Abstract
Context
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a deficit in behavioral inhibition. Recent evidence also suggests a deficit in cortical inhibition via the GABA (γ-aminobutyric acid)–ergic system.
Objective
To investigate the GABAergic component of ADHD using magnetic resonance spectroscopy.
Design
Cross-sectional study.
Setting
Participants were recruited through local schools, local pediatric and other community clinics, and through advertisement in regional publications. Magnetic resonance spectroscopy was performed within the research institute.
Participants
Children (age range, 8–12 years) in a typically developing control group vs a group with ADHD were compared.
Main Outcome Measures
J-difference–edited magnetic resonance spectroscopy at 3 T was used to measure GABA concentration in a volume that included primary somatosensory and motor cortices.
Results
GABA concentration is reduced in children with ADHD compared with typically developing control subjects.
Conclusion
Our finding of reduced GABA concentration in ADHD is concordant with recently reported deficits in short intracortical inhibition in ADHD and suggests a GABAergic deficit in ADHD.
Attention-deficit/hyper-activity disorder (ADHD) is a developmental disorder affecting 3% to 5% of children and is defined by increased inattentiveness, hyperactivity, and impulsivity.1,2 The disorder is commonly linked with deficits of the dopamine and noradrenergic neurotransmitter systems and is treated using stimulant medication; however, ADHD cannot be described by abnormalities of a single neurotransmitter system.
It has been suggested that a deficit in behavioral inhibition lies at the core of ADHD.3 While the link between behavioral inhibition and the principal inhibitory neurotransmitter GABA (γ-aminobutyric acid) is not trivial, recent evidence supports such a link.4–6 GABA concentration can be measured noninvasively in vivo by edited magnetic resonance spectroscopy (MRS),7,8 and it was recently shown that regional GABA concentrations correlate with motor control and impulsivity in healthy adults.5,9
Investigation of GABA in ADHD has been limited. A recent study10 using transcranial magnetic stimulation found reduced short intercortical inhibition (SICI) in school-age children with ADHD; furthermore, the reduced SICI correlated with ADHD symptom severity and with motor skills. Short intercortical inhibition is known to be modulated by GABA-A agonists11 and is thought to be mediated by GABA-A cortical interneurons. Given these findings, we hypothesized that children with ADHD would show reduced GABA concentration in a measurement volume that included primary somatosensory and motor cortices.
METHODS
The study was approved by The Johns Hopkins Medical Institutional Review Board. Before examination, assent was obtained from each participant, and written informed consent was obtained from all parents or legal guardians. Included in the study were 13 children with ADHD (11 boys and 2 girls), with a mean age of 10.2 years (median age, 10.4 years; age range, 8.2–12.5 years). Also included were 19 age-matched typically developing (TD) control subjects (12 boys and 7 girls), with a mean age of 10.6 years (median age, 10.7 years; age range, 8.4–12.8 years). Diagnosis of ADHD was based on the following instruments: the Diagnostic Interview for Children and Adolescents IV (DICA-IV), Conners Parent and Teacher Rating Scales– Revised long form (ADHD-specific broad behavior rating scales), and the ADHD Rating Scale IV home and school versions. For inclusion in the ADHD group, a participant had to meet criteria on the DICA-IV and on at least 1 of 2 parent rating scales and on at least 1 of 2 teacher rating scales. Subtype of ADHD was evaluated using the DICA-IV, parent rating on Conners Parent and Teacher Rating Scales–Revised long form, and the ADHD Rating Scale IV home and school versions. Ten children with ADHD (8 boys and 2 girls) met criteria for ADHD combined subtype, and 3 children (all boys) met criteria for ADHD predominantly inattentive subtype. The mean duration from the date when children in the ADHD group fulfilled the DICA-IV criteria to the date of MRS examination was 2.1 months (range, 0.2–11.0 months), with only 1 participant exceeding 3.2 months. Intellectual ability was assessed using the Wechsler Intelligence Scale for Children III or IV. Children with full-scale IQ scores below 80 were excluded from participation.
