Abstract
Rationale
Myosin light chain (MLC) phosphorylation determines vascular contractile status. In addition to the classic Ca2+-dependent MLC kinase (MLCK), another unidentified kinase(s) also contributes to MLC phosphorylation in living cells. Inhibitor κB kinase 2 (IKK2) deficient mouse embryonic fibroblasts (MEFs) demonstrate abnormal morphology and migration, suggesting that IKK2 may be involved in MLC phosphorylation.
Objective
Therefore, we tested if IKK2 is a MLCK in living cells and the role of IKK2 in mediating vasoconstriction and blood pressure regulation.
Methods and Results
In the present study, we showed that recombinant IKK2 phosphorylated MLC and intact myosin in vitro, and the kinetic parameters were comparable to those of the classic MLCK. Over-expression of IKK2 increased cellular MLC phosphorylation level, and pharmacological inhibition of IKK2 markedly decreased vascular smooth muscle cell (VSMC) MLC phosphorylation, suggesting that IKK2 is a MLCK in living cells. IKK2 inhibitors dose- and time-dependently attenuated vasoconstriction elicited by diverse agonists, suggesting the physiological importance of IKK2 as a MLCK. VSMC-specific IKK2 deficient mice had decreased aortic contractile responses, and reduced hypertensive responses to several vasoconstrictors, compared to wild-type mice, confirming the physiological importance of IKK2 as a MLCK.
Conclusion
Our data provide a novel mechanism whereby IKK2 regulates MLC phosphorylation as a MLCK and thus vascular function and blood pressure.
Keywords: IKK2, MLC, blood pressure, hypertension, vascular smooth muscle, blood vessels, myosin, phosphorylation
INTRODUCTION
Initiation of vascular smooth muscle contraction is primarily regulated at the level of myosin light chain (MLC) Ser19 phosphorylation, which is essential for interaction of actin and myosin and activation of actin-activated myosin ATPase1. The relative activity of MLC kinases (MLCKs) versus MLC phosphatase (MLCP) ultimately determines the phosphorylation level of MLC Ser19, and thus vascular contractile responses.
Previous studies have demonstrated that, in addition to the classic Ca2+-dependent MLCK2, another unidentified kinase(s) also contributes to MLC phosphorylation in living cells. For example, basal MLC phosphorylation in vascular smooth muscle cells (VSMCs) is resistant to MLCK inhibitors ML-73, 4 and ML-95. MLCP inhibition with calyculin A or microcystin-LR induces potent vasoconstriction; these contractile responses are also resistant to MLCK inhibitors6–8, indicating that a kinase(s) other than the classic MLCK is primarily responsible for MLC phosphorylation. The most direct evidence comes from studies in MLCK deficient mice, showing that blood vessels from these mice remain responsive to elevated [Ca2+]i, strongly suggesting that a kinase(s) other than MLCK phosphorylate MLC in VSMCs 9.
Thus far, several kinases other than MLCK have been shown to phosphorylate MLC Ser19 in vitro, including ROCK10, ILK11, PAK12, and ZIPK13. However, it remains controversial whether these kinases phosphorylate MLC in living cells. The Rho-Kinase inhibitor Y-27632 has no effect on calyculin A-induced vasoconstriction and MLC phosphorylation14, suggesting that Rho-Kinase may not be an important MLC kinase in VSMCs. Two potent ZIPK inhibitors, AV25 and SM1, do not relax MLCP inhibition-induced vasoconstriction15, ruling out the possibility that ZIPK phosphorylates MLC in this context. PAK1, one of the most intensively studied PAKs16, contributes to MLC phosphorylation in neurons17, breast carcinoma cells18, Hela cells19, and pulmonary endothelial cells20. However, dominant-negative PAK or shRNA induces myoepithelial cell contraction, in contrast to the expected relaxation21. In guinea pig VSMCs, PAK1 also induces relaxation through phosphorylation and subsequent inhibition of MLCK22, raising doubt on the role of PAKs’ MLCK activity in VSMCs. ILK activity in permeable esophagus smooth muscle cells is increased in response to MLCP inhibition by okadaic acid, and ILK antibody inhibits okadaic acid-induced cell shorting, indicating that ILK may be an important intracellular MLC kinase. However, except for results that ILK phosphorylates MLC in cell free phosphorylation reaction, evidence that ILK is a MLC kinase in VSMCs primarily comes from studies that AV25 and SM1 do not inhibit ILK15. Therefore, the nature of unidentified MLC kinase(s) in VSMCs remains to be determined.
IKK2 was initially identified as a kinase subunit of the IKK complex.23, 24 Its essential role in IκB protein phosphorylation and subsequent NF-κB activation has been well established.25, 26 In addition to IκB proteins, IKK2 phosphorylates other substrates such as insulin substrate 1,27 14-3-3β,28 BCL10,29 FOXO3a,30 and SNAP-23,31 demonstrating its extensive role in cellular responses. The importance of IKK2 in cell function is further illustrated by the fact that IKK2 deficient mice are embryonically lethal due to liver degeneration.26 Notably, IKK2−/− mouse embryonic fibroblasts (MEFs) are abnormal in migration and morphology32, two MLC phosphorylation-dependent cellular responses. Therefore, we tested the hypothesis that IKK2 regulates MLC phosphorylation in VSMCs and that IKK2 is important in mediating vascular contractile responses to several agonists as well as in blood pressure regulation.
METHODS
An expanded Methods section can be found in the online supplement.
Generation of smooth muscle-specific IKK2 deficient mice
IKK2flox/flox mice were generated as previously described33. SM22-Cre+/+ mice were purchased from the Jackson Laboratory. SM22-Cre+/− IKK2flox/flox (IKK2smKo) mice were generated by crossing SM22-Cre+/− IKK2flox/flox and IKK2flox/flox, and the IKK2flox/flox littermates were used as wild-type (WT) control. In preliminary studies we found that IKK2smKo mice had dermatitis. To prevent dermatitis in IKK2smKo mice, we crossed these mice with TNFR1−/− mice obtained from the Jackson Laboratory. Littermate TNFR1−/− SM22-Cre−/− IKK2flox/flox control mice (hereafter presented as TNFR1−/− mice) and TNFR1−/− IKK2smKo mice were generated by crossing TNFR1−/− SM22-Cre+/− IKK2flox/flox and TNFR1−/− SM22-Cre−/− IKK2flox/flox.
Blood pressure measurements
The 20-week-old IKK2smKo mice (n = 7) and littermate control mice (n = 5) were implanted with radiotelemeter probes as previously described34 (model TA11PA-C10; Data Sciences International). Ten days after surgery, we began monitoring 24-hour mean arterial pressure (MAP) and heart rate (HR) for 5 consecutive days.
We also measured blood pressure in younger mice by the tail cuff method. IKK2smKo mice (n = 16) and littermate WT control mice (n = 16) were trained beginning at the age of 6 weeks for two weeks (daily, 5 day/week), and then systolic blood pressure was recorded beginning at the age of 8 weeks for two weeks (daily, 5 days/week). The average systolic blood pressure during this period was recorded.
To measure 24 hour mean arterial blood pressure in TNFR1−/− IKK2smKo, TNFR1−/− IKK2smKo and control littermate mice (n=6/group) were implanted with radiotelemeter probes as previously described34. After 8 days of recovery, mean arterial pressure and heart rate were recorded for 4 consecutive days. For analyzing the acute effects of angiotensin II (0.3 µg/kg), phenylephrine (10 µg/kg), thromboxane (30 µg/kg) and sodium nitroprusside (32 µg/kg), those mice were also implanted with catheter inserted into the jugular vein for drug infusion. After a 30-min stabilizing period, bolus injections of vasoactive drugs were administered at 30 min intervals.
