Abstract
Propofol is a potent intravenous anesthetic agent that rapidly induces sedation and unconsciousness. The potential for propofol dependency, recreational use and abuse has only recently been recognized and several cases of accidental overdose and suicide have emerged. In addition, the first documented case of murder using propofol was reported a few months ago and a high profile case of suspected homicide with propofol is currently under investigation. A number of analytical methods have been employed to detect and quantify propofol concentrations in biological specimens. The reported propofol related deaths and post-mortem blood and tissue levels are reviewed. Importantly, limitations of propofol detection are discussed and future considerations are presented. Because propofol has the potential for diversion with lethal consequences, the forensic scientist must have a basic understanding of its clinical indications and uses, pharmacologic properties, and detection methods. In addition, medical institutions should develop systems to prevent and detect diversion of this potential drug of abuse.
Keywords: forensic, science, propofol, lethal, death, suicide, murder
Propofol is a potent intravenous anesthetic agent that rapidly induces sedation, hypnosis, and unconsciousness (1, 2). Since 1986, it has been widely used in clinical settings to induce and maintain general anesthesia and to provide procedural sedation (2, 3). The potential for propofol dependency, recreational use, and abuse, however, has only recently been recognized and several cases of accidental overdose and suicide have emerged (3). In addition, the first documented case of murder using propofol was reported a few months ago and a high profile case of suspected homicide with propofol is currently being investigated (3, 4). Because of the potential for diversion with lethal consequences, a basic understanding of the clinical indications and uses, pharmacologic properties, and detection methods of propofol are necessary for forensic scientists.
Clinical Indications
Propofol is commonly used in hospital settings around the world as an intravenous anesthetic and sedative agent (2). In 2000, it was the preferred anesthetic induction agent in 96.5% of ambulatory urologic and orthopedic surgical cases in the United Kingdom (5). In a French survey, anesthesiologists used propofol in 50% of patients undergoing cardiac surgery (5).
The drug is administered as a single injection to induce general anesthesia or as a continuous infusion to maintain anesthesia (6). It can also be administered by intermittent bolus injection to target a desired level of sedation (6). Because it induces unconsciousness rapidly, is easily titrated, and results in rapid awakening following administration, propofol has become widely accepted for use in anesthesia (2). In addition, it is routinely used to sedate critically ill patients in the intensive care unit (ICU) (2).
Importantly, propofol is not a controlled substance scheduled by the U.S. Drug Enforcement Agency (7). However, many hospitals restrict its use to specific areas such as anesthetizing locations, ICUs, and the emergency department and limit those capable of administering it to qualified personnel such as anesthesiologists and critical care physicians. In addition to being clinically indicated for anesthesia and sedation, propofol has also been used to treat refractory seizures (status epilepticus), refractory migraine and tension headaches, severe alcohol withdrawal and delirium tremens, and has been used to facilitate rapid opiate detoxification (1, 6).
Pharmacologic Properties
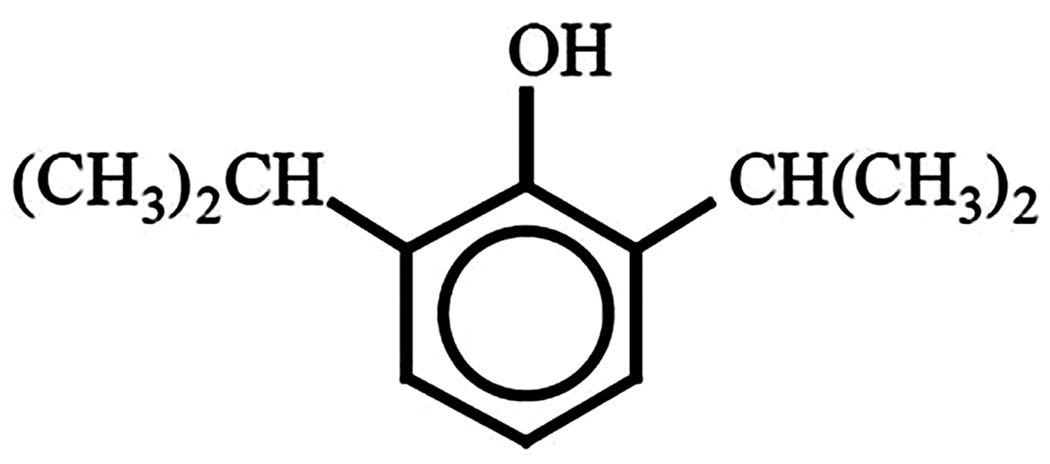
Developed in the 1970s, propofol is structurally distinct from other anesthetic agents (Figure 1) (1, 7). Propofol, or 2, 6-diisopropylphenol, is insoluble in water and the current formulation is an emulsion containing soybean oil, glycerol, and egg lecithin (1). This lipid formulation gives the drug its characteristic and unique white color. In addition, one of two preservatives, either edetate disodium (EDTA) or sodium metabisulfite, is added to inhibit bacterial and fungal growth (1). Preparations containing EDTA are relatively basic (pH 7–8.8) while those containing sulfite are relatively acidic (pH 4.5–6.4) (1). In addition, propofol is currently supplied in two different concentrations (10 mg/mL or 20 mg/mL) (1).
