Abstract
We posit that maternal prenatal nutrition can influence offspring schizophrenia risk via epigenetic effects. In this article, we consider evidence that prenatal nutrition is linked to epigenetic outcomes in offspring and schizophrenia in offspring, and that schizophrenia is associated with epigenetic changes. We focus upon one-carbon metabolism as a mediator of the pathway between perturbed prenatal nutrition and the subsequent risk of schizophrenia. Although post-mortem human studies demonstrate DNA methylation changes in brains of people with schizophrenia, such studies cannot establish causality. We suggest a testable hypothesis that utilizes a novel two-step Mendelian randomization approach, to test the component parts of the proposed causal pathway leading from prenatal nutritional exposure to schizophrenia. Applied here to a specific example, such an approach is applicable for wider use to strengthen causal inference of the mediating role of epigenetic factors linking exposures to health outcomes in population-based studies.
Keywords: Agouti, DNA methylation, epigenetic epidemiology, folate, Mendelian randomization, one-carbon metabolism, prenatal nutrition, psychosis, Reelin, schizophrenia
In light of the considerable interest in the potential role of epigenetic mechanisms in the developmental programming of adult diseases, including psychopathologies, we posit one possibility: prenatal nutrition influences the risk of offspring schizophrenia via epigenetic effects. Briefly, our article is organized as follows. First, we review the evidence which supports independent associations between prenatal nutrition, epigenetic outcomes and schizophrenia. Second, we consider possible biological pathways through which maternal prenatal nutrition might influence schizophrenia risk in offspring via epigenetic effects, focusing upon one-carbon metabolism as an examplar. Finally, we suggest a testable hypothesis pertaining to the role of folate in this pathway and outline viable approaches for test the component parts of the proposed causal pathway leading from prenatal nutritional exposure to schizophrenia (Figure 1).
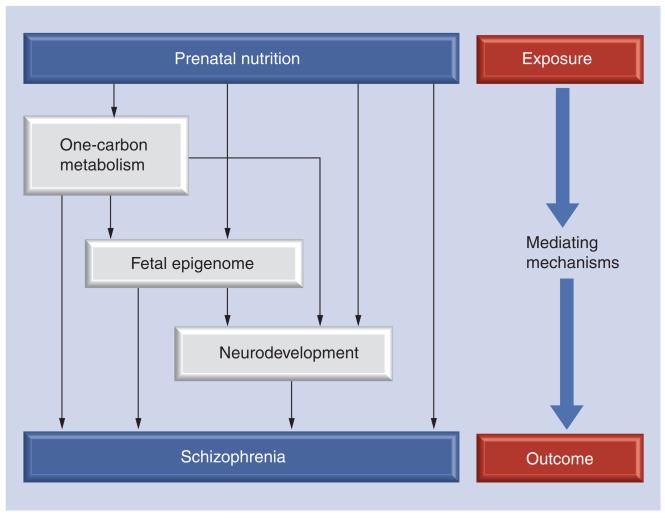
Figure 1. Potential mediating mechanisms linking prenatal nutrition with later schizophrenia development.
Prenatal nutrition may exert its influence on schizophrenia risk via a number of pathways (influencing the kinetics of one-carbon metabolism, epigenetic perturbation of the fetal epigenome or via interfering with neurodevelopment). These possible mediating mechanisms are not mutually exclusive and may be interconnected through some or all of the routes depicted.
It is important to bear in mind that both genetic and epigenetic factors are likely to contribute to the development of schizophrenia spectrum disorders. We know that a degree of genetic liability exists given that the concordance rates amongst monozygotic twins approaches 50% [1]. Furthermore, genome-wide association studies have identified several genetic polymorphisms that are associated with modest, but significant increases in risk [2], and meta-analyses have confirmed candidate gene study associations, such as in the case of the MTHFR gene [3,4]. Such polymorphisms may collectively account for a substantial proportion of genetic liability for psychosis [5]. Rare copy number variants and other genetic mutations, such as 22q11 deletion syndrome, are also strongly associated with schizophrenia risk [6]. However, given that concordance rates amongst monozygotic twins are not 100%, other processes must be involved, and probably involve epigenetic mechanisms [7]. Thus, in addition to genetic liability, exposure to adverse early nutritional environments or social stressors over the life course, or stochastic epigenetic variation, may all influence whether an individual develops schizophrenia [8,9].
Epigenetic processes include various mechanisms that influence chromatin structure and gene expression, such as DNA methylation, histone modifications, chromatin remodeling and the incorporation of specialized histone variants [10]. Under broader definitions, epigenetic mechanisms also include activities of ncRNAs and other regulatory mechanisms. Within the broad scope of epigenetics, we limit attention to DNA methylation, which involves the addition of a methyl group to cytosine nucleotides. Usually (although not always), transcription is impeded in the presence of methylated cytosine residues located in gene promoter regions, and gene expression is reduced. Furthermore, changes in DNA methylation can lead to long-lasting effects on gene expression and phenotype, while some other epigenetic changes tend to be more transient in nature. Finally, DNA methylation is dependent on one-carbon metabolism (Figure 2) which requires essential micronutrients such as folate and vitamin B12.
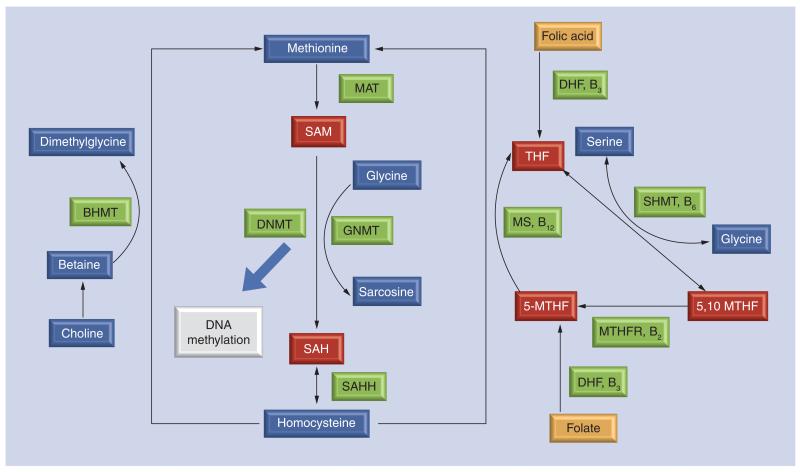
Figure 2. Simplified one-carbon metabolism.
One-carbon metabolism is critical for sustaining many normal cellular functions, including DNA methylation to facilitate normal cell division, growth and differentiation. In this regard, adequate micronutrient availability, including the availability of folate, either through diet or as folic acid supplementation, may be particularly important at certain stages of the life course, particularly prenatally, when the body is undergoing rapid cell division and growth. Technical detail: in one-carbon metabolism, folate from the diet or folic acid supplements are metabolized to DHF via a reductase enzyme in the liver and converted to a series of THF compounds. At both these stages vitamin B3 is a necessary cofactor. Via the addition of a carbon donor (serine or glycine) THF forms 5,10 MTHF, the methylated substrate used by the enzyme MTHFR to produce 5-MTHF, where vitamin B2 is a necessary cofactor. 5-MTHF is used in the reaction to recycle homocysteine to methionine, via single-carbon donation as a result of the interaction between vitamin B12, a necessary cofactor, and MS. Here, choline is synthesized to betaine to facilitate methyl donation to convert homocysteine to methionine via a BMHT. Methylation reactions, including DNA methylation, are then facilitated by the conversion of methionine to SAM, the key methyl donor involved in methylation, via interaction with MAT. Unused methionine in the form of (an inhibitor of DNMTs) is hydrolyzed back to homocysteine via an enyzmatic reaction with SAHH.