Cell culture
A human aortic vascular smooth muscle cell line (HVSMCs, ATCC#: CRL-1999) was purchased from ATCC. The cells were maintained in DMEM supplemented with 10% fetal bovine serum. Treatments were applied to cells approximately 90% confluent in 60 mm dishes.
To measure MLC phosphorylation, cells were serum-starved for two hours and then treated with the indicated compounds followed by washing with ice-cold PBS once. The cell lysates were then prepared with RIBA buffer supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Pierce) on ice. The MLC phosphorylation levels were determined by western blot analysis with mouse anti-Phospho-Myosin Light Chain 2 (Ser19, Cell Signaling Technology) and mouse anti-MLC (Sigma).
Transfection
Lipofectoamine™ 2000 transfection reagent was used to perform transfection with plasmids, according to manufacturer’s instruction. Briefly, HVSMCs (106 cells/ 60 mm dish) were seeded one day before transfection. Transfection complex was prepared at DNA (µg): Lipofectamine™ 2000 (µl) ratio of 1:1.
Western blotting
Western blotting was performed using standard techniques as previously reported35 with primary antibodies as follows: mouse anti-Phospho-Myosin Light Chain 2 (Ser19, Cell Signaling Technology), mouse anti-MLC (Sigma), rabbit anti-IkBα (Cell Signaling Technology), monoclonal anti-β-actin (Sigma), rabbit anti-IKK2 (Sigma), rabbit anti-IKK1 (Sigma), mouse anti-phospho-IkBα (Ser32/36, Cell Signaling Technology), mouse anti-MYPT1 (BD Transduction Laboratories), and rabbit anti-phospho-MYPT1 (Thr696, Upstate). Signals were detected by chemiluminescence and analyzed by densitometry.
Vascular reactivity
Reactivity of rat and mouse aortic rings to several vasoactive agonists was assessed as previously described35. IkB Kinase Inhibitor Peptide, NF-kB Activation Inhibitor II (JSH-23), SC-514 was purchased from EMD Chemicals. All other materials were purchased from Sigma. The experimental protocols for this study were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center and were carried out according to both the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and the guidelines of the Animal Welfare Act. All the mice were housed 2–3 animals per cage at 22 °C (12-h light-dark cycle) with free access to food and water.
Sprague Dawley rats (16 weeks old, Taconic) or mice (8–18 weeks) were anesthetized with pentobarbital sodium, and the thoracic aorta was quickly removed and cleaned in physiological salt solution (PSS), containing (mM): NaCl, 130; NaHCO3, 14.9; KCl, 4.7; KH2PO4, 1.18; MgSO4•7H2O 1.18; CaCl2•2H2O, 1.56; EDTA, 0.026; glucose 5.5. The aorta was cut into 2-mm rings, and the endothelium was mechanically removed by gently rubbing the intimal surface with a stainless steel wire. The aortic rings were then mounted in a muscle bath containing PSS at 37°C and bubbled with 95% O2-5% CO2. Isometric force generation was recorded with either a Multi Myograph System (Danish Myo Technology A/S) or organ chamber.36 A resting tension of 30 mN (rats) or 4 mN (mice) was imposed on each ring, and the rings were allowed to equilibrate for 1 hour. Arterial integrity was assessed first by stimulation of vessels with 80 mM KCl. Endothelium-integrity was assessed by measuring the dilatory response to ACh (10 µM) in PE-contracted vessels (3 µM). The failure of ACh to relax denuded aortic rings was considered proof of endothelium disruption.
To analyze the responses of mesenteric arteries, male WT and IKK2smKo mice (8–10 weeks old) were killed with an overdose of sodium pentobarbital (120 mg/kg i.p.). The mesentery was rapidly removed and placed in ice-cold gassed (95% O2/5% CO2) PSS. The second-order mesenteric arteries were dissected free of adipose and connective tissue and immediately were mounted in a wire myograph (Danish MyoTechnology, Aarhus, Denmark). After an initial 30-min equilibration, vessel wall tension and diameter were normalized in a standardized procedure and stabilized for 1 h. Wire myography was used to assess the KCl-, PE- or U-46619-induced contractile responses of arteries.
In vitro phosphorylation
The assays were established based on HTScan kinase assay kit (Cell signaling technology). 100 ng IKK2 in the kit or other kinases (MLCK and Zip kinase from Upstate and ROCK1 from Abcam) were incubated with 0.75 µM MLC (Calbiochem) or 0.75 µM IκBα (Biomol) at 37°C in a total of 50 µl buffer containing 60 mM HEPES-NaOH (pH 7.5), 3 mM MgCl2, 3 mM MnCl2, 3 µM Na-orthovanadate, 1.2 mM DTT, 10 µM ATP. MLCK phosphorylated MLC in total of 50 µl buffer containing 60 mM HEPES-NaOH (pH 7.5), 3 mM MgCl2, 3 mM MnCl2, 3 µM Na-orthovanadate, 1.2 mM DTT, 10 µM ATP, 0.1 mM CaCl2, and 1 µM calmodulin. Reaction was stopped by adding 50 µl SDS-PAGE sample buffer and boiling for 5 minutes.
Kinetic analyses
Assays were performed by ProQinase. Recombinant, insect cell expressed IKK2 protein was used as full-length, GST-tagged fusion protein (ProQinase #0258-00001 LOT008). Substrate peptide MLC-tide (KKRPQRATSNVFS-amide) was supplied by Penninsula Laboratories. Radiometric Filter Binding Assay was used to determine kinetics. Recombinant IKK2 (50ng/well) was incubated with its substrate MLC-tide (various amounts) and co-substrate ATP (various amounts) containing radioactive 33Pγ- ATP as a tracer in a constant ratio. Phosphorylated peptide were bound to MSPH-Filter plates (Millipore #MSPHN0B10), washed, and bound radioactivity was detected by liquid scintillation counting. In detail, assay components were added to a final volume of 50 µl in a 96 well PP V-bottom plate in assay mix (70 mM HEPES pH7.5; 3 mM MgCl2; 3 mM MnCl2; 3 µM Naorthovanadate; 1,2 mM DTT; 50 µg/ml PEG20,000; ATP and MLC-tide in various amounts; IKK-beta 50 ng/well) in the following order: Assay buffer, MLC-tide, IKK2 and ATP. The assay was mixed and incubated for 20 min at 30°C. The reaction was stopped by addition of 20 µl 10% H3PO4. The MSPH filter plate was washed with 20 µl 100% ethanol followed by 200 µl 150 mM H3PO4, the reaction mixture was transferred to the filter plate and incubated for 30 min at room temperature. The mixture was passed through the filter by application of vacuum, washed 3 times with 200 µl 150 mM H3PO4 and once with 20 µl 100% ethanol. After drying the plate, 50 µl/well of liquid scintillator were added and bound radioactivity determined by measurement in a liquid scintillation counter.
Statistical analysis
Probability values for a type 1 error (α error) of less than 0.05 were considered significant. Student’s t test or ANOVA were used, as appropriate, for statistical analyses with Graphpad Instat 5 software (Graphpad Instat Software, San Diego, CA).