Figure 1.
Structural formula of propofol.
Pharmacodynamics
Propofol acts by depressing the central nervous system (1). It has multiple mechanisms of action involving both pre- and post-synaptic cellular and molecular targets (1). The principle mechanism involves facilitation of inhibitory transmission through post-synaptic activation of the GABAA (gamma-aminobutyric acid) receptor-chloride complex (1). Propofol also inhibits excitatory transmission by modulating the NMDA (N-methyl-D-aspartic acid) subtype of glutamate receptor and slow calcium channels and inhibits voltage-gated sodium channels (1).
Propofol induces hypnosis rapidly. The onset of unconsciousness peaks at 100 seconds following a 2.5 mg/kg injection and lasts for 5–10 minutes (1). It reduces respiratory drive, blunts protective airway reflexes, and can reduce upper airway muscular tone resulting in airway obstruction (1). Without proper management by an experienced provider, a single injection of propofol can result in apnea, respiratory arrest, hypoxia, and death (1). Furthermore, injection of 2.5 mg/kg causes vasodilation that results in a 25–40% reduction in systolic, mean, and diastolic blood pressure (1). This decrease in blood pressure is due to diminished sympathetic vascular tone, effects on vascular smooth muscle calcium flux, and endothelial nitric oxide release and is more pronounced in dehydrated and hypovolemic patients (1). These potent pharmacodynamic effects on the cardiovascular and respiratory systems make propofol potentially dangerous and lethal when injected by inexperienced personnel or self administered.
Pharmacokinetics
Propofol pharmacokinetics are described by a three-compartment pharmacokinetic model: the central compartment (plasma), the rapid-distribution compartment, and the slow-distribution compartment (deep compartment with limited perfusion) (1). Following injection, it is rapidly redistributed and eliminated (1). The initial half-life is 8 minutes, the redistribution half-life is between 30 and 70 minutes, and the elimination half-life is up to 23 hours (1). The longer elimination half-life is due to slow return of propofol from the deep compartment and does not contribute significantly to the clinical effect (1).
