Abstract
Maximizing fat loss while preserving lean tissue mass and function is a central goal of modern obesity treatments. A widely cited rule guiding expected loss of lean tissue as fat-free mass (FFM) states that approximately one-fourth of weight loss will be FFM (i.e., ΔFFM/ΔWeight = ~0.25) with the remaining three-fourths fat mass. This review examines the dynamic relations between FFM, fat mass, and weight changes that follow induction of negative energy balance with hypocaloric dieting and/or exercise. Historical developments in the field are traced with the “Quarter FFM Rule” used as a framework to examine evolving concepts on obesity tissue, excess weight, and what is often cited as “Forbes’ Rule”. Temporal effects in the fractional contribution of FFM to changes in body weight are examined as are lean tissue moderating effects such as aging, inactivity, and exercise that frequently accompany structured low-calorie diet weight loss protocols. Losses of lean tissue with dieting typically tend to be small, raising questions about study design, power, and applied measurement method reliability. Our review elicits important questions related to the fractional loss of lean tissues with dieting and provides a foundation for future research on this topic.
Keywords: Body Composition, Obesity, Mathematical Model, Energy Expenditure, Exercise
INTRODUCTION
Excessive loss of lean tissues in response to a hypocaloric diet poses a range of adverse clinical risks 1, 2. As a result, over a century of research has created a nutrition paradigm that dictates high quality diets, promotion of physical activity, and judicious rates of weight loss aimed at maximizing reductions in body fat while limiting catabolism of functional lean tissues 3. Collectively, these measures are aimed at maintaining a low value for the fraction of weight loss as fat-free mass (ΔFFM/ΔW), a key biomarker of lean tissue erosion during periods of negative energy balance.
This traditional ΔFFM/ΔW paradigm is now extending into a new level of critical review. Drugs being developed that promote weight loss through novel mechanisms that potentially have adverse catabolic effects may have as their first recognizable signal a deviation from control values in the fraction of weight loss as FFM. New anabolic medications designed to limit lean tissue loss with aging are being developed that include FFM and skeletal muscle mass as key biomarkers of efficacy 4, 5. As the biomedical research field expands with advanced data processing capabilities, clinically applicable quantitative energy balance mathematical techniques are being developed that include biomarkers such as ΔFFM/ΔW as key model terms 6, 7. These trends are stimulating an in-depth analysis of biomarkers such as ΔFFM/ΔW that are moving away from previous qualitative discussions to more quantitative reviews.
In that new context, is there an “accepted” proportion of weight loss with dieting that ideally derives from the “functional” FFM compartment? Prentice et al. 8 cautiously suggested that ΔFFM/ΔW “should be about 0.25 at the sorts of fat masses common in dieting women”. The “Quarter FFM Rule” is frequently invoked on dozens of Internet listings, as for example Berkhan writes on the LEANGAINS web site “when an individual loses weight by dietary restriction alone, approximately 75% of weight is lost as fat mass, and 25% of weight is lost as fat-free mass” 9. Berkhan cites an article in this journal as the source 10.
How accurate is the Quarter FFM Rule (i.e., ΔFFM/ΔW=~0.25) and what, if any, theoretical basis is there for its application? How did this widely cited concept originate? When added to dieting, does exercise change the fraction of weight loss as FFM? This report examines these questions that have loosely prevailed for decades and that have not yet been critically examined in a synthetic review. Our study extends earlier meta-analyses on related topics 11–15.
Although we are able to compile a cohesive analysis that addresses some of the posed questions, we also found large gaps in the literature, which have yet to be addressed. We identify these knowledge gaps and thereby provide a path forward for advancing the field.
DIET FAT-FREE MASS EFFECTS
Historical Concept Development
With famines and wars in the recent past, medical scientists working in the middle of the last century and armed with new research tools began to systematically examine the physiological effects of changes in food intake under different environmental conditions. Concepts advanced in several phases over the following decades that today provide us with a broad and still evolving view of how lean tissues respond to changes in energy balance.
Minnesota Approach
Six decades ago in 1953 Ancel Keys and Josef Brozek were the first to rigorously develop the concept of “obesity tissue” with stable composition that can be lost or gained with changes in body weight 16. Keys and Brozek were members of the Minnesota Semi-Starvation Experiment team that with associated small-scale studies provides much of the foundation for our modern understanding of weight loss physiological effects 17. Keys and Brozek envisioned the body as three discrete parts, “tissue of standard normal composition (N)”, “obesity tissue (G)”, and surplus or deficit “extracellular fluid (H)”. Keys and Brozek thus defined body weight as the sum of N, G, and H (Figure 1).
Figure 1.
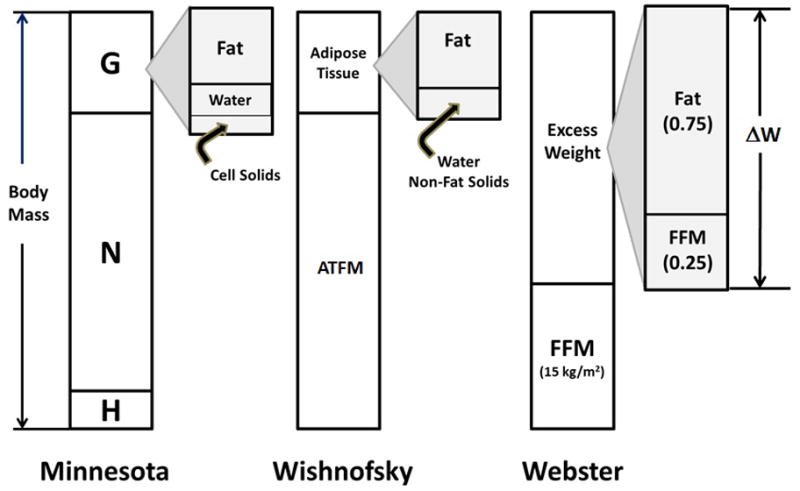
Three models describing “obesity tissue” or “excess weight”. Each model proposes a stable composition of weight loss with dieting that includes established values for fractional weight losses as fat and the indicated fat-free mass (FFM) or related adipose-tissue free mass (ATFM) components. The Minnesota model 16 of body mass consists of three parts, a “core” of “normal” (N) size and composition, excess extracellular fluid (H), and obesity tissue (G) with specified proportions of fat, water, and cell solids. Wishnofsky viewed diet-related body mass changes as deriving solely from adipose tissue fat 20. Webster’s model 23 posits a “core” body mass with zero fat that in women has a FFM index of 15 kg/m2; body mass above this level has a rounded fractional composition of 0.75 fat and 0.25 FFM.
The obesity tissue, G, was assumed to have three primary components, “pure fat, cells or cellular matter (e.g., sarcoplasm, connective tissue, etc.), and extracellular water”. Reviewing their carefully controlled experimental overfeeding studies in men, Keys and Brozek initially defined G as having 62% “pure fat”, 24% cell residue, and 14% extracellular water. By today’s parlance obesity tissue would thus be 62% fat and 38% FFM. The authors derived these values from middle-aged, physically healthy men ranging from 15% underweight to 13% overweight and who gained 2.5 to 21.0 kg during carefully controlled experimental overfeeding studies.