RESULTS
IKK2 phosphorylates MLC in vitro
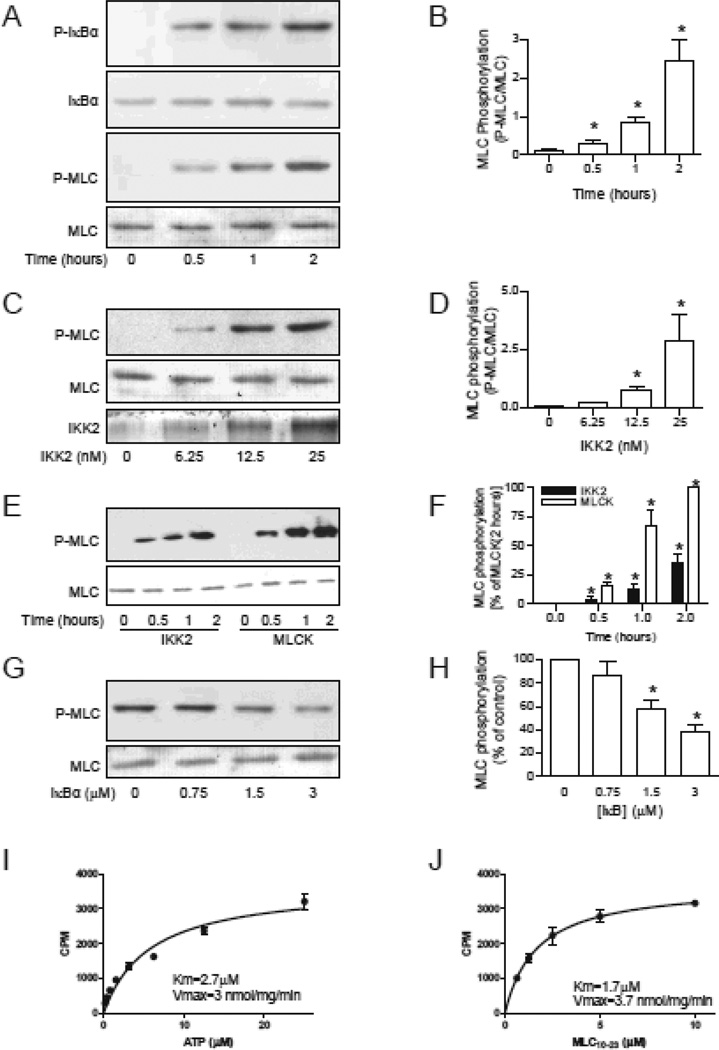
To investigate if IKK2 phosphorylates MLC in vitro, we performed in vitro phosphorylation reactions with recombinant IKK2 and MLC and analyzed MLC Ser19 phosphorylation by western blotting. Consistent with our hypothesis, IKK2 time- and dose-dependently increased MLC phosphorylation at a rate comparable to IκBα phosphorylation (Fig. 1A-D). The velocity of MLC phosphorylation by IKK2 was about 36% of that by the classic MLCK (Fig. 1E and F), the best established MLC kinase in vivo. The maximal MLC phosphorylation by IKK2 was similar to that by the classic MLCK (Online Figure I). To rule out the possibility that recombinant IKK2 was contaminated by the classic MLCK, we assessed the effect of ML-9, a MLCK inhibitor, and EGTA, a Ca2+ chelator that inhibits the classic MLCK activity, on the in vitro phosphorylation reaction. Online Fig. II demonstrates that neither ML-9 nor EGTA inhibited IKK2-induced MLC phosphorylation. Online Fig. III reveals that IKK2 also phosphorylated intact myosin, further supporting that the MLCK activity of IKK2 may be physiologically significant.
Figure 1. IKK2 phosphorylates MLC in vitro.
A and B, MLC (0.75 µM) or IκBα (0.75 µM) was incubated with IKK2 (25 nM) in a total of 50 µl reaction buffer at 37°C for the indicated time. MLC or IκBα was visualized by ponseau s solution, and the phosphorylated MLC or IκBα was analyzed by western blotting. n=5. *P<0.05 vs. 0; One way ANOVA. C and D, the indicated amount of IKK2 were incubated with MLC (0.75 µM) at 37°C for one hour. n=3. *P<0.05 vs. 0; One way ANOVA. E and F, MLC (0.75 µM) were phosphorylated by IKK2 (25 nM) or MLCK (25 nM) for the indicated time. n=3. *P<0.05 vs. 0; # P<0.05 v.s. MLCK; Two way ANOVA. G and H, MLC (0.75 µM) and IKK2 (25 nM) were incubated at 37°C for one hour in the presence of IKK2 peptide substrate (DSGLDSM). n=3. *P<0.05 vs. 0; One way ANOVA. I and J, kinetic analysis was performed with varying concentrations of ATP (I) or substrate MLC10–23 peptide (J).
Further supporting the in vitro MLCK activity of IKK2, Online Fig. IV and V show that SC-514, an IKK2 inhibitor37, decreased MLC phosphorylation by IKK2 but not other MLCKs, including classic MLCK, Zip-kinase, and ROCK1. More importantly, the reaction was also inhibited by IκBα-tide, an IKK2 substrate peptide derived from IκBα (Fig. 1G and H), and the calculated KMLC was comparable to KIκBα (Online Table I). Notably, although IKK1 and IKK2 share the IKK activity, IKK1 did not phosphorylate MLC in vitro (Online Fig. VII).
To determine if the MLCK activity of IKK2 is biologically relevant, we performed kinetic analyses. Fig. 1I and J reveal that the kinetics of MLC phosphorylation by IKK2 were similar to those of IκBα phosphorylation (Vmax: 3.7 and 2 µmol/mg/min; KATP: 2.7 and 0.5 µM; Kpeptide: 1.7 and 1.4 µM; MLC and IκBα, respectively), indicating that the MLCK activity of IKK2 may be biologically important.
IKK2 phosphorylates MLC in living cells
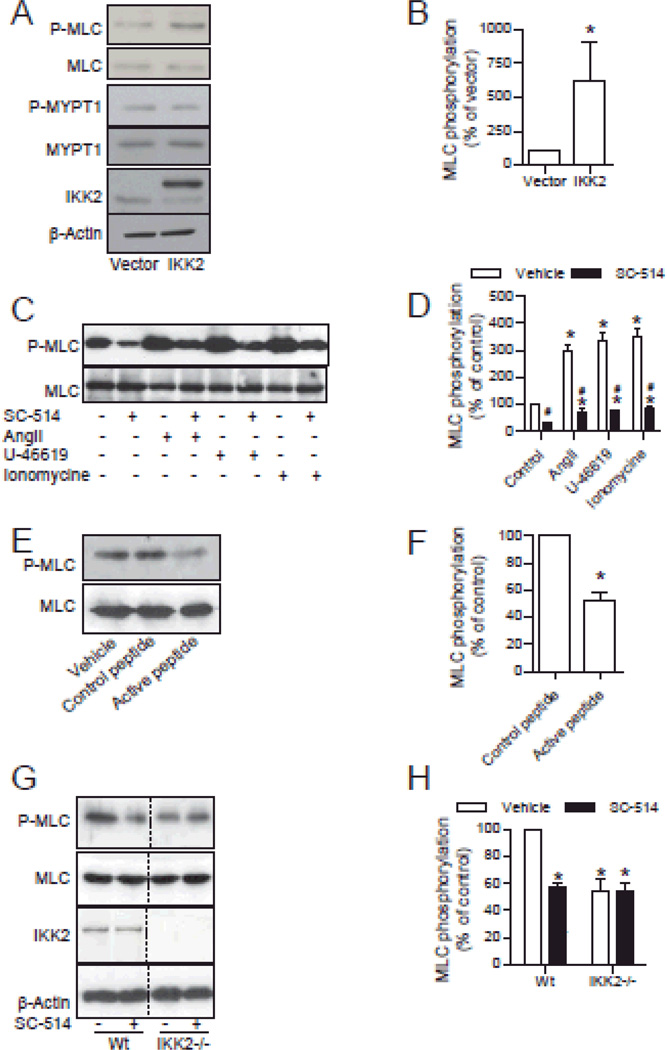
To examine if IKK2 is a MLCK in living VSMCs, we over-expressed IKK2 in human vascular smooth muscle cells (HVSMCs). Consistent with our in vitro results, IKK2 over-expression significantly increased the phosphorylation level of MLC Ser19 but not the regulatory subunit of MLC phosphatase (MLCP) MYPT1 Thr696 (Fig. 2A and B).