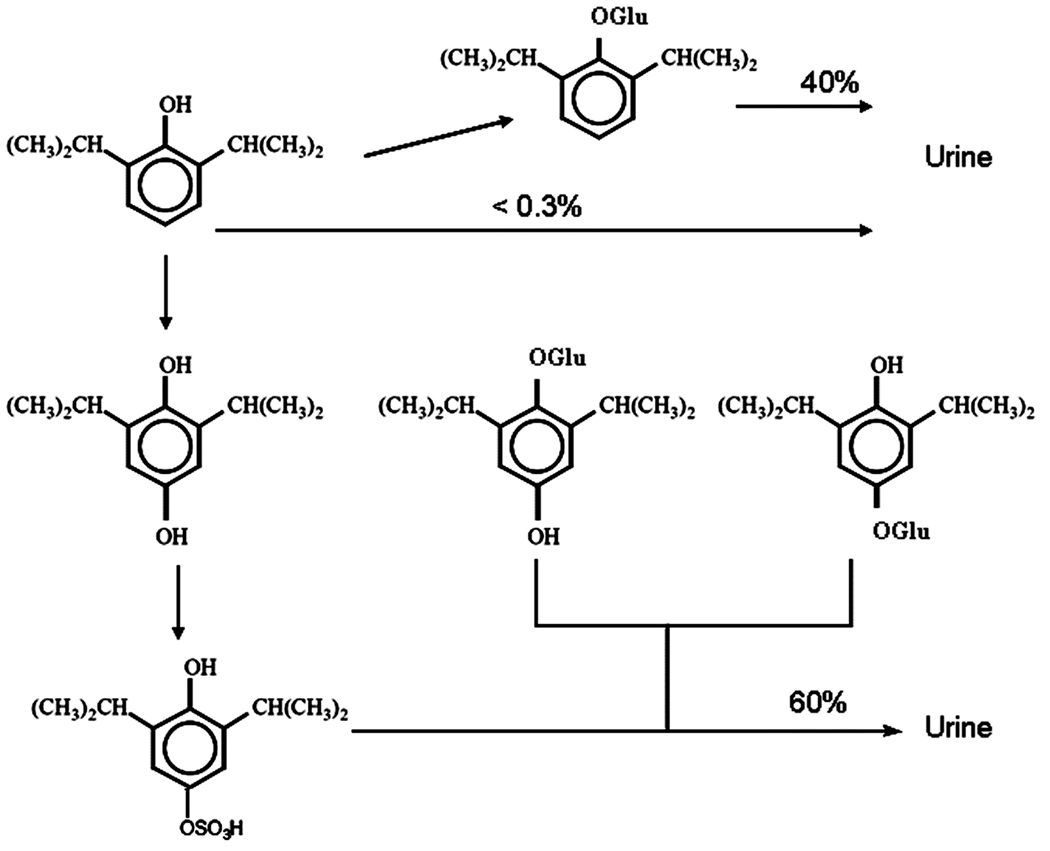
Propofol is rapidly conjugated in the liver to glucuronide and sulfate and excreted as inactive compounds by the kidneys (Figure 2) (1). Less than 1% is excreted as unchanged drug in the urine and 2% is excreted in feces (1). Thus, propofol is cleared rapidly following injection and does not accumulate in the body even in patients with renal or hepatic disease (1).
Figure 2.
Metabolism of propofol. Less than 1% of propofol is excreted unchanged in the urine. Forty percent of propofol is rapidly conjugated by the liver to glucuronide (Glu) and excreted by the kidneys. Alternatively, 60% of propofol is hydroxylated (OH), subsequently conjugated to glucuronide (Glu) or sulfate (OSO3), and then excreted in the urine.
Adverse Effects
When administered by experienced and qualified clinicians, propofol exhibits an attractive safety profile. Despite this, a few severe and potentially life-threatening complications have been associated with its use. These include bacteremia and sepsis, hypertriglyceridemia and pancreatitis, and propofol-infusion syndrome (1).
The lipid emulsion is a medium that supports the growth of bacteria and other microbes at room temperature. Several outbreaks of bacteremia and post-operative infections have been reported following administration of contaminated propofol (1). A variety of organisms were identified to be causative agents and included Staphylococcus aureus, Candida albicans, Moraxella osloensis, Enterobacter agglomerans, and Serratia marcescens (1, 8). Subsequent to these outbreaks, the FDA recommended the addition of a bacteriostatic preservative to the propofol formulation and suggested that practitioners adhere to strict aseptic technique when handling the drug (1, 8). No further outbreaks were reported following addition of the preservative (1).
Hypertriglyceridemia (defined as > 500 mg/dL) is another potential side effect of propofol (1). The lipid emulsion contains 0.1 g of fat per milliliter and can result in hyperlipidemia when infused for greater than 72 hours (as is common in certain ICU settings) (1). The resultant increase in serum triglycerides can lead to pancreatitis (the potentially lethal inflammation of the pancreas) (1). There is a lower risk of hypertriglyceridemia, however, with the higher concentration formulation (20 mg/mL) (1). Pancreatitis has also been reported following a single injection of propofol without change in triglyceride levels (1).