In their initial report 16 Keys and Brozek suggested that G has a stable composition with dietary interventions when subjects range in adiposity from 10% to 25%. The authors acknowledged that no data was available for predicting the composition of obesity tissue when subjects above 25% fat gain body mass. With loss of body mass below the 10% fat level, Keys and Brozek noted that “more and more cellular matter is lost and this is replaced or filled in by extracellular fluid, while the loss of fat becomes a smaller and smaller fraction of the solids lost from the body”. Citing their classic Minnesota Semi-Starvation Experiment 17, “in the region where fat makes up only 5 to 9 % of the body weight, the loss of fat is only about 40 to 50% of the total net loss”.
The authors continued to advance their obesity tissue calculations over the next 15 years with Francisco Grande as an additional collaborator. Grande further reported in 1961 18 that diet related weight loss composition in men differed between the early and later phase of food restriction. Based on experiments conducted in 1954, fat, water, and protein were 25:70:5 (i.e., FFM, 75) and 85:0:15 (i.e., FFM, 15) percent of weight loss at 3 and 21–24 days of diet restriction, respectively. Further evidence for an early and later phase of weight loss response to diet restriction came from the Minnesota Semi-Starvation Experiment published in 1950 17 in which the authors observed 37% of weight loss in men as fat during weeks 1–12 and in the next 12 weeks fat increased to 70% of “gross” weight loss
Brozek and colleagues seminal New York Academy of Sciences publication in 1963 19 further refined estimates of changes in obesity tissue as observed in experimental studies of men. The authors applied three different approaches, one based on their original overfeeding experiments, a second on weight loss experiments in young men, and a third by estimating the “static” difference in body composition between lean and obese men of equivalent height. Results for the three approaches provided ratios for fat, cell residue, and extracellular water, respectively, 64:22:14, 64:32:4, and 73:20:7 (i.e., ΔFFM/ΔW, 0.36, 0.36, 0.27).
Keys, Brozek, and Grande thus introduced the concept of “obesity tissue” and through careful experimental observation defined the composition of “G” by applying three different calculation approaches. The authors noted the fractional composition of weight change as FFM with dietary interventions is larger when men who have low baseline adiposity are placed into negative energy balance. A third observation is that the fractional weight loss as FFM is not “constant” but varies over time with larger changes observed early during the weight loss period.
Wishnofsky Approach
The next notable approach was that of Max Wishnofsky in 1958 20. In one of the most quoted papers in clinical nutrition, Wishnofsky reasoned that with nutritionally balanced low-calorie dieting the main loss in body mass derives from adipose tissue (Figure 1). Experimental observations available at the time appeared to largely support the view that weight loss is accompanied mainly by decrements in adipose tissue 20. Citing Bozenrad’s 1911 study 21, Wishnofsky assumed that adipose tissue is 87% fat (i.e., largely triglyceride) and has an energy density of 3750 kcal/lb or 8250 kcal/kg. With weight gain or loss Wishnofsky surmised that “The calorie deficit will be made up chiefly by the catabolism of fat”. Wishnofsky’s obesity tissue, rounded to 3500 kcal/lb or 7700 kcal/kg, would thus largely be devoid of FFM and ΔFFM/ΔW would be 0.13 if weight loss was solely adipose tissue based on Bozenrad’s chemical analysis 21.
An important aspect of Wishnofsky’s Rule in the current context is that it predicts a constant energy density of weight loss (i.e., 7700 kcal/kg). Similarly, the Quarter FFM Rule predicts a constant energy density of weight loss at ~7400 kcal/kg. The two rules, conceptually harmonious, are connected by the mathematical expression: energy content of weight change (kcal/kg) = 1020 (ΔFFM/ΔW) + 9500 (1 - ΔFFM/ΔW) (Supplementary Material, I). The extent to which these two rules are valid is the subject of not only this review, but of other publications that focus critically on details of Wishnofsky’s Rule and the energy content of weight loss6, 22.
Webster Approach
Almost three decades ago, Webster, Hesp, and Garrow in their landmark 1984 paper 23 established what we now recognize in this review as the Quarter FFM Rule. The authors evaluated fat and FFM in a cohort of women ages 14 to 60 years ranging widely in adiposity using three methods, densitometry, total body water, and total body potassium. Taking the average of these three body composition estimates, Garrow et al. regressed weight adjusted for height (W/Ht2) on fat mass/Ht2 with the resulting model W/Ht2 = 15.5 + 1.28 x fat mass/Ht2. The intercept value of 15.5 kg/m2 was assumed to represent a woman with “zero” fat and “excess” weight would then have a fat fraction of 1/regression model slope of 1.28 (i.e., 0.78) and a FFM fraction of 0.22. Simplifying, the resulting ΔFFM/ΔW is 0.25, giving birth to the Quarter FFM Rule (Figure 1). The authors closed their widely cited paper 23 stating “If it is true that excess weight in obese subjects is 22 per cent lean and 78 per cent fat, then the target for treatment should be that the weight lost by obese patients should not have more than 22 per cent lean”.
The group thus developed a concept of “excess weight” akin to the “obesity tissue” of Keys and colleagues 16. That is, there is a kind of “core” or standard body mass from which “grows” adipose and related tissues. As people move up and down the body mass continuum over time this tissue and associated components are respectively gained or lost.
Forbes Approach
Gilbert Forbes and his colleagues began a series of comprehensive research reports in 1983 with the common theme lean body mass – fat relationships24. Forbes spent decades developing the total body potassium technique for measuring lean body mass, or in modern parlance what approximates to FFM 25–27. The method pioneered by Forbes relies on measured naturally occurring 40K to estimate FFM, with fat mass calculated as the difference between body weight and FFM 27. While the authors provided extensive literature reviews in their 1983 and 1986 papers 24, 28, of most relevance here are the results of their longitudinal experimental studies.
The first experiment published in 1983 involved 6 young men over-fed a high carbohydrate diet for 17–19 days following a 5-day stabilization period 24. Mean body weight and FFM gains were 3.62 and 1.58 kg, respectively, with ΔFFM/ΔW equal to 0.44. The second experiment published in 1986 involved 13 young women and 2 men who participated in a similar overfeeding protocol28. The resulting body weight and FFM gains were (X±SD) 4.44±0.63 kg and 2.23±1.38 kg, respectively, with ΔFFM/ΔW equal to 0.50±0.30. The authors again had conclusively shown that a “partnership” exists between lean and fat mass, both increasing with weight gain.
Forbes gathered the available information on lean-fat relations in what is likely one of the most important synthetic reviews in clinical nutrition 29. The 1987 report included data sets obtained in women who were underweight with anorexia nervosa, normal weight, and obese between the ages of 14 and 50 years and height between 156 – 170 cm. Forbes thus constrained his data set in order to eliminate body composition effects related to sex differences, aging and stature. When Forbes plotted FFM versus fat mass he observed a concave downward curvilinear relationship with the higher slope steep at low values of fat mass and “flattening out at high values” of fat mass. Forbes fit the log-linear curve FFM = A ln(fat mass) + B to stratified group mean data to arrive at the FFM-fat mass relationship known as the Forbes Curve28, 29:
| (1) |
The Forbes curve can be transformed into the ratio relationship
| (2) |
through differentiation (for calculation details see Supplementary Material, II)30, 31 Hall examined ΔFFM/ΔW (31) and he derived a relationship that includes a dependence on both the magnitude and direction of weight change. We present a calculus-based strategy for deriving dFFM/dW that is the subject of this review (Supplementary Material II).