5,10 MTHF: 5,10-methylenetetrahydrofolate; 5-MTHF: 5-methyltetrahydrfolic acid; BMHT: Betaine-homocysteine methyltransferase; DHF: Dihydrofolate; DNMT: DNA methyltransferase; MS: Methionine synthase; THF: Tetrahydrofolate.
Evidence linking maternal prenatal nutrition, DNA methylation & schizophrenia
Prenatal nutrition & DNA methylation in offspring
In animals, it is well established that nutritional exposures during pregnancy can have effects on the epigenome of offspring [11-15]. The laboratory Agouti mouse model is the classic, widely cited exemplar [11,13,16]. It has been shown that when pregnant dams were given supplements comprising methyl donors (e.g., folate, betaine and methionine) this resulted in increased DNA methylation in the embryos at the Avy allele, with offspring coat colors, on average, shifted from the yellow phenotype towards brown or ‘pseudoagouti’ [11,12] phenotype. The yellow coat phenotype in Avy mice is associated with other phenotypic changes, such as overeating, obesity, diabetes, tumorigenesis and reduced longevity [16-18]. The supplements given to pregnant dams, which increase DNA methylation in the embryos, also tend to shift these other phenotypes in a probabilistic manner in offspring back toward pseudoagouti [12,19].
Subsequent studies of the Agouti mouse model provided further evidence that prenatal exposures can change the epigenome of the offspring. An intriguing example is a study of ‘folate rescue’ after prenatal exposure to bisphenol A [20]. This study showed that exposure to bisphenol A led to hypomethylation of the Avy allele and shifted offspring coat color toward yellow. These effects were counteracted by gestational folate supplementation, further suggesting that maternal folate exposure can increase DNA methylation levels in the embryo.
These landmark studies of the Agouti mouse have more recently been complemented by a wider literature of prenatal nutritional intervention studies (including undernutrition, macronutrient deficiency, micronutrient deficiency and overnutrition) in animal models, which provide unequivocal evidence that maternal nutrition marks the fetal epigenome [21,22]. These studies have demonstrated not only a shift in mean methylation of specific loci in response to nutritional manipulation during pregnancy, but an increase in variance in DNA methylation [22].
Several studies in humans now suggest that prenatal nutritional exposures can influence DNA methylation in offspring, although the evidence is considerably more scant than that provided by animal studies. A study based on the Dutch Hunger Winter of 1944–1945 reported on the offspring of nutritionally compromised women around the peak of famine close to the time of conception [23]. At approximately 60 years old, these offspring exhibited less methylation of the IGF2 locus in whole-blood samples than offspring of women who were unexposed or exposed later in gestation. IGF2 is an imprinted gene that plays a crucial role in early development, and continues to play a role in cognitive and other brain processes over the life course [24]. If genuine, it suggests that periconceptional maternal famine exposure can have a lasting effect on offspring DNA methylation over the life course. More recent analysis of global methylation patterns in this cohort have, however, demonstrated no discernible difference in global DNA methylation patterns between offspring of exposed and unexposed mothers [25], perhaps highlighting the complexity and potentially target-specific nature of prenatal exposures upon the fetal epigenome. Finally, a recent study in Gambia has suggested DNA methylation patterns in F1 offspring differ, depending on season of conception, in a context where diet varies greatly according to season [26].
Much of the human literature in this area has focused upon the influence of folate nutrition in the prenatal period and the role it may have in altering epigenetic patterns in the developing fetus. This issue is discussed in more detail in later sections, but is based upon the premise that folate specifically, and one-carbon metabolism more broadly, fuels the addition of methyl groups to DNA (Figure 2). Hence, a direct biological link between folate status during pregnancy and the fetal epigenome provide a logical and biologically plausible framework for investigation. Folate supplementation is associated with substantial shifts in DNA methylation patterns in circulating peripheral blood in women of reproductive age [27] and other adult populations [28,29]. Studies of DNA methylation in offspring following prenatal folic acid supplementation in pregnant women, however, have shown conflicting findings; Steegers-Theunissen et al. reported that offspring of women who took supplements in the periconceptional period had higher IGF2 methylation [30], but in a separate study folic acid use in pregnancy was shown to have no influence on IGF2 methylation, despite an association with hypomethylation of the H19 locus in cord-blood DNA [31]. More recently, Cooper et al. reported that DNA methylation profiling of offspring following periconceptional micro-nutrient supplementation (including folate) indicated shifts in methylation of the IGF2R and GTL2-2 loci, but not at IGF2 [32]. Our own data corroborates these findings; maternal folate status in pregnancy appears to correlate with infant DNA methylation at some loci, but not others [Relton CL, Unpublished Data].
Prenatal nutrition & schizophrenia in offspring
The strongest evidence relating maternal prenatal nutrition to schizophrenia in offspring derives from a series of studies based on exposure to severe famine [33-36]. The first such studies were based on the Dutch Hunger Winter, which began with a Nazi blockade of occupied western Holland in October 1944, ending abruptly following liberation in early May 1945. Over the course of the Hunger Winter, famine progressed from mild to severe as food supplies ran out and individuals were nutritionally compromised. In March and April 1945, famine peaked, reflected in a low fertility rate and a high death rate owing to starvation. The famine was more severe in urban populations compared with rural populations because of more restricted food access in towns and cities. A series of studies with concordant results have linked maternal periconceptional exposure at the famine’s peak with increased risk of three neurodevelopmental outcomes in offspring: neural tube defects (NTDs) [36,37]; schizoid diagnoses at age 18 (males only, at military induction) [38]; and hospitalization for schizophrenia in adulthood [34]. Since the timing of exposure had to be inferred from date of birth, the concordant increase in NTDs is informative here because it is reasonable to infer that this outcome reflected folate deficiency during approximately −4 to +4 weeks gestation (the neural tube closes at 4 weeks).
The second series of studies were based on the Chinese famine that followed the Great Leap Forward. In contrast to the Dutch Hunger Winter, the Chinese famine was much more severe in rural areas, owing to government policies on food distribution. Since the famine was of extended duration, and data were available only for birth year, the Chinese studies cannot precisely define time of exposure. Instead, periconceptional exposure to severe famine is assessed by birth year, where the most severe famine took place in years characterized by sharp reductions in fertility, evidenced by decreased birth rates approximately 10 months later. Two independent studies broadly replicated the findings of the Dutch studies [33,35]. We note here that: the Chinese studies were much larger than the Dutch studies; the results of the two studies in very different regions of China were entirely concordant with the results from the Dutch studies; and one of the Chinese studies included both rural and urban areas, and (as predicted) found this result in the more-exposed rural but not the less-exposed urban area.
