Am J Hypertens. 2009;22:151–155. doi: 10.1038/ajh.2008.331. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. Regional obesity and coronary artery atherosclerosis in females: a non-human primate model. Acta Med Scand Suppl. 1988;723:71–78. doi: 10.1111/j.0954-6820.1987.tb05930.x. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology in Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Kaplan JR, Clarkson TB. Carotid artery atherosclerosis in cholesterol-fed cynomolgus monkeys: the effects of oral contraceptive treatments, social factors and regional adiposity. Arteriosclerosis. 1990;10:358–366. doi: 10.1161/01.atv.10.3.358. [DOI] [PubMed] [Google Scholar]
- Shively CA, Kaplan JR. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am J Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB, Miller LC, Weingand KW. Body fat distribution as a risk factor for coronary artery atherosclerosis in female cynomolgus monkeys. Arteriosclerosis. 1987;7:226–231. doi: 10.1161/01.atv.7.3.226. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB, Kaplan JR. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis. 1989;77:69–76. doi: 10.1016/0021-9150(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis in female primates. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.74. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Sommer G, Kralisch S, Stangl V, Vietzke A, Kähler U, Stepan H, Faber R, Schubert A, Lässner U, Bluher M, Stumvoll M, Fasshauer M. Secretory products from human adipocytes stimulate proinflammatory cytokine secretion from human endothelial cells. J Cell Biochem. 2009;106:729–737. doi: 10.1002/jcb.22068. [DOI] [PubMed] [Google Scholar]
- Stokes WS. Spontaneous diabetes mellitus in a baboon (Papio cynocephalus anubis) Lab Anim Sci. 1986;36:529–532. [PubMed] [Google Scholar]
- Strawn WB, Bondjers G, Kaplan JR, Manuck SB, Schwenke DC, Hansson GK, Shively CA, Clarkson TB. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ Res. 1991;68:1270–1279. doi: 10.1161/01.res.68.5.1270. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–447. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus) Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.77. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]