The Forbes curve predicts in quantitative terms that individuals with greater fat mass have a larger lean mass, that changes in body weight and fat mass are accompanied by changes in lean mass, and that these changes are not constant but in fact the fraction of weight loss or gain as lean mass is larger when baseline fat mass is smaller. While these equations and predictions were based on cross-sectional body composition data, Forbes reviewed earlier longitudinal studies that provided model validation. This validation has withstood the test of time in more rigorous quantitative studies 30–32.
Forbes thus moved the field away from a “constant” ΔFFM/ΔW, a suggestion also made earlier by the Minnesota group 16, 18, and in the process provided a model that supported clinical observations. For example, a widely recognized phenomenon during Forbes time and still well established today is the “protein sparing” effect of adiposity observed when the obese are placed into negative energy balance compared to their normal weight counterparts. In other words, when calorically restricted, protein and FFM losses are relatively smaller in the obese who have a large baseline fat mass than are those of normal weight subjects. Today, Forbes’ Rule and variants serve as key components of dynamic energy balance models that provide weight loss and energy intake predictions 32–34.
Forbes classic equation was developed by examining the relationship between FFM and fat mass in a cross-sectional sample of relatively young women within a specified height range 29. Since then Thomas et al. have updated Forbes model to include a much larger and more diverse sample of men and women who participated in the NHANES survey 30. Body composition was evaluated in the NHANES participants using dual-energy x-ray absorptiometry (DXA). Fat-free mass is plotted against fat mass measured in adult (age ≥18 yrs.) NHANES men and women and the sex-specific Thomas models (Supplementary Material III) can be used to predict FFM from a subject’s fat mass, age, height, and race (download calculator at http://pbrc.edu/calculators/fatfreemass). While predictions of changes in FFM using the Forbes model were slightly better when tested on group mean data 30, the Thomas models allow for evaluation of these effects while considering age, height, and gender as model covariates. The FFM-fat mass functions for an “average” NHANES man (age 45 yrs., height, 174 cm) and woman (46 yrs., 161 cm) are shown as solid lines in the figure for African American (AA) and non-AA subjects. For any level of fat mass AA men and women have a greater FFM than do non-AA subjects, a finding consistent with several earlier small scale studies 35, 36. Taking the slopes of these functions in their mid-range provides us with cross-sectional estimates of ΔFFM/ΔW for men (0.37) and women (0.32) with no race differences. Calculating the “excess weight” using the Webster approach 23 provides almost the identical results: 0.36 for men and 0.31 for women (Supplementary Material, III).
These are the predicted fractions of weight change as FFM should the hypothetical man and woman either gain or lose weight within the mid-range of the functions shown in Figure 1. These estimates assume, as did Webster and colleagues 23, that the human body “remodels” with weight change as predicted from cross-sectional body composition data. Longitudinal studies support that assumption 29, 30 with the proviso that very large excursions in body weight with low calorie dieting or over-feeding are rarely reported that include careful evaluation of body composition with appropriate methodology. Large reductions in body weight are now being reported following bariatric surgery, but questions remain about how these bariatric procedures compare metabolically and hormonally to weight loss with dieting and the validity of applied body composition methods when used in morbidly obese subjects 12, 37. Forbes’ original study29 included women with very low fat mass secondary to anorexia nervosa while such low BMIs and fat mass are infrequent in the general population as reflected in the NHANES body composition sample 30.
Forbes astutely recognized that greater degrees of negative energy balance are accompanied by larger relative lean losses 29 and his observation on a limited data set is supported by the more recent meta-analysis of Chaston et al. 12. There is also a large literature centered several decades ago that describes the relations between diet composition and losses of body protein over the course of a voluntary diet 38. Thus, both energy deficit and diet composition both appear to influence ΔFFM/ΔW, although at present there are no reported adjustments to Forbes’ model that account for these effects.
Metabolic-Hormonal State
Modern concepts relate excess adiposity to metabolic states such as the presence of insulin resistance and circulating inflammatory mediators that impact on patient morbidity and mortality. Additionally, some obese patients seeking diet treatment may have chronic conditions such as rheumatoid arthritis and pulmonary disease that are associated with a low-grade inflammatory state 39. When hospitalized, these chronically ill patients and obese patients in general frequently enter a period of negative energy balance due to a combination of low prescribed energy intakes and the presence of additional metabolic stress 40–42. The question of how the subject’s baseline metabolic milieu impacts FFM and total body protein losses with hypocaloric feeding is a clinically relevant topic that deserves additional scrutiny 43, 44. This area is the focus of considerable discussion and research in the field of specialized hospital nutrition support 45.
The idea that some subjects are more resistant than others to FFM losses during a diet extends back three decades to the studies of Van Itallie and Yang 46. The authors conducted a 64-day weight loss balance study of morbidly obese patients and found that the cumulative nitrogen (i.e., protein) deficit was strongly negatively correlated with the observed decrease in serum triiodothyronine. This observation prompted the authors to suggest that the thyroid hormone response may be a critical protein-sparing adaptation.
Metabolic-hormonal mechanisms operating at the individual level may thus play a role in determining the magnitude of lean tissue losses that follow induction of negative energy balance. Future studies ideally would consider these mechanisms leading to individual variation in lean tissue catabolism over the course of a low calorie diet.
Temporal Trends
A clinical observation made by those who directly manage obesity treatment programs is the rapid weight loss that follows initiation of a hypocaloric diet. The rate of weight loss gradually slows over time and eventually reaches a new steady state weight plateau 33, 47. This clinical observation reflects the disappearance rates of four main compartments at the molecular body composition level, glycogen, protein, water, and fat. The “FFM” compartment collectively represents the sum of glycogen, protein, water, and associated minerals. Benedict in 1915 was among the first to show the variable disappearance rates for the four main compartments with total fasting 48.
Glycogen losses are rapid with onset of a hypocaloric diet and initial losses of nitrogen (i.e., protein) are also rapid and relatively large 47. The glycogen pool is small and quickly stabilizes at a new reduced amount, mainly in liver and skeletal muscle. Adaptations in protein metabolism slows total body losses with a new reduced rate of catabolism reached over several weeks as observed in typical balance studies 47. Since both glycogen and protein associate with water, the dynamic changes in their respective rates of loss are mirrored in the FFM compartment as a whole.
Recognizing these temporal effects of dieting, Forbes in 1970 formalized early descriptive models by providing a dynamic two-component model including an early phase with rapid weight loss lasting days or weeks followed by a later slower weight loss phase lasting months or years (Table 1, equation #1)49. Forbes and his colleagues followed up in 1979 by showing that dynamic changes in total body protein with hypocaloric feeding followed a similar two-component temporal pattern (Table 1, equation #2)50.
Table 1.