In addition, the DICA-IV was used to assess the presence of other psychiatric disorders in all children. Children who met criteria for conduct disorder, mood disorders, generalized anxiety disorder, separation anxiety disorder, social phobia, or obsessive- compulsive disorder were excluded from the study. Children with comorbid oppositional defiant disorder (ODD) were included in the study given evidence from family studies12,13 suggesting that ADHD associated with ODD does not represent a distinct subtype. Five children with ADHD met criteria for ODD. No participant had a history of other neurological disorders, including Gilles de la Tourette syndrome. All children were also administered the basic reading subtest from the Wechsler Individual Achievement Test (WIAT) or the WIAT-II to rule out a learning disability in reading. Children were excluded from the study if they demonstrated a significant discrepancy between full-scale IQ and the WIAT or WIAT-II score or a basic reading subtest score below 85. Of 13 children with ADHD, 7 were being treated with stimulant medication at the time of the study. For those children, medication was withheld the day before and the day of testing. Children with ADHD taking longer-acting medications were excluded from the study. Children were included in the TD comparison group only if they did not meet ADHD diagnostic criteria on any of the administered rating scales and questionnaires. All children in the TD group were free of criteria for psychiatric disorders on the DICA-IV. No children in the TD group were taking psychoactive medications. All children in this study were assessed as right-handed using the Edinburgh Inventory. In addition, participants were administered the Physical Development Scale (Tanner),14 a brief questionnaire describing physical and sexual development. For 7 of 9 female participants, scores established that menarche had not been reached at the time of enrollment, making it unlikely that cyclical hormonal status had an effect on GABA measures in the group of girls.
MRS AND IMAGING
All experimental data were acquired on a 3-T imaging system (Achieva; Philips). J-difference–edited MRS spectra were acquired from a 3×3×3-cm3 voxel centered on the hand knob (as described previously15,16 and as shown in Figure 1A) in a 10-minute experiment. Fourteen-millisecond editing pulses were applied at 1.9 ppm in the “on” experiments and at 7.5 ppm in the “off” experiments. Three hundred twenty transients of 2048 data points (2-kHz spectral width) were acquired as twenty 16- step phase cycles, with the editing pulse frequency switched on alternate-phase cycles. Other experimental parameters included repetition time and echo time of 1800 and 68 milliseconds, respectively; refocusing pulse bandwidth of 1.4 kHz; and VAPOR (variable-power radiofrequency pulses with optimized relaxation delay) water suppression.17
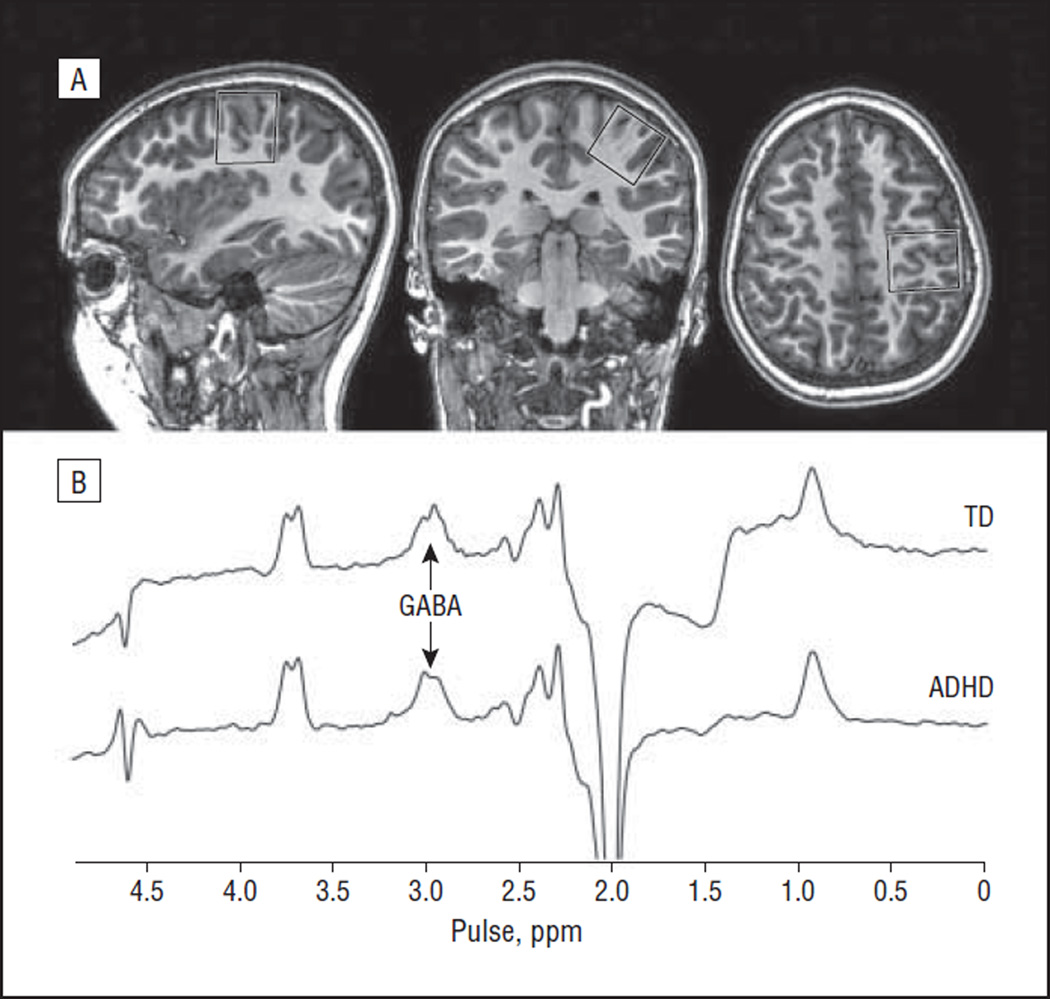
Figure 1.
Edited magnetic resonance spectroscopy of GABA (γ-aminobutyric acid). A, Location of the measurement voxel centered on the hand knob in primary motor cortex (as seen in the axial image). B, Typical edited GABA spectra from 1 child with attention-deficit/hyperactivity disorder (ADHD) and 1 typically developing (TD) child, showing a well-resolved edited peak at 3 ppm. The coedited peak at 3.75 ppm represents total glutamate plus glutamine.
Edited MRS spectra were processed with 4-Hz exponential line broadening; edited GABA signal at 3 ppm was fit using a simple gaussian model,18 and the model was integrated. As a quantitative metric of fit quality, the root mean square residual for this fit was quantified as a percentage of the fitted peak amplitude. The unsuppressed water signal from the same volume was fit using a gaussian-lorentzian model, and the model was integrated. GABA concentration in institutional units was calculated using the following equation: CGABA=Cw × (IGABA/Iw) × (Rw/RGABA) × ([1−αMM]/κ), where the ratio of the integral of edited GABA signal IGABA and water signal Iw was multiplied by the visible water concentration Cw and factors Rw and RGABA to account for T1-weighted and T2-weighted relaxation of the water signal and GABA signal, editing efficiency κ, and the mean macromolecular (MM) signal fraction αMM.
STATISTICAL ANALYSIS
A linear regression analysis was performed using commercially available software (MATLAB Statistics Toolbox Version 7.3.1 [R2009b]; The MathWorks, Inc) to examine the effects of diagnosis and sex on GABA concentration. Owing to the few female participants in one quadrant (2 girls in the ADHD group), no interaction term was included. A t test was performed to assess for a main effect of diagnosis on GABA concentration. As a secondary analysis, an equivalent linear regression was performed to investigate changes in the coedited total glutamate plus glutamine (Glx) signal.