A further strand of evidence linking prenatal nutritional status and schizophrenia risk is provided by, an albeit limited, literature reporting a link between short interpregnancy interval and elevated risk of schizophrenia in offspring [39]. Short interpregnancy interval is associated with maternal nutrient depletion, commonly linked to reduced folate status.
There are many other reports suggesting plausible links between prenatal nutrition and schizophrenia. These include studies of specific micronutrients, such as vitamin D [40,41], the role of homocysteine [42] and studies of candidate genes for psychosis also involved in one-carbon metabolism, such as MTHFR [2,43]. Thus far, however, none of the other findings for schizophrenia have the strength and consistency of the famine findings. In addition, here is indirect evidence from studies linking prenatal nutrition to neurodevelopmental outcomes that can be antecedents of schizophrenia, including poorer cognitive function [44] and language delay in offspring [45].
DNA methylation & schizophrenia
Post-mortem human studies provide evidence that schizophrenia is associated with epigenetic dysregulation in the brain [46-48]. The strongest evidence pertains to alterations in DNA methylation reported in the regulatory regions of several genes of potential relevance to schizophrenia [49-52]. In addition, a genome-wide study examined frontal cortices of psychotic patients and control subjects, and found differential DNA methylation patterns for genes involved in glutamatergic and GABAergic neurotransmission, and in brain development [53]. In most studies, DNA methylation changes were inversely correlated with changes in gene expression. This is consistent with the hypothesis that epigenetic alterations affect brain function via changes in mRNA and protein abundance in specific neuronal or glial cell types.
Here we focus on post-mortem human brain studies of the Reelin gene. Reelin is a critical protein involved in neuronal migration and positioning during neurodevelopment. Furthermore, Reelin continues to be expressed in adult GABAergic neurons, playing a role in synaptic plasticity and memory formation [54]. Reduction of Reelin mRNA in certain brain regions was reported in some of the earliest post-mortem studies of mRNA in schizophrenia [55-57]. Although negative results have also been reported [58], the downregulation of Reelin expression continues to be one of the most consistent findings in this disorder [59-61]. Importantly, in humans, genetic variation in the Reelin gene has not been associated with changes in mRNA expression emphasizing the potential role of epigenetic factors in gene regulation [62,63]. The initial reports did not, however, connect reduced Reelin mRNA levels in the brains of schizophrenic patients to variation in DNA methylation [60,61].
The first evidence that the Reelin gene is regulated through DNA methylation-dependent mechanisms came from in vitro studies with the human neuronal progenitor NT2 cell line [64]. These studies showed that the Reelin promoter is silenced by DNA methylation [64], and could be activated by the agents that induce demethylation of this region [64,65]. It was then hypothesized that the reduced expression of the Reelin gene in schizophrenia may be due to increased methylation of its transcriptionally relevant promoter region [54]. Two [52,66] of the three [52,66,67] studies testing this hypothesis found evidence in support of it. Further post-mortem studies suggested a possible mechanism by which the Reelin gene may be hypermethylated in schizophrenia. DNMT1 was upregulated in cortical GABAergic neurons of individuals with schizophrenia [68,69]. Reelin and DNMT1 colocalize in the same GABAergic neurons. Thus, reduced mRNA levels of Reelin may be due to up regulated DNMT1 activity, which in turn increases promoter methylation [70].
In addition, Reelin is of special interest because some animal models indicate that prenatal nutrition may influence Reelin expression in the brain. For example, in a study of rats, maternal dietary deficiency of methyl donors during pregnancy was associated with reduced Reelin expression in the brain tissue of adult offspring [71]. The mechanisms by which this might occur, however, are still not understood; this study did not detect differences in DNA methylation that might mediate an effect on Reelin expression.
The findings on Reelin illustrate both strengths and remaining gaps in studies of DNA methylation and schizophrenia. Overall, current research strongly supports the view that schizophrenia is associated with changes in DNA methylation in the brain. Post-mortem studies, however, are limited in their capacity to establish the direction of causality. We propose strategies to address this issue in the section entitled ‘establishing the causal role of epigenetic factors as mediators of the influence of prenatal nutrition on schizophrenia risk’.
Although we have not discussed epigenetic findings from peripheral blood DNA in any detail, this does not imply that such studies cannot be useful in schizophrenia research. Indeed, recent evidence has demonstrated that disease-associated differences in DNA methylation can be detected in peripheral blood DNA in monozygotic twins discordant for schizophrenia [72]. Some intriguing reports have been based on peripheral blood [73-75], and forthcoming research of this kind might advance our understanding of epigenetic changes in schizophrenia.
One-carbon metabolism as a plausible pathway
Folate & DNA methylation
Many nutrients have been shown to modulate epigenetic patterns including components of the one-carbon metabolism, as well as retinoic acid, poly phenols, resveratrol and other food components [76]. We have chosen to focus on one-carbon metabolism as a highly relevant exemplar in regard to schizophrenia and prenatal nutrition (Figure 2). As noted earlier, this metabolic pathway is integral to DNA methylation because it provides the methyl donors required for methylation of cytosine nucleotides. Furthermore, among the micronutrients that have been hypothesized to link prenatal nutrition with offspring schizophrenia, most, although not all, play important roles in one-carbon metabolism, notably, choline, vitamin B12 and folate (vitamin B9) [11,12,24,27].
It is possible that deficiency of such micronutrients at critical points of neurodevelopment, including in the prenatal period, may lead to alterations in gene expression that could have adverse downstream effects on health, including mental health and schizophrenia. We examine the human literature that may support such a hypothesis, with particular reference to folate, incorporating evidence for the role of folate on potentially intermediate neurodevelopmental phenotypes, as well as schizophrenia.
Folate, one-carbon metabolism & neurodevelopmental phenotypes
The extent to which folate and wider one-carbon metabolism provide a mechanistic pathway linking adverse nutrition to psychosis is yet to be established. There are, however, indirect clues that folate availability during critical periods of brain development, including prenatally, can alter neurodevelopment in offspring. Interestingly, some of these impairments have also been shown to be associated with later psychosis risk. For example, prenatal maternal folate reduces the risk of NTDs in offspring by between 50 and 75% with adequate and timely prenatal maternal folic acid supplementation [77-84]. The risk of NTDs also appears to be elevated amongst offspring of mothers exposed to the folate antagonist valproate (VPA) during pregnancy [85], used as an anti convulsant in the treatment of epilepsy and bipolar disorder, which can cause folate deficiency [80] as a result of reduced methionine adenosyltransferase activity and plasma vitamin B6 levels [81]. Other types of congenital malformations have been associated with up to twice the risk of schizophrenia spectrum disorders [82,83]. NTDs and schizophrenia appear to have overlapping epidemiological facets [84], raising the possibility that they share a common etiological antecedent, such as prenatal maternal folate deprivation. These malformations may reflect ‘hard’ evidence of abnormal prenatal neurodevelopment associated with a range of functional impairments early in life [83].