Forbes’ Two-Component Weight and Protein Loss Equations.
| Equation | Comments | ||
|---|---|---|---|
| #1 |
|
Model describes voluntary weight loss as consisting of two phases, each with quantifiable kinetic parameters. W(t) represents weight on day t. The coefficients satisfy the property that f1+ f2 = 1 and λ1 ≫ λ2. | |
| #2 |
|
Model describes nitrogen (N; i.e., protein) loss with dieting as consisting of two phases, each with quantifiable kinetic parameters. N(t) represents total body nitrogen (i.e., protein) on day t. |
The relevance of these observations and those made by Grande that are described earlier in this review18 is that the fraction of weight loss as FFM dynamically changes over time. The ΔFFM/ΔW ratio is relatively large during the first days or weeks of dieting and gradually ΔFFM/ΔW assumes a lower stable level as negative energy balance proceeds 47. The early phase half-life is typically less than one-week depending on level of adiposity and thus requires 5–26 days (4–5 half-lives) for completion 47, 49. The half-life for the later weight loss phase ranges from 100 days in lean subjects to 300 days in obese subjects 47, 49. Both weight loss phases are incorporated into modern dynamic energy balance models 7, 32, 33. These effects likely account for considerable variability in reported values for ΔFFM/ΔW as most studies include a single time point measurement reflecting the combined effects of early and later weight loss.
NON-DIET FAT-FREE MASS EFFECTS
Up to this point our review has focused on the fraction of weight loss as FFM with dieting. We have centered our discussion on concepts related to “obesity tissue” or “excess body weight” that have evolved over the past six decades. According to this paradigm body weight and composition move up and down a predictable trajectory with positive and negative energy balance, respectively. Obesity tissue has a definable composition that is either constant or can be predicted and that follows certain “rules”.
Dieting, however, is not the only factor influencing energy balance and body composition over time and here we review three additional considerations, aging, inactivity, and exercise. Each has what might be considered an effect “independent” of diet on dynamic changes in FFM over time. However, teasing apart specific effect sizes is complex as only rarely are experiments done in which one factor is changed while the others are held constant. Our review therefore focuses on effect generalities rather than the quantitative influences of these three factors on ΔFFM/ΔW. Limitations and gaps in the available literature are identified that highlight the need for future research.
Growth and Aging
An increasing number of studies involving weight change are carried out over periods of years or even decades in both children and adults. Hall and colleagues recently incorporated “growth” into their previously reported dynamic models that include partitioning of energy imbalances between fat and FFM 51. Hall’s model predicts a dissociation of ΔFFM from ΔW in obese children during weight management interventions occurring in periods of rapid growth such as during adolescence. Model simulations predict that with weight management the underlying strong anabolic actions associated with adolescent growth lead to FFM accretion, particularly in males, with a corresponding substantial fat loss in the absence of weight change. Longitudinal inter-relations between changes in fat mass, FFM and body weight with interventions during the growth years are thus complex and may not conform to the simple Quarter FFM Rule.
With respect aging, Forbes estimated that at weight equilibrium the global average rate of FFM loss is 1.5 kg/decade52. Goodpaster et al. 53 similarly observed a lean mass loss with approximate weight stability in a large elderly cohort of white and black men (X±SD; -0.87±1.96 kg; -1.19±2.30 kg) and women (-0.31±1.49 kg; −0.30±1.97 kg) followed over three years.
A hypothetical example demonstrates the aging effects on the proportion of weight loss as FFM with dieting. Consider a representative obese 46 year-old white woman who has a height of 161 cm and body weight of 100 kg. We can first examine predicted changes in body composition with a 5% voluntary weight loss over one year using the Thomas NHANES models (30, http://pbrc.edu/calculators/fatfreemass). The sex and race-specific model predicts a FFM loss of 1.7 kg and a ΔFFM/ΔW of 0.34. Had we extended the 5 kg weight loss over ten years instead of one year, the predicted FFM loss is 2.15 kg and the ΔFFM/ΔW increases to 0.43.
Inactivity
Changes in activity patterns are often observed when a subject is brought into a metabolic ward for inpatient study, with convalescence following bariatric surgery, after an orthopedic injury or procedure that impacts on ambulation, and a host of other situations and conditions that might occur over a weight loss study interval. One of the best models for inactivity is experimental bed rest designed to simulate the microgravity effects of spaceflight and the immobility associated with acute and chronic disease 54. As an example, LeBlanc and colleagues evaluated bed rest for 17 weeks in 8 healthy relatively young men with a mean BMI of about 25 kg/m2 55. Subjects ingested a weight maintenance diet while living on a carefully supervised metabolic ward. Total body lean mass, as measured by dual photon absorptiometry, was reduced by 4.1% from baseline with the largest reductions observed in the upper (12.2%) and lower (11.2%) legs. Nitrogen (i.e., protein) balance remained negative throughout the study and losses accounted for a lean mass reduction of (X±SD) 3.9±2.1 kg while mean body weight increased significantly (p<0.05) by 1.8 kg.
Exercise
Exercise imposes a mechanical load on skeletal muscles and bone, the magnitude of which varies with the chosen activity. As such, exercise is the inverse of inactivity and is often viewed as a way to reduce losses of FFM with dieting. Activities vary in type, amount, and protocol duration, making exact estimates of FFM effects complex.
Without Diet
We begin by reviewing what happens to FFM when subjects initiate an exercise program without undergoing a prescribed calorie restriction. This approach involves studies where subjects are “clamped” at baseline intake levels in a tightly controlled experimental environment. The energy intake clamping experiment allows us to evaluate what happens to FFM in response to a negative energy balance solely induced by exercise. These demanding experimental conditions were met in the study of Bouchard and colleagues that evaluated changes on body composition with aerobic exercise while intake was strictly maintained at baseline levels in a confined environment 56. Seven pairs of young male identical twins increased their energy expenditure 580 kcal/d by pedaling on a cycle ergometer over a 93-day period. Subjects lost weight (X±SEM; 5.0±0.6 kg), nearly all of which was fat mass (4.9±0.6 kg) and very little was FFM (0.1±0.3 kg). As typical weight loss of this magnitude (~5 kg) with dieting leads to a FFM loss of at least 1 kg, these results might be interpreted as showing a lean mass “sparing” effect. What we lack to answer this conjecture is a diet control group matched for energy deficit over the same time interval.
A second semi-confined experiment was conducted by Ross et al. 57, 58 that included a supervised aerobic exercise program (brisk walking or light jogging on a treadmill; 517 kcal/d for 12 women and 700 kcal/d for 14 men) with subjects monitored daily to ensure maintenance of baseline intake across the 12 week (men) or 14 week (women) protocol. Adherence to baseline intake was confirmed with mid-point doubly-labeled water measurements. Using whole-body magnetic resonance imaging, the authors observed small mean body mass changes across the two studies (men/women; −0.5/−0.5 kg) reflecting losses of adipose tissue (−1.5/−2.7 kg) and gains in skeletal muscle mass (0.4/ 1.1 kg).
These two study design approaches differ to the extent that dietary intake was strictly controlled even though both included supervised exercise programs. Although many exercise studies request subjects to remain at baseline intake levels or make no recommendations on the amount of food to be eaten, exercise without tightly controlling baseline intake as in the confined and supervised study of Bouchard et al. 56 often elicits a spontaneous and concomitant response in food intake 59–61. Because free-living exercise studies that do not clamp baseline intake or prescribe a dietary restriction most closely reflect exercise interventions performed in ordinary life, understanding changes in FFM within these studies is critical.