RESULTS
Edited spectra were acquired in all 32 age-matched participants; the ages of the ADHD group vs the TD group were not significantly different (P=.31 by t test). The mean (SD) normalized root mean square fit residuals were not significantly different between the ADHD group (13% [6%]) and the TD group (9% [5%]) (P>.05 by t test). Successful application of this method in a pediatric population (even one with ADHD)is not assured a priori because J-difference editing is inherently sensitive to participant movement. Figure 1B shows the edited spectra for 1 participant in the ADHD group and for 1 participant in the TD group, with the GABA peak at 3 ppm labeled.
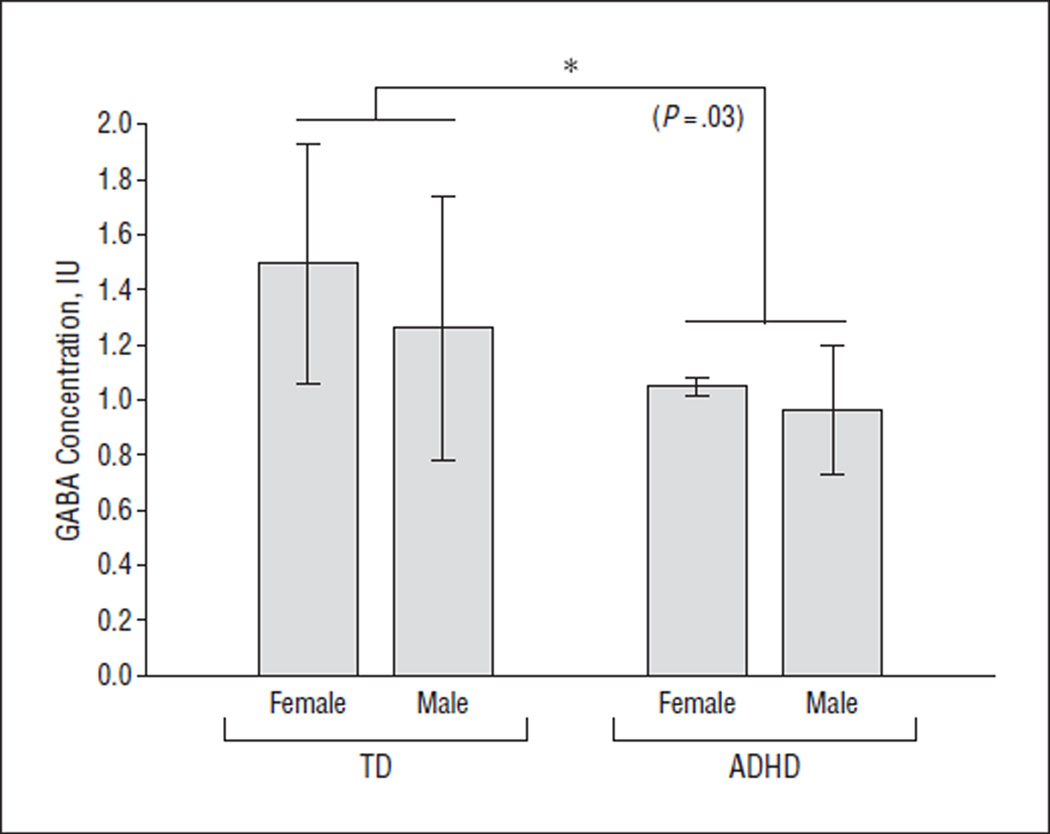
Linear regression analysis revealed a significant effect of diagnosis (β=−0.34, P=.03) but not sex (β=−0.16, P=.33) on GABA concentration, as shown in Figure 2. The mean (SD) GABA concentrations for the subgroups were 0.96 (0.23) IU for ADHD male, 1.05 (0.03) IU for ADHD female, 1.26 (0.47) IU for TD male, and 1.49 (0.43) IU for TD female. The mean (SD) GABA concentrations were significantly lower in the ADHD group (0.98 [0.22] IU) than in the TD group (1.34 [0.47] IU) (P=.01 by t test). No significant effect of age on GABA concentration was observed in univariate or regression analysis (P > .36 for both), although this study was not designed to investigate such an effect given the limited age range (8.2–12.8 years) of the participants. In the ADHD group, no significant difference in GABA concentration was observed between participants who were taken off stimulant medication for the study vs participants who were not receiving medication (P=.67 by t test).