Such functional impairments appear to be associated with prenatal maternal folate consumption. For example, recently published data from a large pregnancy/birth cohort suggest a lower risk of severe language delay in offspring of mothers who took periconceptional folic acid supplementation [45], while data from the Mysore Parthenon birth cohort in south India indicate that children exposed to higher concentrations of folate during pregnancy had better cognitive function at age 9–10 years old [44]. Finally, greater cognitive impairment has been reported in offspring at age 3 years exposed in utero to VPA [86], compared with offspring exposed to other antiepileptics or unexposed controls. Interestingly, similar observations were not independently identified for either vitamin B12 or homocysteine in the same study, two further micronutrients important in one-carbon metabolism (Figure 2) . We know that many of these neurodevelopmental delays in infancy or early childhood are associated with an increased risk of schizophrenia spectrum disorders later in life [87-93]. Such impairments are for the most part subtle, and therefore have low positive predictive value; most people within this range will not go onto to develop schizophrenia.
Further indirect evidence to support a role for folate in neurodevelopment comes from one study which observed that perinatal folate supplementation was associated with a reduced risk of autism in offspring [94]. There are also reports that in utero exposure to the folate antagonist VPA is associated with increased risk of autism in offspring [95,96].
To date, no prospective pregnancy cohort has been able to directly examine the relationship between periconceptional maternal folate and later schizophrenia risk in offspring, largely due to the large numbers required to study psychoses, long lag between exposure and outcome, and the requirement for prospectively collected, accurate data on very early prenatal exposure. A few large ongoing pregnancy cohorts, however, have the necessary data from early pregnancy, are following the children, and will very soon be able to examine adolescents for schizophrenia-like symptoms and then young adults for diagnoses (e.g., [97]). A few previous smaller studies have examined hypotheses pertaining to later periods of gestation. One study using a nested case–control design in the Child Health and Development Study birth cohort, reported an increased risk of schizophrenia in the offspring of mothers whose maternal serum had elevated levels of homocysteine, a potential, although not conclusive, marker of low folate status, in the third trimester of pregnancy [42]. Data from the ALSPAC birth cohort study was used to investigate the association between psychotic-like symptoms at age 12 years and maternal folate status measured at week 18, but found no evidence to support this hypothesis [98]. As yet, no studies have used innovative methodological approaches, which will be required to determine the causal role of epigenetic changes in any relationship between prenatal nutrition and psychosis. We discuss some of these approaches in the next section. We note that the approach we propose can be used not only in large pregnancy cohorts, but also in other designs, because it involves two steps, and it is not strictly necessary for the two steps to be conducted within the same large cohort.
Establishing the causal role of epigenetic factors as mediators of the influence of prenatal nutrition on schizophrenia risk
Temporal relationships & conventional epidemiological & experimental approaches
A clear temporal relationship exists between the postulated role of prenatal nutrition in modulating the risk of schizophrenia and the potential mediating influence of epigenetic mechanisms. Although case–control series can offer evidence that epigenetic perturbation is associated with disease, one cannot deduce the direction of causation. Animal studies, and where practicable human studies, that can provide biological samples taken early in the life course will be required to clarify the relationship between epigenetic change and disease pathogenesis, especially if samples are available before the onset of the disease of interest.
The dynamic nature of epigenetic variation poses challenges; an epigenetic change induced in utero may not necessarily need to persist across the life course to precipitate adult-onset disease, it may merely set in motion a chain of biological events that culminate in schizophrenia. This chain of events may include early neurodevelopmental pathologies that act as a key intermediate phenotype prompted by epigenetic changes induced in utero. Approaches that can delineate the temporal association of epigenetic change with the development of the adult phenotype are required. These may include the analysis of longitudinal cohort studies [97,99], or more realistically, synthesized life course studies (i.e., drawn from several cohorts of different age brackets) [100], which focus upon the respective steps in the pathway depicted in Figure 1 in turn. Experimental approaches can include the use of animal models, including intervention studies [101], permitting time course experiments and tissue-specific analyses of both epigenetic patterns and gene expression. In vitro approaches using cultured human tissues or cell lines can also provide useful tools when focusing on specific mechanistic issues, for example, the relationship between DNA methylation and gene expression at a specific locus following controlled exposure to a particular nutrient [102].
Novel epidemiological approaches to strengthen causal inference
A novel method that has the potential to improve our understanding of the role of epigenetic processes in mediating the effect of prenatal nutrition on later risk of schizophrenia is based upon a method that has been popularized in the analysis of causal relationships in observational epidemiology known as Mendelian randomization (MR). The MR approach (described in detail elsewhere [103]) uses one or more genetic variants as proxies for an environmentally modifiable exposure (including diet) to establish whether an exposure is causally related to disease [103]. This approach offers major advantages because the utilization of genetic variants as proxies for environmentally modifiable exposures allows the problematic issues of confounding, reverse causation and measurement error to be circumvented [104]. In addition, genetic variants reflect long-term, life-long levels of exposure as opposed to the snapshot measure often acquired in exposure measurement.
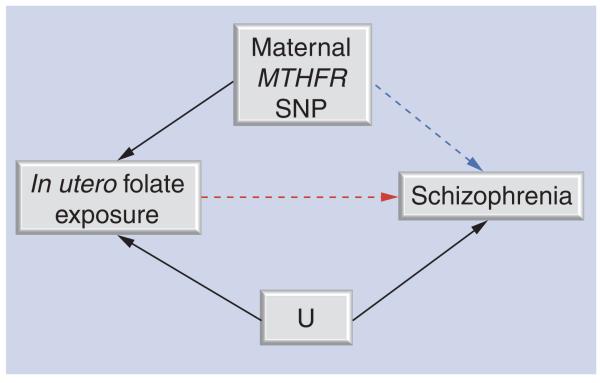
The application of the MR approach to interrogate the relationship between prenatal folate nutrition and schizophrenia risk is outlined in Figure 3. Maternal MTHFR genotype is used as a proxy of reduced availability of in utero folate (or more specifically elevated homocysteine) [105], and the relationship with schizophrenia assessed. This basic MR principle has been applied in the interrogation of the association between offspring NTD risk and maternal MTHFR genotype [104].
Figure 3. Mendelian randomization.
Using a genetic variant as a proxy to establish whether prenatal exposure to folate/homocysteine is causally related to schizophrenia. Maternal MTHFR 677C>T (rs1801133) genotype is used here as a proxy for folate/homocysteine exposure in utero. Genotype is independent of U. Genotype will only influence schizophrenia if the exposure→schizophrenia association is causal.
U: Unmeasured confounders.