A good example of such a study is INFLAME 62 that included a 4-month carefully supervised exercise program (16 KKW in 3–5 sessions/wk) but that included no dietary guidance or monitoring of calorie intake as in the study of Ross et al. 57, 58 Men and women (n=162; X±SD: age, 49.7±10.9 yrs.; BMI, 31.8±4.0 kg/m2) were randomized into non-exercise control or supervised exercise groups. There were no significant differences between exercisers and controls for median (IQR) changes in weight (−0.6 [−1.2, 0] kg vs. 0.1 [−0.5, 0.7] kg) or FFM (−0.06 [−0.4, 0.3] kg vs. −0.04 [−0.14, 0.3] kg) while the exercise group had a small but significant (p=0.02) reduction in fat mass compared to the control group (−0.6 [−1, −0.1] vs. 0.2 [−0.3, 0.6] kg).
Several other well-done meta-analyses and reviews reflect this limited control of food intake over the course of an exercise-body composition study. Garrow and Summerbell 11 in their meta-analysis included evaluation of 28 publications with 425 sedentary and 491 actively exercising subjects. Aerobic exercise “without” dietary restriction led to a 2.6 kg mean weight loss with no change in FFM over 31 weeks in overweight men compared to 0.4 kg weight and 0.3 kg FFM gains in sedentary controls. Overweight women who exercised lost 1.4 kg in 12 weeks compared to sedentary controls with neither group showing any change in FFM. Garrow and Summerbell found that resistance training increased FFM by ~2 kg in overweight men and ~1 kg in women even though weight remained unchanged.
Forbes wrote three influential reviews relating exercise to changes in body composition63–65. His analyses included studies in both animals and humans and Forbes concluded that it was possible to gain lean mass during exercise if energy intake increased. These insights were combined recently to develop a Forbes-like curve specific to exercise which reflect quantifications of lean loss during exercise if intake is maintained and lean gain if intake is increased 61.
While these collective non-diet exercise studies varied in design and experimental rigor, we see that commonalities exist across all three approaches: most reported reductions in body fat mass with no or small increases in lean tissues (i.e., FFM and skeletal muscle mass).
With Diet
Does exercise when combined with a low calorie diet reduce the fraction of weight loss as FFM compared to that of diet alone?
To ideally answer this question a study needs to include two groups matched on the degree of negative energy balance, one treated with diet alone and the other with exercise alone or diet and exercise combined. Three studies with this design serve as good examples, the first of which is the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE I) study protocol reported by Redman et al. 66 that tested the effects of a diet alone (25% energy deficit; n=12) or diet plus aerobic exercise (each 12.5% energy deficit; n=12) for six months in overweight men and women. Subjects assigned to the exercise group participated in a structured exercise program (treadmill, stationary cycle, or stair stepper) 5 days/week adjusted to maintain a total daily energy deficit of 25%. The authors confirmed that both groups similarly approached the energy deficit goal across the six-month study period using the intake-balance method 67. By month six weights had declined by (X±SD) 10.4±0.9% for the diet alone group and by 10.1±0.9% for diet plus exercise group (p=NS), about 8.3 kg for both groups. There were no between-group significant differences in changes from baseline in total body fat or FFM as measured by DXA.
The experiments by Ross et al. 57, 58 mentioned earlier that included a supervised exercise program had two additional groups, one that was assigned to weight loss with a diet and the other to weight maintenance with aerobic exercise. The groups were matched for calorie deficit (men −700 kcal/d; women, −500 kcal/d). Body weight decreased by a mean of 7.8 kg in the diet-only group of men (n=14) compared to 7.5 kg in the exercise weight loss group (n=16). Men in the diet-only group lost 4.8 kg of adipose tissue compared to 6.1 kg in the exercise group (p<0.05). The men treated with diet alone lost 1.7 kg of skeletal muscle, more than that observed in the exercise weight loss group (1.3 kg), although the difference was not statistically significant. Women treated with diet alone (n= 15) reduced their weight by 6.5 kg, including 4.1 kg of adipose tissue and 0.5 kg of skeletal muscle. The exercise weight loss group had a weight loss of 5.9 kg, 6.7 kg of which was adipose tissue (p<0.05 vs. diet-only group) and none was skeletal muscle.
A large body of literature exists on the FFM changes observed in subjects managed with low calorie diets combined with exercise. Three meta-analyses serve as a good combined source of this information with some possible overlap.
Garrow and Summerbell in their 1995 meta-analysis 11 used regression analysis to explore the main determinants of FFM changes with dieting. Their results indicated that for a weight loss of 10 kg with dieting alone men and women lose 2.9 kg and 2.2 kg of FFM, respectively. When a low calorie diet was combined with exercise FFM losses were reduced to 1.7 kg in both the men and women.
The 2007 meta-analysis of Chaston et al. 12 included 26 overweight or obese cohorts with dietary and behavioral interventions that reported a mean weight loss >10 kg. The magnitude of caloric restriction was significantly (p=0.006) associated with the percent of weight loss as FFM (r2=0.31) with no additional variance explained by initial BMI, magnitude of weight loss, sex, or reported exercise. The ΔFFM/ΔW was greater (p=0.08) in men (X±SD; 27±7%) compared to women (20±7%).
Citing three randomized 16-week supervised low-calorie diet (−1000 kcal/d deficit) combined with exercise studies from the same research team68–70, Chaston et al. 12 pooled data from sedentary (n=42), aerobic exercise (n=41; 50–85% maximum heart rate, 15–60 min, 5-days/week), and resistance training (n=44; designed to increase strength by 30–45%) groups. The study arms were not matched for magnitude of negative energy balance. Compared to controls, both aerobic exercise and resistance training substantially reduced the magnitude of ΔFFM/ΔW from 0.27±0.06 to 0.13±0.04 and 0.17±0.14, respectively. Viewing results from all three studies, loss in weight was similar across the sedentary, aerobic exercise, and resistance training groups and ranged from 10–14 kg. Loss in adipose tissue mass was consistently greater in the groups with prescribed exercise than in the diet-only groups. Skeletal muscle losses in the sedentary diet-only group ranged from about 1–2 kg with losses reduced to one-half or less in the exercise groups.
Weinheimer and colleagues in their 2010 report examined FFM changes in 52 studies of dietary and behavioral interventions in overweight and obese middle-aged and older adults 13. Most of the evaluated exercise studies included aerobic activities with an observed reduction in FFM losses to about one-half that of diet-only groups, similar that observed by Chaston et al 12.
In a study of extreme weight loss in response to a high degree of calorie restriction and high dose of exercise, severely obese subject’s lean mass decreased horizontally across the Forbes Curve found by comparing actual group mean weight loss to predicted weight loss in a dynamic model utilizing the Forbes Curve 71, 72.
Viewed across all available studies and recognizing inherent design limitations, exercise added to a low-calorie diet program appears to have similar body composition effects to those observed in the non-diet exercise studies: increases in relative fat loss and no change or a reduction in FFM losses.