Figure 2. IKK2 phosphorylates MLC in HVSMCs.
A and B, HVSMCs were transfected with flag-tagged IKK2 or vector. MLC phosphorylation and flag-tagged IKK2 expression were analyzed by western blotting. n=3. *P<0.05 vs. vector; student t-test. C and D, after 15 minutes of incubation with SC-514 (30 µM) or vehicle, HVSMCs were treated with the indicated agonist for 3 minutes (angiotensin II, 0.1 µM) or 20 minutes (U-46619, 1 µM and ionomycin, 1 µM), MLC phosphorylation was analyzed by western blotting. n=3. *P<0.05 vs. control; # P<0.05 vs. vehicle; one way ANOVA. E and F, HVSMCs were treated with inhibitory peptide (A 14-amino acid peptide corresponding to the active IκB phosphorylation recognition sequence fused to the hydrophobic region of the fibroblast growth factor signal peptide to aid in cellular delivery) or control peptide [A 14-amino acid peptide corresponding to the mutated recognition sequence of IκB (Ser32→Ala and Ser36→Ala) fused to the hydrophobic region of the fibroblast growth factor signal peptide to aid in cellular delivery] for 30 minutes, MLC phosphorylation was then analyzed by western blotting. n=3. *P<0.05 vs. control; student t-test. G and H, wild-type and IKK2−/− MEFs were incubated with SC-514 (30 µM) for 15 minutes, MLC phosphorylation and IKK2 expression in wild-type and IKK2−/− MEFs was analyzed by western blotting. n=3. *P<0.05 vs. control; student's t-test.
We next analyzed if endogenous IKK2 is a MLCK in living cells. Fig. 2C and D and Online Fig. VIII show that SC-514 treatment markedly attenuated the basal MLC phosphorylation and MLC phosphorylation in response to angiotensin II, U-46619 (a thromboxane mimetic), or ionomycin in HVSMCs. Notably, if compared to the basal level, those agonists markedly increased MLC phosphorylation even in the presence of SC-514 (Fig. 2C and D), suggesting that IKK2 mainly contributes to the basal but not agonist-induced MLC phosphorylation. We also tested the MLC phosphorylation effect of a cell-permeant IKK2 inhibitory peptide.38, 39 Consistent with the SC-514 results, the IKK2 peptide inhibitor markedly decreased the basal MLC phosphorylation in HVSMCs (Fig. 2E and F).
To exclude the possibility that IKK2 inhibitors decrease MLC phosphorylation through non-specific actions, we analyzed the MLC phosphorylation effect of SC-514 in IKK2 deficient MEFs32. Fig. 2G and H show that SC-514 markedly decreased MLC phosphorylation levels in wild-type but not IKK2−/− MEFs, strongly supporting that SC-514 decreases MLC phosphorylation via targeting IKK2. Notably, consistent with its proposed role in MLC phosphorylation, IKK2−/− MEFs had lower basal MLC phosphorylation level (Fig. 2G and H).
IKK2 is implicated in vasoconstriction
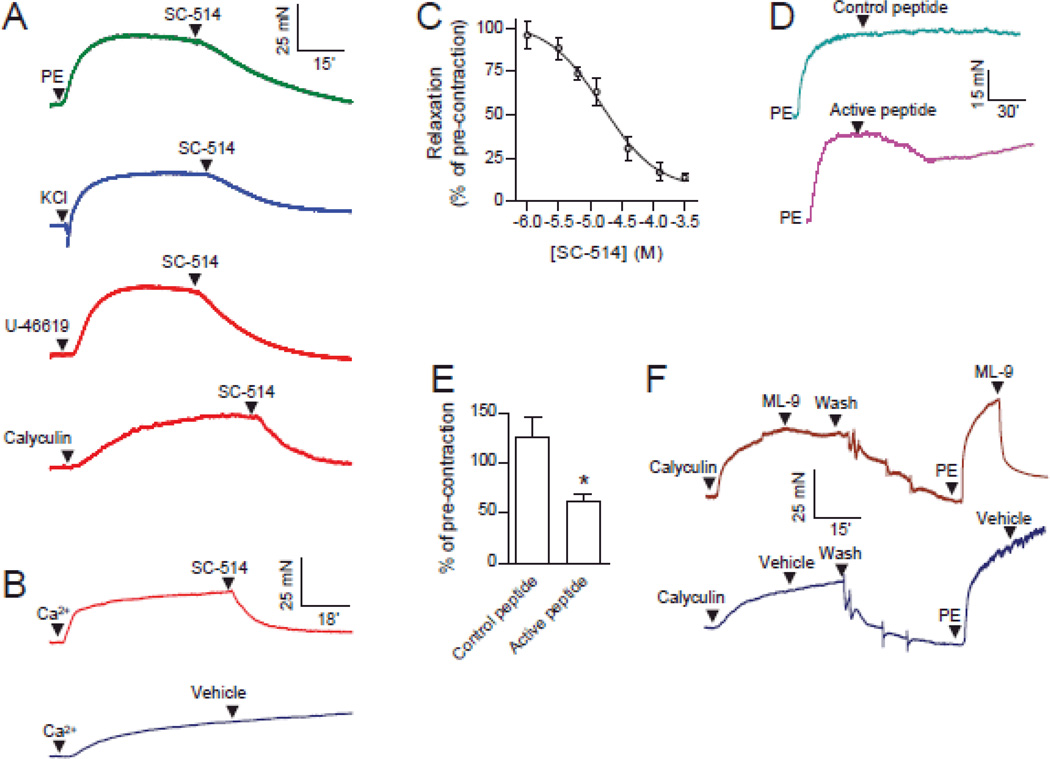
To assess the physiological importance of the MLCK activity of IKK2, we analyzed the role of IKK2 in vasoconstriction, a MLC phosphorylation-dependent physiological process. Fig. 3A shows that SC-514 caused relaxation in endothelium denuded rat aortic rings pre-contracted with phenylephrine, KCl, U-46619 (a thromboxane mimetic), or calyculin A (MLCP inhibitor). To establish a high level of intracellular Ca2+ we treated endothelium-denuded aortic rings with the Ca2+ ionophore, ionomycin. This contraction was markedly relaxed by SC-514 (Fig. 3B), suggesting that SC-514 does not cause relaxation by lowering intracellular Ca2+. We next used SC-514 to further document the role of IKK2 in vasoconstriction. Fig. 3C reveals that SC-514 relaxed phenylephrine-induced contraction with a half maximal inhibitory concentration (IC50) comparable to that of inhibiting IKK2 in vitro.37, 40 Consistent with the effect of SC-514, IKK2 inhibitory peptide but not control peptide significantly relaxed phenylephrine-induced contraction (Fig. 3D and E), confirming the involvement of IKK2 in vasoconstriction. In contrast, a classic MLCK inhibitor ML-9 potently relaxed PE-induced endothelium denuded rat aortic ring contraction, but had little effect on calyculin A-induced contraction (11±7% of pre-contraction, n = 4, Fig. 3F), suggesting that SC-514 did not primarily target the classic MLCK for its vasodilator actions at least in calyculin A-induced contraction. However, we cannot completely exclude the possibility that SC-514 relaxed PE-induced contraction through inhibition of MLCK.