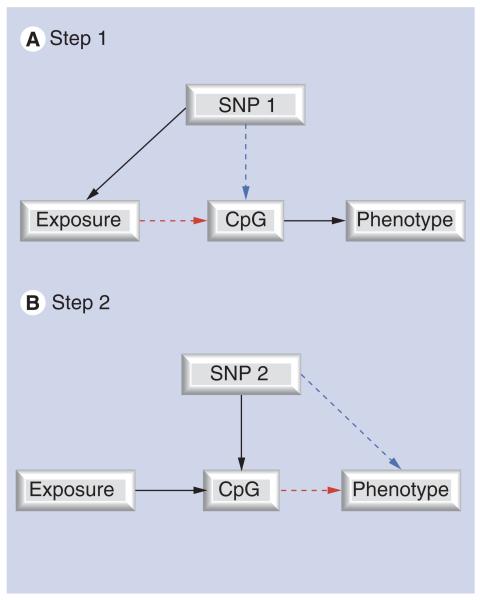
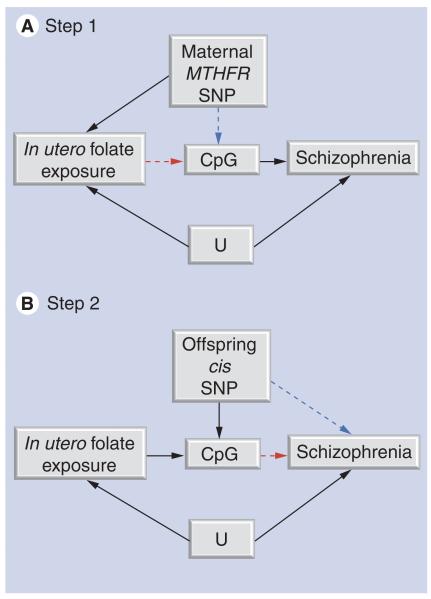
MR can be further developed to assess the potential mediating role of DNA methylation [106], in an approach we term ‘two-step epigenetic MR’ [107] (Figures 4 & 5). The two-step epigenetic MR approach first uses a genetic proxy for the exposure of interest to assess the relationship between exposure and DNA methylation. A second step then utilizes a genetic proxy for DNA methylation to interrogate the causal relationship between DNA methylation and outcome. This approach can be applied to assess whether the effect of in utero exposures on offspring outcomes are mediated by DNA methylation, using maternal genotype as a proxy for maternal exposure in the first step (folate and one-carbon pathway intermediates) and offspring genotype as a proxy for DNA methylation in the second step (a neurodevelopmental outcome or schizophrenia).
Figure 4. The principle of two-step epigenetic Mendelian randomization.
The principle of Mendelian randomization is applied to assess the mediating role of DNA methylation. Genetic variants can be used in a two-step framework. (A) In step 1, a genetic variant is used to proxy for the environmental exposure of interest. (B) In step 2, a separate genetic variant is used to proxy for DNA methylation.
Figure 5. Two-step epigenetic Mendelian randomization applied to prenatal nutrition and schizophrenia risk.
(A) In step 1, maternal MTHFR 677C>T (rs1801133) genotype acts as a proxy for prenatal folate/homocysteine influences on fetal DNA methylation. MTHFR genotype will only influence DNA methylation if folate/homocysteine is causally related to methylation levels at the locus (CpG) studied. (B) In step 2, a separate genetic variant that correlates with offspring DNA methylation levels (commonly a cis variant in close proximity to the CpG site being measured) is utilized. The offspring genotype will only be associated with schizophrenia if the relationship between DNA methylation and schizophrenia is causal.
U: Unmeasured confounders.
This method is founded upon the well-established framework of MR, which now has an extensive literature attesting its application [108]. There are, however, as yet few examples of two-step epigenetic MR other than its use in interrogating causality with respect to epigenetic mediation of the association between postnatal growth and childhood adiposity [109]. The method has limitations, such as the lack of availability of established proxies for DNA methylation (although these will largely be cis variants that can be explored once the differentially methylated CpG sites of interest have been identified) and the tissue specificity of DNA methylation. Strengths and weaknesses of both MR and two-step epigenetic MR approaches are detailed elsewhere [106]. This approach does, however, present a tractable method for exploring causal relationships in the context of developmental programming.
It has been suggested that schizophrenia spectrum disorders could largely be explained by epigenetic causes [7], and that these causes are primarily stochastic in nature [110]. The two-step MR approach we outline here would be able to test such a hypothesis, given that under such a scenario one would still expect a proportion of cases to occur as a result of nonstochastic processes, such as via genes which modify methylation status or environmental exposures which do the same. Because such factors would mimic the underlying stochastic epigenetic causes hypothesized to lead to schizophrenia, the two-step epigenetic MR approach could be used to verify this hypothesis.
MR-based approaches are not the only tool for strengthening causal inference when considering intrauterine influences on offspring health. A number of other epidemiological approaches can be applied, including the comparison of siblings discordant for maternal exposures, such as use of folic acid supplements [111,112] or the comparison of paternal and maternal exposures with offspring outcomes [99,100]. In the latter design, similar effect sizes for maternal and paternal exposure would detract from the evidence regarding a putative intrauterine effect [113]. Additional strategies, such as the comparison of cohorts with markedly different confounding structures [114], or the use of ecological studies comparing disease incidence before or after a particular intervention (such as folate fortification), could be applied, with DNA methylation in offspring being considered as an intermediate phenotype. In summary, both conventional and recently developed epidemiological approaches have the potential to make a major contribution to understanding the causal role of epigenetic mechanisms in mediating the effect of in utero exposures on later health [115].
Conclusion
Post-mortem human studies have revealed differences in DNA methylation patterns in the brains of people with schizophrenia and controls. Other studies in human populations have shown that prenatal maternal nutrition deprivation is linked with a range of neurodevelopmental abnormalities in offspring, including schizophrenia and some of its antecedents, such as language delay and cognitive and social impairment. There is strong animal evidence and some human evidence to support the possibility that adverse prenatal nutrition can lead to epigenetic changes for some offspring phenotypes, putatively via one-carbon metabolism. We have suggested a novel epidemiological methodology, utilizing a two-step epigenetic MR approach, which can be employed to test whether DNA methylation changes following prenatal maternal exposure to adverse nutritional environments may be induced in this way to increase the risk of complex disorders such as schizophrenia in offspring.
Future perspective
Several risk factors for schizophrenia and other psychoses appear to occur early in life, including in the prenatal period. Putative exposures include nutritional deprivation, as discussed here, infections, such as maternal influenza or toxoplasmosis, and a range of early adverse social traumas. To elucidate the pathways through which exposure to these factors may increase schizophrenia risk in offspring requires sophisticated study designs to overcome some significant challenges. With particular regard to the present hypothesis, we will need to employ novel methodologies capable of prospectively measuring exposure status (maternal prenatal nutrition relevant to one-carbon metabolism), epigenetic markers (in blood or brain, global or gene-specific) and outcomes in offspring (i.e., schizophrenia) several decades later. Conventional epidemiological approaches would need to be prospective, extremely large, long (several decades) and measure a wide range of possible confounding factors, both genetic and environmental. Such studies will be feasible only in a limited number of large pregnancy/birth cohorts with a rich array of biological as well as follow-up data. We have presented one alternative possibility, using a two-step epigenetic Mendelian randomization approach to establish associations between exposure, DNA methylation and outcome in a framework that can offer some balance for confounders and potentially strengthens causal inference. While we anticipate psychiatric studies will begin to utilize this technique in the coming years, we also note that it is not without limitations [106], including the need for large sample sizes and some notable statistical genetic issues; thus, we see this field as a growing area both for theoretical and empirical research.
Executive summary.
Background
-
■
Prenatal nutrition is hypothesized to be linked to increased offspring schizophrenia risk via epigenetic processes, such as DNA methylation.
-
■
Epigenetic processes include various mechanisms that influence chromatin structure and gene expression, such as DNA methylation, histone modifications and chromatin remodeling.