In Non-Obese Subjects
While our focus is on weight loss with dieting and exercise in subjects who are overweight or obese, lean subjects also lose weight under some relevant circumstances such as with the onset of anorexia nervosa, voluntary calorie restriction for promotion of longevity 73, and during military field operations 2, 74.
An example is the intense activity levels combined with extreme energy restriction as part of United States Army Ranger training 2, 74. Friedl reports a ΔFFM/ΔW of 0.40 over 8 weeks with a 10 kg weight loss in 50 normal weight men (baseline fat, 15%)2. A significant (p<0.001) positive correlation (n=105, R2=0.42) in these Ranger studies is observed between ΔFFM/ΔW and baseline %fat 74, revealing that greater relative FFM loss is experienced by the leaner men. This observation confirms the similar finding of Keys et al. 17 of a significant positive correlation between ΔFFM/ΔW and baseline %fat in men after 12 weeks of a hypocaloric diet in the Minnesota Semi-Starvation Experiment (n=32, R2, 0.23, p<0.01).
Forbes in his reviews 63–65 also described how larger amounts of baseline body fat yielded less loss of FFM during exercise, while smaller amounts of body fat generated greater FFM losses.
An important proviso when considering weight loss in lean subjects is the development of edema. Recall that the Minnesota model 16 included three components, tissue of normal composition (N), obesity tissue (G), and surplus or deficit of extracellular fluid (H). Keys et al. measured H in the Minnesota Semi-Starvation Experiment 17 and the authors observed fat mass “replacement” by edema fluid (4–9 kg) as men progressively lost weight approaching 24 weeks of semi-starvation. Obviously any change in the “H” component of body weight will directly impact the ΔFFM/ΔW ratio that must therefore be interpreted cautiously with large weight losses in lean subjects.
STUDY DESIGN
The kinds of studies needed to isolate independent intervention effects on lean tissues are described throughout this review. Here we comment on two additional study design considerations that emerge from our literature review, sample size and selected body composition measurement methods.
Sample Size
A consistent finding across all reviewed studies is the relatively small changes in FFM observed with dieting and other intervening factors. Subjects in low calorie diet studies often weigh on average about 100 kg and typical reductions in FFM for six-month protocols range between 1 and 3 kg. An isocaloric exercise intervention might reduce FFM loss, for example, by one-half to 0.5 to 1.5 kg. Detection of such small differences in ΔFFM pose challenges to most available body composition methods75, 76 and demand close attention to measurement protocols and a priori sample size selection.
An example from the literature provides a frame of reference for establishing sample size. The CALERIE I Study of Redman et al. 66 mentioned earlier included two groups matched for level of calorie restriction (~25%), one prescribed diet alone (n=12) and the other diet combined with aerobic exercise (n=12). Body composition was evaluated over the 6-month protocol with DXA. Does the isocaloric replacement of diet with exercise prevent loss of FFM? To a priori test this hypothesis, how many subjects per group would be needed to detect a between-group difference in FFM of an empirically selected level of 0.5–1.0 kg? The six-month ΔFFM (X±SD) in the diet only and diet plus exercise groups was −2.14±0.86 and −1.09±0.93 kg (P=NS), respectively. Assuming a 2-sided test and a 0.05 level of significance, to achieve 80% power a study would need 31–120 subjects per group or 41–161 to reach 90% power (Table 2). These are relatively large samples compared to the prevailing literature and may explain why definitive answers to our questions posed earlier in the review are often lacking.
Table 2.
Study power estimates for detecting FFM differences in a representative 6-month diet-exercise study*.
| Power | Detectable FFM Δ (kg) | Subjects/Group |
|---|---|---|
| 80% (90%) | 1.0 | 31 (41) |
| 80% (90%) | 0.75 | 54 (72) |
| 80% (90%) | 0.5 | 120 (161) |
Assumes 2-sided test, 0.05 level of significance.
Body Composition Approach
Most studies reporting body composition changes in randomized trials apply practical and available methods for measuring FFM. By inference investigators often assume FFM represents the “healthy” functional tissue of the body, notably total body protein. The FFM compartment, however, includes all of body mass except fat and thus caution in interpretation is needed. Weight loss with voluntary dieting is accompanied by dynamic changes in glycogen stores, fluid balance, protein kinetics, and many other tissue compositional effects that are collectively described by measured FFM. Moreover, inactivity, aging, exercise, and metabolic stress each produce characteristic changes in these compartments that often differ from those of dieting. The net result is that ΔFFM as observed across weight loss promoted by diet versus that by exercise, for example, must be cautiously interpreted as the makeup of lean tissue changes in each group may be very different. Advances in body composition methodology provide a new opportunity to explore these kinds of effects beyond that of the traditional FFM compartment 75, 76.
CONCLUSIONS
Three clear conclusions emerge from our in-depth review: the widely cited Quarter FFM Rule is at best an approximation that has a limited mechanistic basis; the proportion of weight loss as lean tissue varies over time and is determined by multiple factors including level of energy intake, diet composition, sex, baseline adiposity, the presence of inactivity or type and level of added activity, and potentially the subject’s metabolic state or hormonal response (Figure 2); and additional appropriately powered and designed studies are needed to isolate some of these factors as independent ΔFFM/ΔW determinants. Approximations of ΔFFM/ΔW for the second weight loss phase (i.e., past the early rapid weight loss period) can now be made with the NHANES Thomas model 30 for subjects treated with low calorie diets and a preliminary model is also available for predicting ΔFFM/ΔW with addition of aerobic exercise 61. Hall’s dynamic system of equations 32 also advances Forbes’ simplified rule by modeling macronutrient balance and the resulting changes in body composition at the multi-component molecular-level, including organ mass changes. Our review provides a framework for exploring the lean tissue effects that accompany weight loss and creates a basis for designing future clinical studies aimed at furthering our understanding of voluntary calorie restriction.
Figure 2.
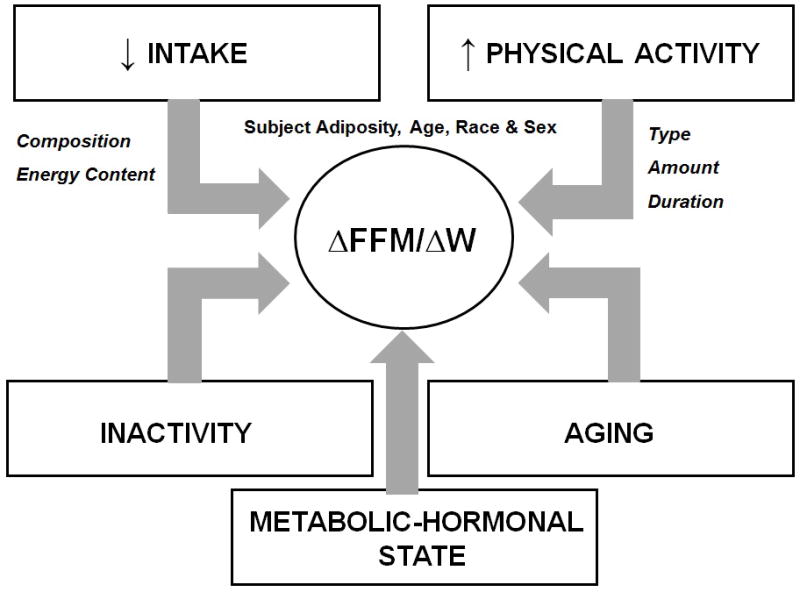
The main recognized or potential contributing factors that influence the fractional loss of FFM (ΔFFM/ΔW) with dieting.