Figure 3. IKK2 contributes to diverse vascular contractile responses.
A, endothelium-denuded rat aortic rings were contracted by the indicated vasoconstrictors (1 µM PE, phenylephrine; 120 mM KCl, potassium chloride; 1 µM U-46619, a thromboxane A2 agonist; 1 µM calyculin A, a MLCP inhibitor), and then 30 µM SC-514 was added. A representative recording from at least three independent experiments is presented. B, endothelium denuded rat aortic rings were treated with ionomycin (1.5 µM) in calcium-free PSS for 30 minutes, and contracted with CaCl2 (0.4 mM), and the IKK2 inhibitor SC-514 (100 µM) or vehicle was added. A representative result from four independent experiments is presented. C, endothelium denuded rat aortic rings were contracted by PE (1µM), and the IKK2 inhibitor SC-514 was added in an accumulative manner. Results were expressed as percentage of pre-contraction. D and E, phenylephrine (PE, 1 µM) pre-contracted endothelium denuded rat aortic rings were treated with IKK2 inhibitory or control peptide (the same peptides as in Fig. 2E and F. 50 µg/ml). The representative recordings (C) and the quantification data (D) are presented. n=3. *P<0.05 vs. control; student t-test. F, endothelium denuded rat aortic rings were contracted with calyculin A, followed by ML-9 (100µM) was added. After washing, the rings were contracted with PE again and followed by ML-9 (100µM). A representative result from three independent experiments is presented.
IKK2 inhibition induces apoptosis in diverse cell types.41 However, the vasodilator action of IKK2 inhibitors is unlikely due to decreased viability of smooth muscle cells, as the IKK2 inhibitory peptide-induced decrease in vasoconstriction recovered in hours (Fig. 3D), and the vascular effect of 30 µM SC-514 was completely reversible by washing (Online Fig. IX). In addition, although NF-κB is the best known downstream molecule from IKK2, it is not involved in the vasodilator action of IKK2 inhibitors, as the action was observed within seconds and JSH-23, an NF-κB antagonist that inhibits NF-κB binding to DNA but not IKK2 activation42, did not have a similar vasodilator action (Online Fig. X).
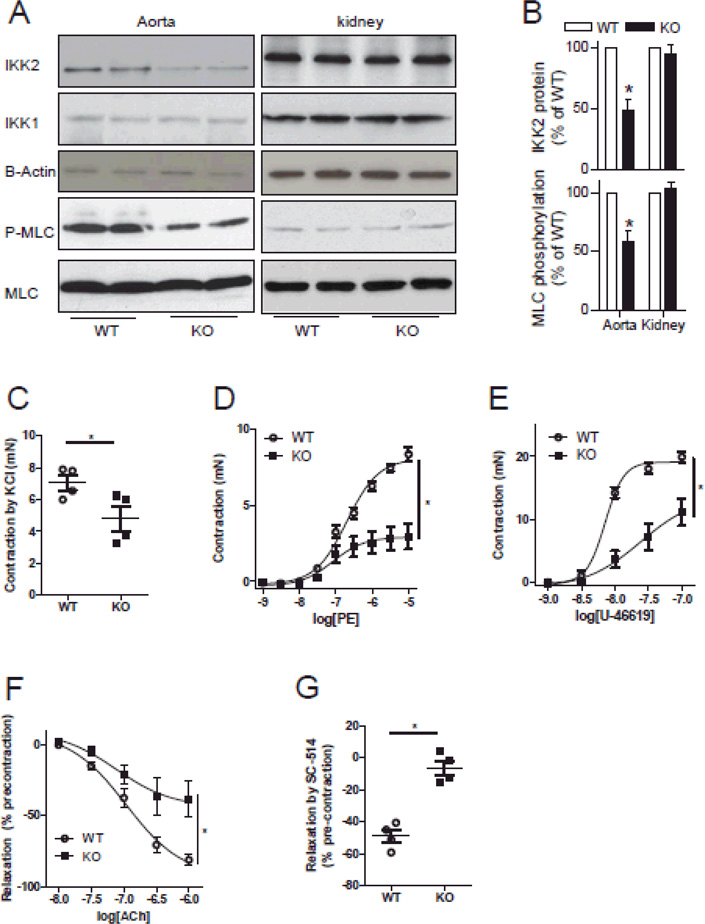
To confirm the role of IKK2 in vasoconstriction, we generated smooth muscle-specific IKK2 deficient mice (IKK2smKo). Western blot analysis demonstrated that IKK2smKo mice had a 58% decrease in aortic IKK2 protein expression (Fig. 4A and B). In agreement with the proposed role of IKK2 in vasoconstriction, aortic MLC phosphorylation in IKK2smKo mice was deceased by 41% (Fig. 4A and B). In contrast, IKK2smKo mice had comparable IKK2 expression and MLC phosphorylation in the kidney (Fig. 4A and B). Further supporting the importance of IKK2 in vasoconstriction, IKK2 deficiency significantly reduced aortic vasoconstrictor responses to KCl or phenylephrine (Online Fig. XIA and B), and SC-514 had markedly decreased vasodilator action in IKK2 deficient mouse aortic rings (Online Fig. XIC). Given the important role of resistant arteries in blood pressure regulation, we further documented the vascular contractile responses in mesenteric arteries. Fig. 4C-E revealed that IKK2 deficiency in vascular smooth muscle cells markedly reduced contraction of mesenteric arteries induced by KCl, phenylephrine, or U-46619. Notably, mesenteric arteries from IKK2smKo mice had significantly decreased relaxation responses to acetylcholine and SC-514 (Fig. F and G)
Figure 4. IKK2 deficiency reduces vascular contractile responses.
A and B, Thoracic aortas of IKK2smKo mice and their littermate WT controls were quickly isolated from animals, placed on ice and the peri-vascular adipose tissue was rapidly removed, and snap-frozen with liquid nitrogen. IKK2 expression and MLC phosphorylation in these aortas were analyzed by western blot. n=6/group. *P<0.05 vs. WT; student t-test. C-E, Contractile responses of mesenteric arterial rings from IKK2smKo and their littermate WT controls to KCl, PE, and U-46619. F and G, mesenteric arterial rings were contracted with PE (1µM) and then relaxed by acetylcholine or SC-514 (100 µM). n=4/group; *P<0.05 vs. WT; student t-test or two way ANOVA.
IKK2 deficiency decreases vascular contractile response and basal blood pressure
To investigate the physiological role of IKK2, we measured 24 hour mean arterial blood pressure of IKK2smKo mice using telemetry as previously described.34 Unexpectedly, no significant change in mean arterial blood pressure was observed (WT vs IKK2smKo: 99 ± 1 vs. 102 ± 6 mmHg; n=5 or 7/group; p = 24.6%, student’s t test). However, 20-week old IKK2smKo mice developed severe dermatitis (data not shown), which may have tended to increase blood pressure as a consequence of inflammation43, 44 and thus oppose the blood pressure lowering effect of smooth muscle-specific IKK2 deficiency. Supporting this concept, we observed lower systolic blood pressure in 8–10 week old IKK2smKo mice (WT vs IKK2smKo: 105 ± 9 vs 98 ± 10 mmHg by the tail cuff method; n=16/group; P<0.05, student’s t test).
