Abstract
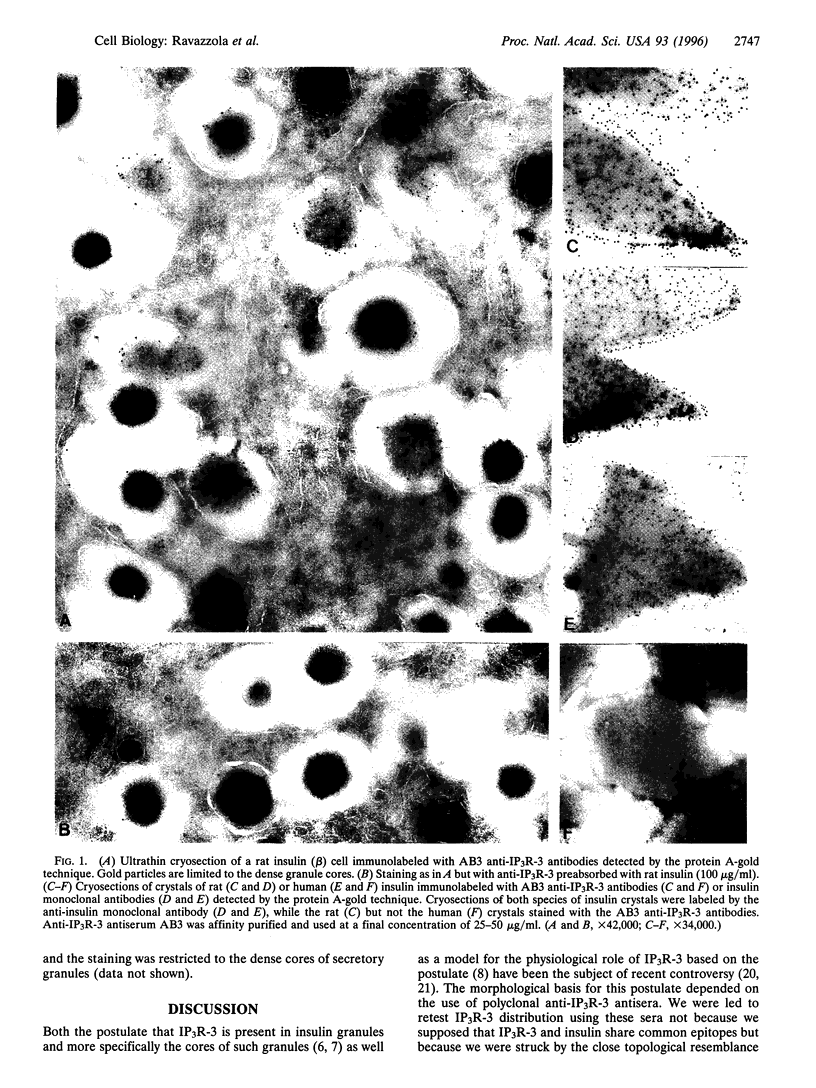
It has been reported that the inositol 1,4,5-trisphosphate receptor subtype 3 is expressed in islet cells and is localized to both insulin and somatostatin granules [Blondel, O., Moody, M. M., Depaoli, A. M., Sharp, A. H., Ross, C. A., Swift, H. & Bell, G. I. (1994) Proc. Natl. Acad. Sci. USA 91, 7777-7781]. This subcellular localization was based on electron microscope immunocytochemistry using antibodies (affinity-purified polyclonal antiserum AB3) directed to a 15-residue peptide of rat inositol trisphosphate receptor subtype 3. We now show that these antibodies cross-react with rat, but not human, insulin. Accordingly, the anti-inositol trisphosphate receptor subtype 3 (AB3) antibodies label electron dense cores of mature (insulin-rich) granules of rat pancreatic beta cells, and rat granule labeling was blocked by preabsorption of the AB3 antibodies with rat insulin. The immunostaining of immature, Golgi-associated proinsulin-rich granules with AB3 antibodies was very weak, indicating that cross-reactivity is limited to the hormone and not its precursor. Also, the AB3 antibodies labeled pure rat insulin crystals grown in vitro but failed to stain crystals grown from pure human insulin. By immunoprecipitation, the antibodies similarly displayed a higher affinity for rat than for human insulin. We could not confirm the labeling of somatostatin granules using AB3 antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbruster B. L., Carlemalm E., Chiovetti R., Garavito R. M., Hobot J. A., Kellenberger E., Villiger W. Specimen preparation for electron microscopy using low temperature embedding resins. J Microsc. 1982 Apr;126(Pt 1):77–85. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blondel O., Bell G. I., Moody M., Miller R. J., Gibbons S. J. Creation of an inositol 1,4,5-trisphosphate-sensitive Ca2+ store in secretory granules of insulin-producing cells. J Biol Chem. 1994 Nov 4;269(44):27167–27170. [PubMed] [Google Scholar]
- Blondel O., Bell G. I., Seino S. Inositol 1,4,5-trisphosphate receptors, secretory granules and secretion in endocrine and neuroendocrine cells. Trends Neurosci. 1995 Apr;18(4):157–161. doi: 10.1016/0166-2236(95)93894-4. [DOI] [PubMed] [Google Scholar]