Supplementary Material
Acknowledgments
DMT is supported by a Herman and Margret Sokol Institute for Pharmaceutical Life Sciences Fellowship and NIH grant R15DK090739. LMR is supported by NIH grants R00HD060762 and U01 DK094418.
Abbreviations
- ATFM
adipose-tissue free mass
- BMI
body mass index
- FFM
fat-free mass
- FFMI
fat-free mass index
- G
obesity tissue
- H
extracellular fluid
- Ht
height
- IQR
inter-quartile ratio
- LBM
lean body mass
- N
tissue of standard or normal composition
- NHANES
National Health and Nutrition Examination Survey
- W
weight
Footnotes
Potential Conflicts of Interest: None of the investigators report conflicts of interest for this study.
References
- 1.Phillips SM, Zemel MB. Effect of protein, dairy components and energy balance in optimizing body composition. Nestle Nutrition Institute workshop series. 2011;69:97–108. doi: 10.1159/000329288. discussion 08–13. [DOI] [PubMed] [Google Scholar]
- 2.Friedl K. Variability of fat and lean tissue loss during physical exertion with energy deficit. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 3.Hansen D, Dendale P, Berger J, van Loon LJ, Meeusen R. The effects of exercise training on fat-mass loss in obese patients during energy intake restriction. Sports Med. 2007;37:31–46. doi: 10.2165/00007256-200737010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Prado CM, Antoun S, Sawyer MB, Baracos VE. Two faces of drug therapy in cancer: drug-related lean tissue loss and its adverse consequences to survival and toxicity. Current opinion in clinical nutrition and metabolic care. 2011;14:250–4. doi: 10.1097/MCO.0b013e3283455d45. [DOI] [PubMed] [Google Scholar]
- 5.Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Current diabetes reviews. 2012;8:131–43. doi: 10.2174/157339912799424573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas DM, Martin CK, Lettieri S, et al. Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–37. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prentice AM, Goldberg GR, Jebb SA, Black AE, Murgatroyd PR, Diaz EO. Physiological responses to slimming. The Proceedings of the Nutrition Society. 1991;50:441–58. doi: 10.1079/pns19910055. [DOI] [PubMed] [Google Scholar]
- 9.Berkhan M. Intermittent Fasting For Weight Loss Preserves Muscle Mass? 2011. [Google Scholar]
- 10.Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:e593–601. doi: 10.1111/j.1467-789X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 11.Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. European journal of clinical nutrition. 1995;49:1–10. [PubMed] [Google Scholar]
- 12.Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–50. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 13.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutrition reviews. 2010;68:375–88. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 14.Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36:239–62. doi: 10.2165/00007256-200636030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Heymsfield S. Physical activity, weight loss, and maintenance of lean mass. 2. Champaign, IL: Human Kinetics; 2010. [Google Scholar]
- 16.Keys A, Brozek J. Body fat in adult man. Physiological reviews. 1953;33:245–325. doi: 10.1152/physrev.1953.33.3.245. [DOI] [PubMed] [Google Scholar]
- 17.University of Minnesota. Laboratory of Physiological Hygiene., Keys A. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 18.Grande F, Henschel A. Nutrition and energy balance in body composition studies. Washington, D.C: National Academy of Sciences-National Research Council; 1961. [Google Scholar]
- 19.Brozek J, Grande F, Anderson JT, Keys A. Densitometric Analysis of Body Composition: Revision of Some Quantitative Assumptions. Annals of the New York Academy of Sciences. 1963;110:113–40. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 20.Wishnofsky M. Caloric equivalents of gained or lost weight. Am J Clin Nutr. 1958;6:542–6. doi: 10.1093/ajcn/6.5.542. [DOI] [PubMed] [Google Scholar]
- 21.Bozenraad O. Ueber den Wassergehalt des menschlichen Fettgewebes unter verschiedenen bedingungen. Deutsch Arch f kiln Med. 1911;103:120–23. [Google Scholar]
- 22.Heymsfield SB, Gonzalez MC, Pereira AZ, Thomas DM. Time to correctly predict the amount of weight loss with dieting. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster JD, Hesp R, Garrow JS. The composition of excess weight in obese women estimated by body density, total body water and total body potassium. Human nutrition Clinical nutrition. 1984;38:299–306. [PubMed] [Google Scholar]
- 24.Brown MR, Klish WJ, Hollander J, Campbell MA, Forbes GB. A high protein, low calorie liquid diet in the treatment of very obese adolescents: long-term effect on lean body mass. Am J Clin Nutr. 1983;38:20–31. doi: 10.1093/ajcn/38.1.20. [DOI] [PubMed] [Google Scholar]
- 25.Forbes GB, Gallup J, Hursh JB. Estimation of total body fat from potassium-40 content. Science. 1961;133:101–2. [PubMed] [Google Scholar]
- 26.Wang ZM, Pierson RN, Jr, Heymsfield SB. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr. 1992;56:19–28. doi: 10.1093/ajcn/56.1.19. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Pi-Sunyer FX, Kotler DP, Wang J, Pierson RN, Jr, Heymsfield SB. Magnitude and variation of ratio of total body potassium to fat-free mass: a cellular level modeling study. American journal of physiology Endocrinology and metabolism. 2001;281:E1–7. doi: 10.1152/ajpendo.2001.281.1.E1. [DOI] [PubMed] [Google Scholar]
- 28.Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. The British journal of nutrition. 1986;56:1–9. doi: 10.1079/bjn19860080. [DOI] [PubMed] [Google Scholar]
- 29.Forbes GB. Lean body mass-body fat interrelationships in humans. Nutrition reviews. 1987;45:225–31. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas D, Das SK, Levine JA, et al. New fat free mass - fat mass model for use in physiological energy balance equations. Nutr Metab (Lond) 2010;7:39. doi: 10.1186/1743-7075-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall KD. Body fat and fat-free mass inter-relationships: Forbes’s theory revisited. The British journal of nutrition. 2007;97:1059–63. doi: 10.1017/S0007114507691946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. American journal of physiology Endocrinology and metabolism. 2010;298:E449–66. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas DM, Martin CK, Heymsfield SB, Redman LM, Schoeller DA, Levine JA. A simple model predicting individual weight change in humans. Journal of Biological Dynamics. 2011;5:579–99. doi: 10.1080/17513758.2010.508541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr. 2010;92:1326–31. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva AM, Shen W, Heo M, et al. Ethnicity-related skeletal muscle differences across the lifespan. American journal of human biology : the official journal of the Human Biology Council. 2010;22:76–82. doi: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones A, Jr, Shen W, St-Onge MP, et al. Body-composition differences between African American and white women: relation to resting energy requirements. Am J Clin Nutr. 2004;79:780–6. doi: 10.1093/ajcn/79.5.780. [DOI] [PubMed] [Google Scholar]
- 37.Allison DB, Nathan JS, Albu JB, Heymsfield SB, Duprat LJ, Pi-Sunyer FX. Measurement challenges and other practical concerns when studying massively obese individuals. The International journal of eating disorders. 1998;24:275–84. doi: 10.1002/(sici)1098-108x(199811)24:3<275::aid-eat5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.Bell JD, Margen S, Calloway DH. Ketosis, weight loss, uric acid, and nitrogen balance in obese women fed single nutrients at low caloric levels. Metabolism: clinical and experimental. 1969;18:193–208. doi: 10.1016/0026-0495(69)90039-0. [DOI] [PubMed] [Google Scholar]