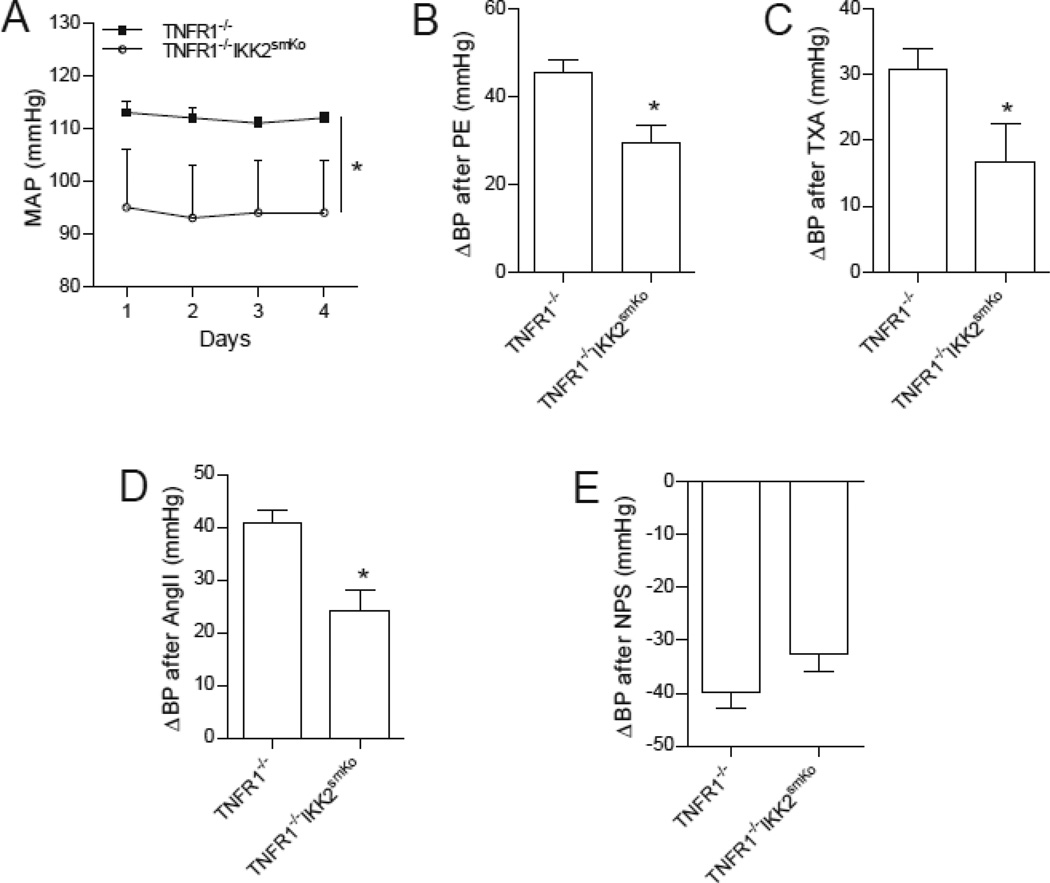
IKK2 deficiency-induced dermatitis is TNFR1-dependent.45 To further test the role of IKK2 in blood pressure regulation, we generated a mouse model with whole body TNFR1 deficiency and smooth muscle-specific IKK2 deficiency (TNFR1−/− IKK2smKo). As expected, these mice did not develop dermatitis. Supporting the important role of IKK2 in blood pressure regulation, TNFR1−/− IKK2smKo mice had lower 24 hour mean blood pressure as measured by telemetry (Fig. 5A). To verify that IKK2 regulates blood pressure through regulating MLC phosphorylation, we assessed the acute blood pressure responses to three vasoconstrictors (thromboxane, phenylephrine, and angiotensin II) each with a different mechanism of action. Fig. 5B-D demonstrates that TNFR1−/− IKK2smKo mice had markedly reduced hypertensive responses to those vasoconstrictors. In contrast, the vasodilator sodium nitroprusside, a nitric oxide donor, produced similar decreases in blood pressure in TNFR1−/− and TNFR1−/− IKK2smKo mice (Fig. 5E).
Figure 5. IKK2 deficiency reduces vascular contractile responses.
A, average mean arterial pressures, measured 24 hrs/day for 4 days by telemetry, in TNFR1−/− IKK2smKo (n=8) and their littermate TNFR1−/− controls (n=6).*P<0.05 vs. TNFR1−/−; two way ANOVA. B-E, acute increases in mean arterial pressure (ΔMAP) of TNFR1−/− IKK2smKo and their littermate TNFR1−/− controls in response to phenylephrine (PE, 10 µg/kg, B), thromboxane A2 agonist (TXA, 30 µg/kg, C), angiotensin II (AngII, 0.3 µg/kg, D), and nitroprusside sodium (NPS, 32 µg/kg, E). n=6/group. *P<0.05 v.s. TNFR1−/−; student t-test.
DISCUSSION
In the present study, we demonstrate that IKK2 is an important MLCK in VSMCs, as evidenced by the following results: 1) IKK2 phosphorylates MLC and intact myosin in vitro with kinetics comparable to that by the classic MLCK; 2) over-expression of IKK2 increases cellular MLC phosphorylation level while inhibition of IKK2 reduces cellular MLC phosphorylation level; 3) the effects of IKK2 inhibitors on vascular MLC phosphorylation and contractile responses are not mediated by the classic MLCK and MLCP; 4) VSMC-specific IKK2 deficiency decreases aortic MLC phosphorylation and contractile responses to several agonists, lowers basal blood pressure, and reduces the hypertensive responses to vasoconstrictors.
Previous studies have provided compelling evidence for the existence of a MLC kinase(s) other than the classic MLCK in living cells.3–5 In addition to the classic MLCK, several kinases, including Rho-Kinase10, ILK11, PAK12, and ZIPK13, can phosphorylate MLC in vitro. However, except for ILK, the MLCK activity of those kinases has been shown not to be of physiological significance.14, 15, 21 To our knowledge, this is the first study demonstrating that a kinase other than the classic MLCK regulates VSMC MLC phosphorylation as a MLCK, contributes to vascular contractile responses, and is important in blood pressure regulation.
Previous studies have shown that the basal MLC phosphorylation is independent of the classic MLCK, as its inhibitors have no effect on the basal MLC phosphorylation.3–5 However, the factors that contribute to basal MLC phosphorylation in VSMCs have not been previously elucidated. Our data demonstrate that IKK2 contributes importantly to basal MLC phosphorylation in VSMCs. Given that various vasoconstrictor agonists induce further MLC phosphorylation, in excess of basal MLC phosphorylation, IKK2 may be implicated in diverse vascular contractile responses. This conclusion is consistent with our results demonstrating that IKK2 inhibition markedly reduced basal MLC phosphorylation and vasoconstrictor responses to all tested agonists. However, the mechanisms whereby IKK2 regulates cellular MLC phosphorylation in different physiological and pathophysiological conditions remain to be determined.