- Blondel O., Moody M. M., Depaoli A. M., Sharp A. H., Ross C. A., Swift H., Bell G. I. Localization of inositol trisphosphate receptor subtype 3 to insulin and somatostatin secretory granules and regulation of expression in islets and insulinoma cells. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7777–7781. doi: 10.1073/pnas.91.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem. 1993 May 25;268(15):11356–11363. [PubMed] [Google Scholar]
- Colaco C. Glycation in neuropathies. Trends Neurosci. 1995 Aug;18(8):341–342. doi: 10.1016/0166-2236(95)93925-n. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Snyder S. H. Inositol 1,4,5-trisphosphate-activated calcium channels. Annu Rev Physiol. 1992;54:469–488. doi: 10.1146/annurev.ph.54.030192.002345. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Mikoshiba K. Inositol 1, 4, 5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J Neurochem. 1995 Mar;64(3):953–960. doi: 10.1046/j.1471-4159.1995.64030953.x. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Mutkoski R., Dodson G., Orci L. Resistance of the insulin crystal to lysosomal proteases: implications for pancreatic B-cell crinophagy. Diabetologia. 1987 May;30(5):348–353. doi: 10.1007/BF00299029. [DOI] [PubMed] [Google Scholar]
- Irminger J. C., Vollenweider F. M., Neerman-Arbez M., Halban P. A. Human proinsulin conversion in the regulated and the constitutive pathways of transfected AtT20 cells. J Biol Chem. 1994 Jan 21;269(3):1756–1762. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Pozzan T. IP3 receptors and secretory granules. Trends Neurosci. 1995 Aug;18(8):340–341. doi: 10.1016/0166-2236(95)93924-m. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Jul 25;265(21):12679–12685. [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Perrelet A. (Pro)insulin associates with Golgi membranes of pancreatic B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6743–6746. doi: 10.1073/pnas.81.21.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Rutter G. A., Theler J. M., Li G., Wollheim C. B. Ca2+ stores in insulin-secreting cells: lack of effect of cADP ribose. Cell Calcium. 1994 Aug;16(2):71–80. doi: 10.1016/0143-4160(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Storch M. J., Petersen K. G., Licht T., Kerp L. Recognition of human insulin and proinsulin by monoclonal antibodies. Diabetes. 1985 Aug;34(8):808–811. doi: 10.2337/diab.34.8.808. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Yoo S. H., Albanesi J. P. Inositol 1,4,5-trisphosphate-triggered Ca2+ release from bovine adrenal medullary secretory vesicles. J Biol Chem. 1990 Aug 15;265(23):13446–13448. [PubMed] [Google Scholar]