- 39.van den Oever IA, van Sijl AM, Nurmohamed MT. Management of cardiovascular risk in patients with rheumatoid arthritis: evidence and expert opinion. Therapeutic advances in musculoskeletal disease. 2013;5:166–81. doi: 10.1177/1759720X13491025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruberg RL. Protein and energy requirements of the hospitalized patient. The Ohio State medical journal. 1981;77:382–4. [PubMed] [Google Scholar]
- 41.Van Loan MD, Strawford A, Jacob M, Hellerstein M. Monitoring changes in fat-free mass in HIV-positive men with hypotestosteronemia and AIDS wasting syndrome treated with gonadal hormone replacement therapy. AIDS. 1999;13:241–8. doi: 10.1097/00002030-199902040-00012. [DOI] [PubMed] [Google Scholar]
- 42.Hoffer LJ. Clinical nutrition: 1. Protein-energy malnutrition in the inpatient. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2001;165:1345–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Schulman RC, Mechanick JI. Metabolic and nutrition support in the chronic critical illness syndrome. Respiratory care. 2012;57:958–77. doi: 10.4187/respcare.01620. discussion 77–8. [DOI] [PubMed] [Google Scholar]
- 44.Pingleton SK. Nutrition in chronic critical illness. Clinics in chest medicine. 2001;22:149–63. doi: 10.1016/s0272-5231(05)70031-9. [DOI] [PubMed] [Google Scholar]
- 45.Alley DE, Koster A, Mackey D, Cawthon P, et al. Hospitalization and change in body composition and strength in a population-based cohort of older persons. Journal of the American Geriatrics Society. 2010;58:2085–91. doi: 10.1111/j.1532-5415.2010.03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang MU, Van Itallie TB. Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. The Journal of clinical investigation. 1976;58:722–30. doi: 10.1172/JCI108519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heymsfield SB, Thomas D, Nguyen AM, et al. Voluntary weight loss: systematic review of early phase body composition changes. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:e348–61. doi: 10.1111/j.1467-789X.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 48.Benedict FG. An Experiment on a Fasting Man. Science. 1912;35:865. doi: 10.1126/science.35.909.865. [DOI] [PubMed] [Google Scholar]
- 49.Forbes GB. Weight loss during fasting: implications for the obese. Am J Clin Nutr. 1970;23:1212–9. doi: 10.1093/ajcn/23.9.1212. [DOI] [PubMed] [Google Scholar]
- 50.Forbes GB, Drenick EJ. Loss of body nitrogen on fasting. Am J Clin Nutr. 1979;32:1570–4. doi: 10.1093/ajcn/32.8.1570. [DOI] [PubMed] [Google Scholar]
- 51.Hall KD, Butte NF, Swinburn BA, Chow CC. Quantifying the Dynamics of Childhood Growth and Obesity. Lancet Diabetes Endocrinol. 2013;1:97–105. doi: 10.1016/s2213-8587(13)70051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forbes GB. Longitudinal changes in adult fat-free mass: influence of body weight. Am J Clin Nutr. 1999;70:1025–31. doi: 10.1093/ajcn/70.6.1025. [DOI] [PubMed] [Google Scholar]
- 53.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 54.Krasnoff J, Painter P. The physiological consequences of bed rest and inactivity. Advances in renal replacement therapy. 1999;6:124–32. doi: 10.1016/s1073-4449(99)70030-0. [DOI] [PubMed] [Google Scholar]
- 55.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol. 1992;73:2172–8. doi: 10.1152/jappl.1992.73.5.2172. [DOI] [PubMed] [Google Scholar]
- 56.Bouchard C, Tremblay A, Despres JP, et al. The response to exercise with constant energy intake in identical twins. Obes Res. 1994;2:400–10. doi: 10.1002/j.1550-8528.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 57.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 58.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–98. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 59.Martins C, Kulseng B, Rehfeld JF, King NA, Blundell JE. Impact of Chronic Exercise on Appetite Control in Overweight and Obese Individuals. Med Sci Sports Exerc. 2012 doi: 10.1249/MSS.0b013e31827d1618. [DOI] [PubMed] [Google Scholar]
- 60.King NA, Caudwell P, Hopkins M, et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity (Silver Spring) 2007;15:1373–83. doi: 10.1038/oby.2007.164. [DOI] [PubMed] [Google Scholar]
- 61.Thomas DM, Bouchard C, Church T, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012 doi: 10.1111/j.1467-789X.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Church TS, Earnest CP, Thompson AM, et al. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc. 2010;42:708–16. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forbes GB. Exercise and lean weight: the influence of body weight. Nutrition reviews. 1992;50:157–61. doi: 10.1111/j.1753-4887.1992.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 64.Forbes GB. Exercise and body composition. J Appl Physiol. 1991;70:994–7. doi: 10.1152/jappl.1991.70.3.994. [DOI] [PubMed] [Google Scholar]
- 65.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Annals of the New York Academy of Sciences. 2000;904:359–65. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- 66.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. The Journal of clinical endocrinology and metabolism. 2007;92:865–72. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Racette SB, Das SK, Bhapkar M, et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. American journal of physiology Endocrinology and metabolism. 2012;302:E441–8. doi: 10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janssen I, Ross R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes Relat Metab Disord. 1999;23:1035–46. doi: 10.1038/sj.ijo.0801038. [DOI] [PubMed] [Google Scholar]
- 69.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–8. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 70.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–91. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 71.Johannsen DL, Knuth ND, Huizenga R, et al. Metabolic slowing with massive weight loss despite preservation of fat-free mass. The Journal of clinical endocrinology and metabolism. 2012;97:2489–96. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall KD. Diet versus exercise in “the biggest loser” weight loss competition. Obesity(Silver Spring) 2013;21:957–9. doi: 10.1002/oby.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Redman LM, Huffman KM, Landerman LR, et al. Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. The Journal of clinical endocrinology and metabolism. 2011;96:E312–21. doi: 10.1210/jc.2010-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoyt RW, Friedl KE. Field studies of exercise and food deprivation. Current opinion in clinical nutrition and metabolic care. 2006;9:685–90. doi: 10.1097/01.mco.0000247472.72155.7c. [DOI] [PubMed] [Google Scholar]
- 75.Galgani JE, Smith SR, Ravussin E. Assessment of EchoMRI-AH versus dual-energy X-ray absorptiometry to measure human body composition. Int J Obes (Lond) 2011;35:1241–6. doi: 10.1038/ijo.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baracos V, Caserotti P, Earthman CP, et al. Advances in the science and application of body composition measurement. JPEN J Parenter Enteral Nutr. 2012;36:96–107. doi: 10.1177/0148607111417448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