Evidence linking maternal prenatal nutrition, DNA methylation & schizophrenia
-
■Prenatal nutrition & DNA methylation in offspring
-
–In animals, there is strong evidence that prenatal nutrition can affect offspring phenotype via DNA methylation, with the Agouti mouse model providing a classic example.
-
–In humans, famine studies suggest that periconceptional exposure to famine results in DNA methylation changes in offspring, notably at the IGF2 locus, which has a role in early development.
-
–
-
■Prenatal nutrition & schizophrenia in offspring
-
–Compromised periconceptional nutrition in humans is associated with an increased risk of offspring schizophrenia, as well as a number of other putatively related neurodevelopmental impairments, including cognitive function, social function, language delay and autism.
-
–
-
■DNA methylation & schizophrenia
-
–Post-mortem human studies have shown DNA methylation changes in the brain of people with schizophrenia, particularly with regard to the Reelin gene, coding for critical neurodevelopmental protein.
-
–There is a small amount of animal evidence to suggest that Reelin expression in offspring may be influenced by prenatal nutritional availability.
-
–Post-mortem studies have limited ability to establish causation, as epigenetic changes could be a consequence of the disorder, not a cause.
-
–
One-carbon metabolism as a plausible pathway
-
■Folate & DNA methylation
-
–One-carbon metabolism is essential for normal DNA methylation.
-
–DNA methylation is dependent on a number of key essential nutrients, including folate.
-
–
-
■Folate, one-carbon metabolism & neurodevelopmental phenotypes
-
–Periconceptional folate supplementation reduces the risk of neural tube defects, and has been associated with a reduced risk of language delay and cognitive impairments in offspring, known antecedents of schizophrenia.
-
–No direct link between folate availability and schizophrenia has yet been tested. It is difficult to study in conventional epidemiological frameworks, owing to sample size requirements, biological sample requirements, the need for extensive control for confounders and long length of follow-up.
-
–
Establishing the causal role of epigenetic factors as mediators of the influence of prenatal nutrition on schizophrenia risk
-
■Temporal relationships and conventional epidemiological & experimental approaches
-
–The dynamic nature of epigenetic variation poses challenges, as in utero epigenetic changes which lead to adverse neurodevelopmental outcomes need not persist over the life course.
-
–
-
■Novel epidemiological approaches to strengthen causal inference
-
–We propose a novel technique using a two-step Mendelian randomizaton approach to establish whether DNA methylation is associated with the exposure and outcome of interest.
-
–The approach utilizes one or more genetic variants as proxies for an environmentally modifiable exposure (including diet) to establish whether an exposure is causally related to disease.
-
–The two-step approach first tests whether the genetic proxy for an environmental exposure is associated with DNA methylation, then whether a genetic proxy for DNA methylation is associated with outcome of interest.
-
–The approach offers advantages in balancing (unmeasured) confounders, reverse causation and measurement error, but is in its infancy and problems of large sample sizes and other statistical/genetic issues (such as population stratification, pleiotropy or linkage disequilibrium) are not eliminated.
-
–
Conclusion
-
■
There is growing evidence from different perspectives that prenatal maternal nutrition may be associated with increased risk of schizophrenia in offspring mediated by epigenetic modifications. Novel methodological approaches are required to test this.
Acknowledgments
Financial & competing interests disclosure
This work was funded by the Wellcome Trust (grant number WT085540MA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Cardno AG, Marshall EJ, Coid B, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch. Gen. Psychiatry. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr. Res. 2008;104:96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am. J. Epidemiol. 2007;165:1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- 4.Peerbooms OLJ, van Os J, Drukker M, et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav. Immun. 2011;25:1530–1543. doi: 10.1016/j.bbi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Decandia TR, Ripke S, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat. Genet. 2012;44:247–250. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Schizophrenia Consortium Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petronis A, Gottesman II, Kan P, et al. Monozygotic twins exhibit numerous epigenetic differences: clues to twin discordance? Schizophr. Bull. 2003;29:169–178. doi: 10.1093/oxfordjournals.schbul.a006988. [DOI] [PubMed] [Google Scholar]
- 8.Petronis A. The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol. Psychiatry. 2004;55:965–970. doi: 10.1016/j.biopsych.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Petronis A, Paterson AD, Kennedy JL. Schizophrenia: an epigenetic puzzle? Schizophr. Bull. 1999;25:639–655. doi: 10.1093/oxfordjournals.schbul.a033408. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect Agouti gene expression in A(vy)/a mice. FASEB J. 1998;12:949–957. [■ Showed that feeding mouse dams a methyl-supplemented diet led to epigenetic changes in Agouti expression in offspring, providing clues that one-carbon metabolism was important in epigenetic modifications, particularly DNA methylation.] [PubMed] [Google Scholar]
- 12.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 2002;132(8 Suppl.):2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 13.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandovici I, Smith NH, Nitert MD, et al. Maternal diet and aging alter the epigenetic control of a promoter–enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;44:401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 16.Morgan HD, Sutherland HGE, Martin DIK, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 1999;23:314–318. doi: 10.1038/15490. [■ Demonstrates inheritance of the Avy modification of the Agouti gene is due to epigenetic modifications.] [DOI] [PubMed] [Google Scholar]
- 17.Wolff GL, Roberts DW, Galbraith DB. Prenatal determination of obesity, tumor susceptibility, and coat color pattern in viable yellow (Avy/a) mice. The yellow mouse syndrome. J. Hered. 1986;77:151–158. doi: 10.1093/oxfordjournals.jhered.a110206. [DOI] [PubMed] [Google Scholar]
- 18.Wolff GL, Roberts DW, Mountjoy KG. Physiological consequences of ectopic agouti gene expression: the yellow obese mouse syndrome. Physiol. Genomics. 1999;1:151–163. doi: 10.1152/physiolgenomics.1999.1.3.151. [DOI] [PubMed] [Google Scholar]
- 19.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes. 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl Acad. Sci. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seki Y, Williams L, Vuguin PM, Charron MJ. Minireview: epigenetic programming of diabetes and obesity: animal models. Endocrinology. 2012;153(3):1031–1038. doi: 10.1210/en.2011-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CC, Cropley JE, Cowley MJ, Preiss T, Martin DI, Suter CM. A sustained dietary change increases epigenetic variation in isogenic mice. PLoS Genet. 2011;7:e1001380. doi: 10.1371/journal.pgen.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen DY, Stern SA, Garcia-Osta A, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumey L, Terry MB, Delgado-Cruzata L, et al. Adult global DNA methylation in relation to pre-natal nutrition. Int. J. Epidemiol. 2011;41(1):116–123. doi: 10.1093/ije/dyr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterland RA, Kellermayer R, Laritsky E, et al. Season of conception in rural Gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crider KS, Quinlivan EP, Berry RJ, et al. Genomic DNA methylation changes in response to folic acid supplementation in a population-based intervention study among women of reproductive age. PLoS ONE. 2011;6:e28144. doi: 10.1371/journal.pone.0028144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stidley CA, Picchi MA, Leng S, et al. Multivitamins, folate, and green vegetables protect against gene promoter methylation in the aerodigestive tract of smokers. Cancer Res. 2010;70:568–574. doi: 10.1158/0008-5472.CAN-09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pufulete M, Al-Ghnaniem R, Khushal A, et al. Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut. 2005;54:648–653. doi: 10.1136/gut.2004.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS ONE. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyo C, Murtha AP, Schildkraut JM, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6:928–936. doi: 10.4161/epi.6.7.16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper WN, Khulan B, Owens S, et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J. 2012;26(5):1782–1790. doi: 10.1096/fj.11-192708. [DOI] [PubMed] [Google Scholar]