Figure 2.
Comparison of GABA (γ-aminobutyric acid) concentration, showing the statistically significant effect of attention-deficit/hyperactivity disorder (ADHD) diagnosis. Asterisk indicates the statistically significant difference (P <.05); IU, institutional units; and TD, typically developing.
Quantification of the coedited Glx peak (at 3.75 ppm) is shown in Figure 1B. No significant difference in Glx was observed between the ADHD group and the TD group (P=.28 by t test).
COMMENT
Our finding of significantly reduced GABA concentration in children with ADHD is consistent with the initial hypothesis of this study and is concordant with reduced SICI in ADHD.10 Cerebral cortical inhibitory function, via GABAergic transmission, may be vital for filtering sensory information and selecting appropriate behavioral responses.9 As such, understanding and quantifying GABAergic function in children who are developing motor and behavioral control could provide vital insights into the neurobiological underpinnings of ADHD.
GABAergic inhibition is implicated in several developmental disorders, including ADHD and autism spectrum disorder. As the principal inhibitory neurotransmitter in the mature brain, GABA is initially excitatory in the developing brain. In addition to this role reversal, it has been suggested that the GABAergic system is particularly susceptible to damage during abnormal development because GABAergic neurons arise from a different region of the neural tube than glutamatergic neurons, with which they must later integrate.19
Although good evidence for a GABAergic abnormality, the observation of reduced GABA concentration in ADHD as measured by MRS does not inform us as to the location or distribution of that reduction. For example, we do not know whether this result reflects a reduced density of GABAergic neurons or a reduced concentration of GABA within a normal density of GABAergic neurons. Nor does MRS provide us with any information on the cellular compartmentalization of the GABA from which we detect signals.
It is common to interpret changes in GABA in the context of the effect on excitation-inhibition balance. Previous MR spectroscopy work in ADHD has shown elevated Glx,20,21 suggesting an exacerbated effect on excitation-inhibition balance, although Glx should cautiously be interpreted in terms of excitatory neurotransmission owing to the extensive metabolic role of glutamate. Our finding of no significant difference in Glx concentration between the 2 study groups is consistent with a recent meta-analysis22 of previous MRS results in ADHD.
Although no significant difference in data quality (quantified through the normalized fitting residual) was observed between the 2 study groups, the mean residuals were slightly greater for the ADHD group. This would be expected because children with ADHD are likely to have greater difficulty staying still during the experiment. If the fitting residual is included as a regressor, the significance of diagnosis as a factor explaining GABA concentration is increased. This suggests that the group difference in measured GABA concentration tends to be masked by, rather than caused by, inaccuracies of fitting.
Several methodological limitations are relevant to the edited detection of GABA by MRS. The volume of acquisition (3×3×3 cm3) contains a range of tissues, including precentral and postcentral gray and white matter, and its large size is required owing to the low concentration of GABA, the inherent insensitivity of MRS, and a compromise between volume and experiment time. In addition, GABA signal detected by the PRESS (point-resolved spectroscopy) sequence at 3 T contains a significant contribution from coedited macromolecular signals. Although it is possible that the effect observed could arise from macromolecular differences, this study was based on a GABA deficit hypothesis, and we interpret the differences seen as likely arising from changes in GABA concentration.
As with any case-control study, limitations existed in the extent to which the ADHD group and the TD group were matched. However, age does not statistically explain significant variance in GABA concentration, so it seems unlikely that imperfect matching drives the observed group difference. It is also important to consider medication effects as a potential explanation for our findings. Seven of 13 children with ADHD had been prescribed stimulant medication, which was withheld the day before and the day of testing. We cannot rule out the possibility that chronic administration or withdrawal of stimulants may have influenced GABA measurements in these participants (although no direct pharmacological reason exists to believe that either will affect GABA concentration), and the absence of a difference between medicated and unmedicated participants supports this. However, given the small sample for this comparison, follow-up analyses with larger cohorts of children actively prescribed and not prescribed stimulants will provide opportunity to address this question.