Propofol-infusion syndrome (PRIS) is characterized by myocardial failure, severe metabolic acidosis, bradyarrhythmias, rhabdomyolysis, acute renal failure, hypotension, dyslipidemias, and cardiac arrest and is associated with a mortality rate of > 80% (1, 9). PRIS was first described in 1992 in a cohort of 5 critically ill children sedated with propofol via infusion for > 48 hours (10). Since then, 55 cases have been reported, involving both adults and children (9). Risk factors for PRIS include younger age, presence of airway infection, severe head trauma, high dose propofol infusion (> 5 mg/kg/hr) for > 48 hours, and concomitant use of high dose corticosteroids and vasopressor agents (1, 11). Although PRIS results in cytolysis of skeletal and cardiac myocytes, the underlying etiology remains unknown (1, 11). Recent work, however, suggests that propofol may interfere with mitochondrial function by impairing fatty acid oxidation and interrupting oxidative phosphorylation via a nitric oxide mediated mechanism (1, 11). Because PRIS is recognized as a potential risk of propofol sedation, the most common approach is prevention. This is achieved by avoiding propofol sedation for children in the ICU less than 16 years of age and limiting the dose and duration of infusion in critically ill adults (1, 11). Furthermore, monitoring serum pH, triglyceride, and creatine kinase levels has been advocated (1).
Abuse Potential
Evidence for Abuse
Drug abuse is defined as: “the use of a psychoactive substance in a manner detrimental to the individual or society but not meeting criteria for substance or drug dependence” (12). Prior to 1992, the abuse potential of propofol was completely unappreciated (3). Since then, 38 cases of propofol abuse occurring between 1992 and 2007 have been published (3). Propofol has increasingly become a drug of abuse largely because it is easily accessible (not a controlled substance), its onset of action is rapid following injection, and the duration of action is ultra-short without long term side effects (3). These properties make detection of the abuser quite difficult.
Medical professionals and healthcare workers represent the largest cohort of known propofol abusers (3). Of these personnel, anesthesiologists and nurse anesthetists are the most common offenders with the current incidence of abuse approximating 10 cases per 10,000 anesthesia providers per decade (1, 3, 13). The most likely rationale for this is familiarity with the drug and unregulated access to it. Of concern, recent studies suggest that propofol diversion and abuse are on the rise (13).
The majority of cases of propofol abuse involve use of the drug for recreational purposes, stress relief, and to alleviate insomnia (5). In one report, propofol dependency was described in a lay person following prescription for the treatment of tension headaches (6). In the cases of known propofol abuse, the average age was 30.2 +/− 6.4 years with most of the abusers being male (5). In many cases, propofol was used in addition to other drugs of abuse and the user had a history of drug dependency (5).
Physical dependence has not been described in propofol abusers, however, psychological dependence is quite common due to the associated euphoria, stress and tension relief, sexual fantasies and dreams, and sexual disinhibition experienced following injection and upon awakening (3, 5). These effects of propofol lead to drug craving and loss of control over the amount and frequency of drug injected as well as continued use of propofol despite adverse consequences, thus, meeting the definition of psychological dependence (5, 14). Chronic propofol abuse can result in tolerance and repeated injections exceeding 100 times per day have been reported (15).
All drugs of abuse increase dopaminergic activity in the mesocorticolimbic reward circuit within the brain (5). Anesthetic and subanesthetic doses of propofol have been shown to increase extracellular dopamine concentration in the main component of the reward circuit (5). Such an effect may underlie the abuse potential of propofol (5). Interestingly, propofol shares similarities with ketamine, another intravenous anesthetic agent with abuse potential (5). Both drugs inhibit NMDA receptors in the brain in a similar manner and both are known to cause visual hallucinations (5).
The potential for propofol abuse has been studied using animal models. Rats demonstrate a conditioned preference for propofol compared to control vehicle by spending more time in a drug-paired compartment (16, 17). This so-called place preference has been shown with both subanesthetic and anesthetic doses (16, 17). Propofol-induced place preference in these studies was likely due to contextual cues associated with administration and suggests a reward effect (5).