- 33.St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [■ First independent replication (from the Chinese famine) of the Dutch Hunger Winter findings that offspring of prenatally nutritionally compromised mothers had a higher risk of schizophrenia.] [DOI] [PubMed] [Google Scholar]
- 34.Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch. Gen. Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [■■ First strong evidence from a study of a human population that prenatal maternal dietary restriction was associated with an increased risk of schizophrenia in offspring later in life.] [DOI] [PubMed] [Google Scholar]
- 35.Xu MQ, Sun WS, Liu BX, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959-1961 Chinese famine. Schizophr. Bull. 2009;35:568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: the story of the Dutch Famine Study. Am. J. Epidemiol. 1998;147:213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- 37.Stein Z. Famine And Human Development: The Dutch Hunger Winter of 1944–1945. Oxford University Press; NY, USA: 1975. [■■ Study that laid the platform for subsequent investigations of schizophrenia and epigenetic changes related to prenatal famine.] [Google Scholar]
- 38.Hoek HW, Susser E, Buck KA, Lumey LH, Lin SP, Gorman JM. Schizoid personality disorder after prenatal exposure to famine. Am. J. Psychiatry. 1996;153:1637–1639. doi: 10.1176/ajp.153.12.1637. [DOI] [PubMed] [Google Scholar]
- 39.Gunawardana L, Davey Smith G, Zammit S, et al. Pre-conception inter-pregnancy interval and risk of schizophrenia. Br. J. Psychiatry. 2011;199:338–339. doi: 10.1192/bjp.bp.111.092916. [DOI] [PubMed] [Google Scholar]
- 40.McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr. Res. 2003;63:73–78. doi: 10.1016/s0920-9964(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 41.McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr. Res. 1999;40:173–177. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 42.Brown AS, Bottiglieri T, Schaefer CA, et al. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch. Gen. Psychiatry. 2007;64:31–39. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Friso S, Choi SW, Girelli D, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veena SR, Krishnaveni GV, Srinivasan K, et al. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10-year-old children in south India. J. Nutr. 2010;140:1014–1022. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roth C, Magnus P, Schjolberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306:1566–1573. doi: 10.1001/jama.2011.1433. [■ Birth cohort data linking lack of prenatal maternal folic acid supplementation directly to language delays in offspring. Language delays have been shown to be an antecedent of schizophrenia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdolmaleky HM, Thiagalingam S. Can the schizophrenia epigenome provide clues for the molecular basis of pathogenesis? Epigenomics. 2011;3:679–683. doi: 10.2217/epi.11.94. [DOI] [PubMed] [Google Scholar]
- 47.Grayson DR. Schizophrenia and the epigenetic hypothesis. Epigenomics. 2010;2:341–344. doi: 10.2217/epi.10.22. [DOI] [PubMed] [Google Scholar]
- 48.Roth TL, Lubin FD, Sodhi M, Kleinman JE. Epigenetic mechanisms in schizophrenia. Biochim. Biophys. Acta. 2009;1790:869–877. doi: 10.1016/j.bbagen.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdolmaleky HM, Yaqubi S, Papageorgis P, et al. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr. Res. 2011;129:183–190. doi: 10.1016/j.schres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Abdolmaleky HM, Cheng KH, Faraone SV, et al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum. Mol. Genet. 2006;15:3132–3145. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwamoto K, Bundo M, Yamada K, et al. DNA methylation status of SOX10 correlates with its downregulation and oligodendrocyte dysfunction in schizophrenia. J. Neurosci. 2005;25:5376–5381. doi: 10.1523/JNEUROSCI.0766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mill J, Tang T, Kaminsky Z, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am. J. Hum. Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grayson DR, Chen Y, Costa E, et al. The human reelin gene: transcription factors (+), repressors (-) and the methylation switch (+/-) in schizophrenia. Pharmacol. Ther. 2006;111:272–286. doi: 10.1016/j.pharmthera.2005.01.007. [■■ Provides a comprehensive review on DNA methylation-dependent regulation of Reelin gene expression and its implication for an epigenetic hypothesis of schizophrenia.] [DOI] [PubMed] [Google Scholar]
- 55.Impagnatiello F, Guidotti AR, Pesold C, et al. A decrease of Reelin expression as a putative vulnerability factor in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch. Gen. Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 57.Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol. Psychiatry. 2000;5:654–663. 571. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 58.Lipska BK, Peters T, Hyde TM, et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum. Mol. Genet. 2006;15:1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- 59.Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol. Psychiatry. 2003;8:769–821-31. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- 60.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Eastwood SL, Harrison PJ. Cellular basis of reduced cortical reelin expression in schizophrenia. Am. J. Psychiatry. 2006;163:540–542. doi: 10.1176/appi.ajp.163.3.540. [DOI] [PubMed] [Google Scholar]
- 62.Tost H, Lipska BK, Vakkalanka R, et al. No effect of a common allelic variant in the reelin gene on intermediate phenotype measures of brain structure, brain function, and gene expression. Biol. Psychiatry. 2010;68:105–107. doi: 10.1016/j.biopsych.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ovadia G, Shifman S. The genetic variation of RELN expression in schizophrenia and bipolar disorder. PLoS ONE. 2011;6:e19955. doi: 10.1371/journal.pone.0019955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Sharma RP, Costa RH, Costa E, Grayson DR. On the epigenetic regulation of the human reelin promoter. Nucleic Acids Res. 2002;30:2930–2939. doi: 10.1093/nar/gkf401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kundakovic M, Chen Y, Costa E, Grayson DR. DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol. Pharmacol. 2007;71:644–653. doi: 10.1124/mol.106.030635. [DOI] [PubMed] [Google Scholar]
- 66.Abdolmaleky HM, Cheng KH, Russo A, et al. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- 67.Tochigi M, Iwamoto K, Bundo M, et al. Methylation status of the reelin promoter region in the brain of schizophrenic patients. Biol. Psychiatry. 2008;63:530–533. doi: 10.1016/j.biopsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- 69.Veldic M, Caruncho HJ, Liu WS, et al. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc. Natl Acad. Sci. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa E, Chen Y, Dong E, et al. GABAergic promoter hypermethylation as a model to study the neurochemistry of schizophrenia vulnerability. Expert Rev. Neurother. 2009;9:87–98. doi: 10.1586/14737175.9.1.87. [DOI] [PubMed] [Google Scholar]