Although our results indicate that IKK2 plays a key role in basal MLC phosphorylation, its role in stimulating further MLC phosphorylation in response to vasoconstrictors is less clear. We found that vasoconstrictors increased MLC phosphorylation even in the presence of an IKK2 inhibitor, suggesting that IKK2 may not be essential for agonist-induced MLC phosphorylation. In addition, since IKK2 inhibition relaxed all tested contractions (agonist-induced MLC phosphorylation can be through diverse pathways and thus less likely to be inhibited by a single inhibitor, whereas basal MLC phosphorylation definitely contributes to all contractions), it is more likely that the contribution of IKK2 to agonist-induced MLC phosphorylation may be trivial. However, this remains to be determined. Unfortunately, the methods commonly used to determine the phosphorylation level of cellular MLC, such as the one used in the present study and urea-glycerol gel, are only semi-quantitative. It may be necessary to assess the contribution of IKK2 to agonist-induced MLC phosphorylation with more quantitative methods, such as isotope tracers, in the future. Our finding that pharmacological blockade of IKK2 or VSMC-specific genetic deficiency of IKK2 markedly attenuates vasoconstrictor responses to several agonists with different modes of action and reduces blood pressure suggests that IKK2 may be important in agonist-induced as well as basal MLC phosphorylation in VSMCs.
Another interesting aspect of IKK2 as a MLCK is that in contrast to the Ca2+-dependent activation of the classic MLCK, the MLCK activity of IKK2 appears to be Ca2+-independent, as the Ca2+ chelator EGTA does not have an inhibitory effect on the IKK2-induced MLC phosphorylation in vitro. This is consistent with our results demonstrating that IKK2 plays an important role in cellular basal MLC phosphorylation. Notably, although the MLCK activity of IKK2 is Ca2+-independent, it could theoretically enable Ca2+-dependent MLC phosphorylation and contraction in the absence of classic MLCK. The MLCK activity of IKK2 is antagonized by the active MLCP in the absence of agonists, which maintains MLC phosphorylation at a low level. As agonists increase [Ca2+]i and subsequently inhibit the antagonizing MLCP through recently identified Ca2+-dependent signaling pathways46, IKK2 then can increase MLC phosphorylation through its MLCK activity and consequently induce contraction.
Therefore, it will be particularly interesting to determine if the vasoconstriction of MLCK deficient mice is dependent on the MLCK activity of IKK2 in future studies.
Consistent with the proposed role of IKK2 in the regulation of MLC phosphorylation, our data also show that VSMC-specific IKK2 deficiency on the genetic background of TNFR1 deficiency results in a significant reduction in blood pressure. Furthermore, mice with VSMC-specific IKK2 deficiency had reduced hypertensive responses to all vasoconstrictor agonists tested. These observations suggest that IKK2 may be a potential target for anti-hypertensive treatment. However, further studies are needed to assess the blood pressure lowering effect of IKK2 inhibitors in diverse hypertensive animal models.
In conclusion, our study reveals that IKK2 is an important MLCK in living cells and is critical in vascular smooth muscle contractile regulation both in vitro and in vivo. Our observations therefore provide a novel pathway for physiological regulation of vascular smooth muscle contraction that may be an important target for antihypertensive therapy.
Supplementary Material
Novelty and Significance.
What is Known?
Phosphorylation of the myosin light chain (MLC) plays a central role in regulating vascular smooth muscle contractile responses.
In vascular smooth muscle cells, MLC is phosphorylated by the classic MLC kinase (MLCK), although MLC could be phosphorylated by other, not yet identified, kinase(s) as well.
What New Information Does This Article Contribute?
MLC is phosphorylated by IKK2.
IKK2 is a vascular smooth muscle cell MLCK.
IKK2 regulates vascular tone and blood pressure through its MLCK activity.
MLC phosphorylation regulates diverse myosin II-dependent cellular responses, and the classic MLCK is the only kinase identified to date that can phosphorylate MLC in living cells. Here we report that IKK2 acts as an intracellular MLCK. These findings reveal a novel pathway for the physiological regulation of vascular smooth muscle mediated by IKK2. Although our studies were focused on vasoconstriction, a broader implication of our work is that IKK2 may also regulate other cellular responses such as vascular cell motility and morphology, independently of its effects on NF-κB. Because abnormal contractility of smooth muscle has been implicated in various diseases, such as hypertension and asthma these findings suggest that IKK2 may be a novel target for regulating smooth muscle contractility..
ACKNOWLEDGEMENTS
We thank Dr. Fei Chen (Health Effects Laboratory Division, National Institute for Occupational Safety and Health) for kindly providing us IKK2 deficient MEFs.
SOURCES OF FUNDING
This work was supported by a grant to J.E. Hall from the National Heart, Lung and Blood Institute (PO1 HL-51971). Zhekang Ying was also supported by a postdoctoral fellowship from the American Heart Association (11POST7640030) and the National Natural Science Foundation of China (Grant No. 81270342).
Nonstandard Abbreviations and Acronyms
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- IKK2
inhibitor κB kinase 2
- MEFs
mouse embryonic fibroblasts
- VSMC
vascular smooth muscle cell
- MLCP
myosin light chain phosphatase
- ROCK
Rho-associated protein kinase
- ILK
integrin linked kinase
- PAK
p21 activated kinase
- ZIPK
zipper-interacting protein kinase
- IκB
inhibitor-κB
- BCL10
B-cell lymphoma/leukemia 10
- FOXO3a
forkhead box O3
- SNAP-23
synaptosomal-associated protein 23
- EGTA
ethylene glycol tetraacetic aci
- HVSMCs
human vascular smooth muscle cells (HVSMCs)
- WT
wildtype
- TNFR1
tumor necrosis factor receptor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Hai CM, Murphy RA. Ca2+, crossbridge phosphorylation, and contraction. Annual review of physiology. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- 2.Hong F, Haldeman BD, Jackson D, Carter M, Baker JE, Cremo CR. Biochemistry of smooth muscle myosin light chain kinase. Archives of biochemistry and biophysics. 2011;510:135–146. doi: 10.1016/j.abb.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe H, Takahashi R, Zhang XX, Goto Y, Hayashi H, Ando J, Isshiki M, Seto M, Hidaka H, Niki I, Ohno R. An essential role of myosin light-chain kinase in the regulation of agonist- and fluid flow-stimulated ca2+ influx in endothelial cells. Faseb J. 1998;12:341–348. doi: 10.1096/fasebj.12.3.341. [DOI] [PubMed] [Google Scholar]
- 4.Baek I, Jeon SB, Kim J, Seok YM, Song MJ, Chae SC, Jun JE, Park WH, Kim IK. A role for rho-kinase in ca-independent contractions induced by phorbol-12, 13-dibutyrate. Clinical and experimental pharmacology & physiology. 2009;36:256–261. doi: 10.1111/j.1440-1681.2008.05045.x. [DOI] [PubMed] [Google Scholar]
- 5.Padro T, Pena E, Garcia-Arguinzonis M, Llorente-Cortes V, Badimon L. Low-density lipoproteins impair migration of human coronary vascular smooth muscle cells and induce changes in the proteomic profile of myosin light chain. Cardiovascular research. 2008;77:211–220. doi: 10.1093/cvr/cvm045. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara H, Ozaki H, Sato K, Hori M, Karaki H, Watabe S, Kato Y, Fusetani N, Hashimoto K, Uemura D, et al. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-a. The Journal of pharmacology and experimental therapeutics. 1989;250:388–396. [PubMed] [Google Scholar]
- 7.Suzuki A, Itoh T. Effects of calyculin a on tension and myosin phosphorylation in skinned smooth muscle of the rabbit mesenteric artery. British journal of pharmacology. 1993;109:703–712. doi: 10.1111/j.1476-5381.1993.tb13631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber LP, Van Lierop JE, Walsh MP. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. The Journal of physiology. 1999;516(Pt 3):805–824. doi: 10.1111/j.1469-7793.1999.0805u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somlyo AV, Wang H, Choudhury N, Khromov AS, Majesky M, Owens GK, Somlyo AP. Myosin light chain kinase knockout. Journal of muscle research and cell motility. 2004;25:241–242. doi: 10.1023/b:jure.0000038362.84697.c0. [DOI] [PubMed] [Google Scholar]