Reinforcing effects of propofol, as assessed by the potential for self-administration, have also been evaluated with animal models. Reinforcement is the ability of a stimulus to increase the frequency of certain behaviors (5). Although studies in mice did not yield evidence for reinforcement, longer duration self-administration studies in rats and baboons demonstrated reinforcing effects at subanesthetic doses (18, 19).
The reward potential of propofol has also been evaluated in human studies. Discrete-trial choice procedure has been used to assess the potential for positive reinforcement of well known drugs of abuse such as alcohol, amphetamines, and marijuana (5). In a clinical trial, 12 healthy volunteers were assessed using discrete-trial choice procedure for a subanesthetic dose of propofol (20). Each person received two blinded injections of propofol (0.6 mg/kg) and soy-based lipid emulsion control (20). In the next sampling sessions, subjects chose the drug they wished to receive. Fifty-percent of volunteers chose propofol based on pleasant subjective effects suggesting that in certain people, propofol may have reward potential (20).
Detecting Diversion
Diversion is defined as the transfer of a controlled substance from a lawful to an unlawful channel of distribution or use (21). Because propofol is not a controlled substance, access to it is often unregulated or minimally restricted in hospitals and veterinary care centers (7). Potential areas where the drug can be diverted within the hospital include anesthetizing locations, ICUs, hospital pharmacy stock, and the emergency department. Most hospitals do not control or monitor clinical propofol use and, in one study, 71% of university hospital-based pharmacies did not secure or account for it as is done with other potential drugs of abuse (13, 22). Such lax systems enhance the potential for diversion.
Since the potential for propofol diversion and abuse is now being recognized around the world, many hospital-based pharmacies and anesthesia departments are establishing systems to prevent diversion (7, 22). Recently, The University of Iowa Hospitals and Clinics (UIHC) identified a case of propofol diversion and abuse by an ICU employee (7). In response, the pharmaceutical care department at UIHC changed the status of propofol to that of a controlled substance and established locked storage locations for the drug (7). In addition, UIHC utilizes Pyxis MedStation Systems (CareFusion Corp.). This technology allows the pharmacist to define user access privileges, employs unique user identification and passwords, and can utilize a fingerprint identification system for access. Furthermore, this automated medication dispensing system assists the pharmacy in accounting for numbers of drug vials and keeps an electronic record of all user-specific transactions.
Other institutions have focused on detection of drug diversion. Some have advocated for comparison of automated medication dispensing system or pharmacy records with the written or electronic anesthesia record to detect discrepancies (22). Unfortunately, many of these discrepancies are due to errors in documentation of dosage administered and drug wasted (22). Another approach, however, involves detecting and analyzing atypical drug transactions (22). Specifically, this method focuses on the frequency, timing, and location of pharmaceutical transactions (22). Frequent removal of a drug an hour or more after the end of a procedure and accessing a drug in an anesthetizing location different from the location of a procedure have been found to be consistent with diversion (22).
Although few hospitals currently attempt to prevent or detect propofol diversion, many pharmacists and anesthesiologists now recognize the risk of propofol abuse and are calling for tighter regulation of the drug. Some have requested that propofol be designated as a controlled substance by the FDA and others have even recommended routine propofol drug testing in suspected or high risk individuals (13).
Methods of Detection
Propofol can be detected in whole blood, plasma, serum, tissues, urine, breast milk, hair, and exhaled breath (23–30). A variety of analytical methods have been developed, tested, and reported for the detection and quantification of propofol in biological samples (25). The most generally accepted and commonly used techniques include high-performance liquid chromatography (HPLC) and gas chromatography (GC) (24, 25).
HPLC with ultraviolet (UV), fluorescence, or electrochemical detection are commonly used to quantify propofol in whole blood and plasma (25, 31, 32). Sample preparation for HPLC and GC requires liquid-liquid extraction or solid-phase extraction (31). Liquid-liquid extraction is tedious and time consuming and sensitivity may be affected by interference with plasma constituents (31). HPLC with UV detection is not very sensitive for analysis of low levels of propofol in blood, however solid-phase extraction has been shown to amplify its sensitivity (25). Recently, a HPLC method coupled with tandem mass spectrometry (MS) detection has been developed and validated for propofol detection in plasma (32).