- 71.Konycheva G, Dziadek MA, Ferguson LR, et al. Dietary methyl donor deficiency during pregnancy in rats shapes learning and anxiety in offspring. Nutr. Res. 2011;31:790–804. doi: 10.1016/j.nutres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Dempster EL, Pidsley R, Schalkwyk LC, et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum. Mol. Genet. 2011;20:4786–4796. doi: 10.1093/hmg/ddr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carrard A, Salzmann A, Malafosse A, Karege F. Increased DNA methylation status of the serotonin receptor 5HTR1A gene promoter in schizophrenia and bipolar disorder. J. Affect Disord. 2011;132:450–453. doi: 10.1016/j.jad.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Zhang J, Zhang L, Shen Y, Xu Q. Effects of MAOA promoter methylation on susceptibility to paranoid schizophrenia. Hum. Genet. 2011 doi: 10.1007/s00439-011-1131-5. [DOI] [PubMed] [Google Scholar]
- 75.Melas PA, Rogdaki M, Ösby U, Schalling M, Lavebratt C, Ekström TJ. Epigenetic aberrations in leukocytes of patients with schizophrenia: association of global DNA methylation with antipsychotic drug treatment and disease onset. FASEB J. 2012;26:2712–2718. doi: 10.1096/fj.11-202069. doi:10.1096/fj.11-202069. [DOI] [PubMed] [Google Scholar]
- 76.Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012;71:75–83. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- 77.MRC vitamin study research group Prevention of neural tube defects: results of the medical research council vitamin study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 78.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 79.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative project for neural tube defect prevention. N. Engl. J. Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 80.Hendel J, Dam M, Gram L, Winkel P, Jorgensen I. The effects of carbamazepine and valproate on folate metabolism in man. Acta Neurol. Scand. 1984;69:226–231. doi: 10.1111/j.1600-0404.1984.tb07805.x. [DOI] [PubMed] [Google Scholar]
- 81.Úbeda N, Alonso-Aperte E, Varela-Moreiras G. Acute valproate administration impairs methionine metabolism in rats. J. Nutr. 2002;132:2737–2742. doi: 10.1093/jn/132.9.2737. [DOI] [PubMed] [Google Scholar]
- 82.Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch. Gen. Psychiatry. 1999;56:234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- 83.Waddington JL, Brown AS, Lane A, et al. Congenital anomalies and early functional impairments in a prospective birth cohort: risk of schizophrenia-spectrum disorder in adulthood. Br. J. Psychiatry. 2008;192:264–267. doi: 10.1192/bjp.bp.107.035535. [DOI] [PubMed] [Google Scholar]
- 84.Zammit S, Lewis S, Gunnell D, Davey Smith G. Schizophrenia and neural tube defects: comparisons from an epidemiological perspective. Schizophr. Bull. 2007;33:853–858. doi: 10.1093/schbul/sbl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samren EB, van Duijn CM, Koch S, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 86.Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N. Engl. J. Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 88.Gunnell D, Harrison G, Rasmussen F, Fouskakis D, Tynelius P. Associations between premorbid intellectual performance, early-life exposures and early-onset schizophrenia. Cohort study. Br. J. Psychiatry. 2002;181:298–305. doi: 10.1192/bjp.181.4.298. [DOI] [PubMed] [Google Scholar]
- 89.Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr. Bull. 2000;26:351–366. doi: 10.1093/oxfordjournals.schbul.a033458. [DOI] [PubMed] [Google Scholar]
- 90.Malmberg A, Lewis G, David A, Allebeck P. Premorbid adjustment and personality in people with schizophrenia. Br. J. Psychiatry. 1998;172:308–313. doi: 10.1192/bjp.172.4.308. discussion 314-305. [DOI] [PubMed] [Google Scholar]
- 91.Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch. Gen. Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 92.Isohanni M, Miettunen J, Maki P, et al. Risk factors for schizophrenia. Follow-up data from the Northern Finland 1966 birth cohort study. World Psychiatry. 2006;5:168–171. [PMC free article] [PubMed] [Google Scholar]
- 93.Sorensen HJ, Mortensen EL, Schiffman J, Reinisch JM, Maeda J, Mednick SA. Early developmental milestones and risk of schizophrenia: a 45-year follow-up of the Copenhagen perinatal cohort. Schizophr. Res. 2010;118:41–47. doi: 10.1016/j.schres.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt RJ, Hansen RL, Hartiala J, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22:476–485. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rasalam AD, Hailey H, Williams JHG, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 96.Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod. Toxicol. 2009;28:1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 97.Magnus P, Irgens LM, Haug K, et al. Cohort profile: the Norwegian mother and child cohort study (MoBa) Int. J. Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 98.Glaser B, Ades AE, Lewis S, et al. Perinatal folate-related exposures and risk of psychotic symptoms in the ALSPAC birth cohort. Schizophr. Res. 2010;120:177–183. doi: 10.1016/j.schres.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Madrigano J, Baccarelli A, Mittleman MA, et al. Aging and epigenetics: longitudinal changes in gene-specific DNA methylation. Epigenetics. 2012;7:63–70. doi: 10.4161/epi.7.1.18749. doi:10.4161/epi.7.1.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McKay JA, Wong YK, Relton CL, Ford D, Mathers JC. Maternal folate supply and sex influence gene-specific DNA methylation in the fetal gut. Mol. Nutr. Food Res. 2011;55:1717–1723. doi: 10.1002/mnfr.201100150. [DOI] [PubMed] [Google Scholar]
- 102.Bell JT, Pai AA, Pickrell JK, et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol. 2011;12:R10. doi: 10.1186/gb-2011-12-1-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davey Smith G. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. 2011;6:27–43. doi: 10.1007/s12263-010-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 105.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 106.Relton CL, Davey Smith G. Two step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int. J. Epidemiol. 2012;41:161–176. doi: 10.1093/ije/dyr233. [■■ Proposes a methodology to test epigenetic modifications as mediators of relationships between environmental exposures and outcome, underpinned by a Mendelian randomization approach.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Relton CL, Davey Smith G. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med. 2010;7:e1000356. doi: 10.1371/journal.pmed.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Timpson NJ, Wade KH, Davey Smith G. Mendelian randomization: application to cardiovascular disease. Curr. Hypertens. Rep. 2012;14:29–37. doi: 10.1007/s11906-011-0242-7. [DOI] [PubMed] [Google Scholar]
- 109.Groom A, Potter C, Swan DC, et al. Postnatal growth and DNA methylation are associated with differential gene expression of the TACSTD2 gene and childhood fat mass. Diabetes. 2012;61:391–400. doi: 10.2337/db11-1039. [■ First empirical application of a two-step Mendelian randomization approach.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- 111.Donovan SJ, Susser E. Commentary: advent of sibling designs. Int. J. Epidemiol. 2011;40:345–349. doi: 10.1093/ije/dyr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Susser E, Eide MG, Begg M. Invited commentary: the use of sibship studies to detect familial confounding. Am. J. Epidemiol. 2010;172:537–539. doi: 10.1093/aje/kwq196. [DOI] [PubMed] [Google Scholar]
- 113.Davey Smith G. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin. Pharmacol. Toxicol. 2008;102:245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 114.Brion MJ, Lawlor DA, Matijasevich A, et al. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int. J. Epidemiol. 2011;40:670–680. doi: 10.1093/ije/dyr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Relton CL, Davey Smith G. Is epidemiology ready for epigenetics? Int. J. Epidemiol. 2012;41:5–9. doi: 10.1093/ije/dys006. [DOI] [PMC free article] [PubMed] [Google Scholar]