- 10.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by rho-associated kinase (rho-kinase) The Journal of biological chemistry. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of cpi-17 and mlc20 via preferential activation of the pi3k/ilk pathway. The Biochemical journal. 2006;396:193–200. doi: 10.1042/BJ20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin ii regulatory light chain by p21-activated kinase (gamma-pak) Journal of muscle research and cell motility. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Nomura F, Hoshino K, Copeland NG, Gilbert DJ, Jenkins NA, Akira S. Death-associated protein kinase 2 is a new calcium/calmodulin-dependent protein kinase that signals apoptosis through its catalytic activity. Oncogene. 1999;18:3471–3480. doi: 10.1038/sj.onc.1202701. [DOI] [PubMed] [Google Scholar]
- 14.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 15.Wilson DP, Sutherland C, Borman MA, Deng JT, Macdonald JA, Walsh MP. Integrin-linked kinase is responsible for ca2+-independent myosin diphosphorylation and contraction of vascular smooth muscle. The Biochemical journal. 2005;392:641–648. doi: 10.1042/BJ20051173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bokoch GM. Biology of the p21-activated kinases. Annual review of biochemistry. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 17.Rubio MD, Haroutunian V, Meador-Woodruff JH. Abnormalities of the duo/ras-related c3 botulinum toxin substrate 1/p21-activated kinase 1 pathway drive myosin light chain phosphorylation in frontal cortex in schizophrenia. Biological psychiatry. 2012;71:906–914. doi: 10.1016/j.biopsych.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coniglio SJ, Zavarella S, Symons MH. Pak1 and pak2 mediate tumor cell invasion through distinct signaling mechanisms. Molecular and cellular biology. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brzeska H, Szczepanowska J, Matsumura F, Korn ED. Rac-induced increase of phosphorylation of myosin regulatory light chain in hela cells. Cell motility and the cytoskeleton. 2004;58:186–199. doi: 10.1002/cm.20009. [DOI] [PubMed] [Google Scholar]
- 20.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. American journal of respiratory cell and molecular biology. 2002;26:453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 21.Raymond K, Cagnet S, Kreft M, Janssen H, Sonnenberg A, Glukhova MA. Control of mammary myoepithelial cell contractile function by alpha3beta1 integrin signalling. The EMBO journal. 2011;30:1896–1906. doi: 10.1038/emboj.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirth A, Schroeter M, Kock-Hauser C, Manser E, Chalovich JM, De Lanerolle P, Pfitzer G. Inhibition of contraction and myosin light chain phosphorylation in guinea-pig smooth muscle by p21-activated kinase 1. The Journal of physiology. 2003;549:489–500. doi: 10.1113/jphysiol.2002.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive ikappab kinase that activates the transcription factor nf-kappab. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 24.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. Ikk-1 and ikk-2: Cytokine-activated ikappab kinases essential for nf-kappab activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 25.Perkins ND. Integrating cell-signalling pathways with nf-kappab and ikk function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 26.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The ikkbeta subunit of ikappab kinase (ikk) is essential for nuclear factor kappab activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa b kinase complex. The Journal of biological chemistry. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 28.Gringhuis S, Garcia-Vallejo J, Van Dijk W. Convergent action of ikb kinase beta and protein kinase ctheta modulate mrna stability through phosphorylation of 14-3-3beta complexed with tristetraprolin. Mol Biol Cell. 2005;25:6454–6463. doi: 10.1128/MCB.25.15.6454-6463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegener E, Oeckinghaus A, Papadopoulou N, Lavitas L, Schmidt-Supprian M, Ferch U, Mak TW, Ruland J, Heissmeyer V, Krappmann D. Essential role for ikappab kinase beta in remodeling carma1-bcl10-malt1 complexes upon t cell activation. Mol Cell. 2006;23:13–23. doi: 10.1016/j.molcel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. Ikappab kinase promotes tumorigenesis through inhibition of forkhead foxo3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Verma IM. Phosphorylation of snap-23 by ikappab kinase 2 regulates mast cell degranulation. Cell. 2008;134:485–495. doi: 10.1016/j.cell.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen F, Lu Y, Castranova V, Li Z, Karin M. Loss of ikkbeta promotes migration and proliferation of mouse embryo fibroblast cells. The Journal of biological chemistry. 2006;281:37142–37149. doi: 10.1074/jbc.M603631200. [DOI] [PubMed] [Google Scholar]
- 33.Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. Ikkbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating tnfalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- 34.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ying Z, Jin L, Palmer T, Webb RC. Angiotensin ii up-regulates the leukemia-associated rho guanine nucleotide exchange factor (rhogef), a regulator of g protein signaling domain-containing rhogef, in vascular smooth muscle cells. Molecular pharmacology. 2006;69:932–940. doi: 10.1124/mol.105.017830. [DOI] [PubMed] [Google Scholar]
- 36.LaMarca BD, Chandler DL, Grubbs L, Bain J, McLemore GR, Jr, Granger JP, Ryan MJ. Role of sex steroids in modulating tumor necrosis factor alpha-induced changes in vascular function and blood pressure. American journal of hypertension. 2007;20:1216–1221. doi: 10.1016/j.amjhyper.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS. A selective ikk-2 inhibitor blocks nf-kappa b-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. The Journal of biological chemistry. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- 38.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human i kappa b-alpha on serines 32 and 36 controls i kappa b-alpha proteolysis and nf-kappa b activation in response to diverse stimuli. The EMBO journal. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaroop N, Chen F, Wang L, Dokka S, Toledo D, Rojanasakul Y. Inhibition of nuclear transcription factor-kappab by specific ikappab kinase peptide inhibitor. Pharmaceutical research. 2001;18:1631–1633. doi: 10.1023/a:1013051019098. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, Itai A, Matsuda H. A novel nf-kappab inhibitor, imd-0354, suppresses neoplastic of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105:2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]
- 41.Luo JL, Kamata H, Karin M. Ikk/nf-kappab signaling: Balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin HM, Kim MH, Kim BH, Jung SH, Kim YS, Park HJ, Hong JT, Min KR, Kim Y. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of nf-kappab without affecting ikappab degradation. FEBS Lett. 2004;571:50–54. doi: 10.1016/j.febslet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 43.Laffer CL, Elijovich F. Inflammation and therapy for hypertension. Current hypertension reports. 2010;12:233–242. doi: 10.1007/s11906-010-0125-3. [DOI] [PubMed] [Google Scholar]
- 44.Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, Kitas GD. Hypertension in rheumatoid arthritis. Rheumatology (Oxford, England) 2008;47:1286–1298. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 45.Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, Krieg T, Rajewsky K, Haase I. Tnf-mediated inflammatory skin disease in mice with epidermis-specific deletion of ikk2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 46.Ying Z, Giachini FR, Tostes RC, Webb RC. Pyk2/pdz-rhogef links ca2+ signaling to rhoa. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1657–1663. doi: 10.1161/ATVBAHA.109.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.