Taken in combination with reduced SICI, our finding of reduced GABA provides strong evidence for a GABAergic deficit in ADHD. Should further research establish strong relationships between specific ADHD symptoms (such as reduced motor control) and deficits in cortical inhibition, GABAergic therapy may have a role in the future treatment of ADHD.
Acknowledgments
Funding/Support: This work was supported by grants R01 MH078160, R01 MH085328, and P41 RR15241 from the National Institutes of Health and by The Johns Hopkins University School of Medicine Institute for Clinical and Translational Research National Institutes of Health/ National Center for Research Resources Clinical and Translational Science Award program UL1 RR025005.
Role of the Sponsors: The sources of funding had no further role in study design or conduct; collection, management, analysis, or interpretation of data; or preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Edden had full access to all the data in the study, performed the statistical analysis, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
REFERENCES
- 1.Tannock R. Attention deficit hyperactivity disorder: advances in cognitive, neurobiological, and genetic research. J Child Psychol Psychiatry. 1998;39(1):65–99. [PubMed] [Google Scholar]
- 2.Brown RT, Freeman WS, Perrin JM, Stein MT, Amler RW, Feldman HM, Pierce K, Wolraich ML. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. [Accessed March 29, 2012.];Pediatrics. 2001 107(3):E43. doi: 10.1542/peds.107.3.e43. [DOI] [PubMed] [Google Scholar]
- 3.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, Sumner P. Dorsolateral prefrontal γ-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70(9):866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20(19):1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marenco S, Savostyanova AA, van der Veen JW, Geramita M, Stern A, Barnett AS, Kolachana B, Radulescu E, Zhang F, Callicott JH, Straub RE, Shen J, Weinberger DR. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology. 2010;35(8):1708–1717. doi: 10.1038/npp.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of γ-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13(7):825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76(7):615–621. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109(1):127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 12.Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, Spencer T, Norman D, Kolodny R, Kraus I, Perrin J, Keller MB, Tsuang MT. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry. 1992;49(9):728–738. doi: 10.1001/archpsyc.1992.01820090056010. [DOI] [PubMed] [Google Scholar]
- 13.Faraone SV, Biederman J, Chen WJ, Milberger S, Warburton R, Tsuang MT. Genetic heterogeneity in attention-deficit hyperactivity disorder (ADHD): gender, psychiatric comorbidity, and maternal ADHD. J Abnorm Psychol. 1995;104(2):334–345. doi: 10.1037/0021-843X.104.2.334. [DOI] [PubMed] [Google Scholar]
- 14.Petersen AC. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17(1):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 15.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of γ-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31(1):204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 16.Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31(46):16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106(20):8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx 1 and Dlx 2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19(1):27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- 20.Courvoisie H, Hooper SR, Fine C, Kwock L, Castillo M. Neurometabolic functioning and neuropsychological correlates in children with ADHD-H: preliminary findings. J Neuropsychiatry Clin Neurosci. 2004;16(1):63–69. doi: 10.1176/jnp.16.1.63. [DOI] [PubMed] [Google Scholar]
- 21.Hammerness P, Biederman J, Petty C, Henin A, Moore CM. Brain biochemical effects of methylphenidate treatment using proton magnetic spectroscopy in youth with attention-deficit hyperactivity disorder: a controlled pilot study. CNS Neurosci Ther. 2012;18(1):34–40. doi: 10.1111/j.1755-5949.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlov E, Philipsen A, Matthies S, Drieling T, Maier S, Bubl E, Hesslinger B, Buechert M, Henning J, Ebert D, Tebartz Van Elst L. Spectroscopic findings in attention-deficit/hyperactivity disorder: review and meta-analysis. World J Biol Psychiatry. 2009;10(4, pt 2):355–365. doi: 10.1080/15622970802176032. [DOI] [PubMed] [Google Scholar]