GC with flame ionization detection, MS detection, capillary GC, and head space GC with solid-phase microextraction have also been utilized for propofol quantification in blood and plasma (24, 31). GC/MS and head space GC with solid-phase microextraction have been employed for propofol detection in exhaled breath (30, 33).
Propofol Releated Deaths
Propofol abuse and recreational use often lead to death because of the rapid onset of unconsciousness and apnea following injection (3). Thirty-seven percent of the 38 published cases of abuse between 1992 and 2007 were fatalities (3). The majority of these propofol related deaths were the result of accidental overdose or intentional suicide. Recently, however, the first case of first degree murder with the use of propofol was reported (3). Of interest, blood levels of propofol in the majority of propofol related deaths were within or below the therapeutic range (1.3 – 6.8 µg/mL), indicating that the mechanism of death was likely due to respiratory depression with subsequent hypoxia (3, 34). It is important to also recognize that peri-mortem and post-mortem metabolism and redistribution may occur (35). Thus, blood and tissue toxicological analysis must be interpreted carefully in these cases (3). Furthermore, as with other drugs of abuse, a specific “lethal level” may not be necessary to conclude that death was caused by propofol injection. Table 1 summarizes the post-mortem blood levels of propofol in the reported cases of propofol related deaths.
TABLE 1.
Post-mortem blood levels of propofol in related deaths.
| Propofol Related Deaths | Blood Level of Propofol (µg/mL) |
|---|---|
| Accidental Deaths | |
| 26 year old male ICU nurse, known propofol abuser | 5.3 |
| 27 year old male nurse anesthetist, chronic propofol abuser | 0.026 |
| 44 year old female nurse anesthetist, chronic propofol abuser | 0.039 |
| 21 year old male layperson, propofol abuser | 0.071 |
| 38 year old female anesthesiologist, known propofol abuser | 2.4 |
| Suicides | |
| 29 year old female radiologist | 0.22 |
| 37 year old male physician | 2.5 |
| Homicide | |
| 24 year old female | 4.3 |
Accidental Deaths
A 26 year old male ICU nurse who was a known propofol abuser was found dead in his apartment surrounded by several empty or partially empty propofol vials and two syringes (36). His autopsy demonstrated several fresh and partially scarred needle marks on his arms, wrists, and hands. He had aspirated stomach contents and had a fatty liver (36). Propofol was detected in hair (1.05 – 3.5 µg/g), blood (5.3 µg/mL), urine (5.4 µg/mL), brain (7.6–8.1 µg/g), and liver (27 µg/g) by GC/MS (36).
A 27 year old male nurse anesthetist was found dead at home with three empty propofol vials next to him and unused vials were found in his car (37). Several needle marks in his skin suggesting chronic abuse were noted and postmortem examination demonstrated pulmonary hemorrhage and pancreatitis (37). Propofol was detected in his blood (0.026 µg/mL), bile (0.25 µg/mL), and urine by GC/MS (37).
A 44 year old female nurse anesthetist was found dead at home with an empty vial of midazolam and a syringe near her body (29). Toxicological analysis revealed propofol (0.039 µg/mL), midazolam, and ethanol in her blood (29). Segmental analysis of a 6 cm-long strand of hair demonstrated propofol and midazolam in each 2 cm-long segment indicating repetitive abuse for 6 months prior to her death (29).
A 21 year old male layperson purchased propofol online using an internet bidding system (38). He was found to have an indwelling intravenous catheter placed to facilitate several daily injections (38). Propofol was detected in the blood (0.071 µg/mL) in the postmortem analysis (38).
A 38 year old known propofol abusing female anesthesiologist was found dead in the hospital dormitory kneeling on the floor facing downward with three empty vials of propofol and two empty used syringes next to her body (39). Autopsy demonstrated numerous needle marks with fresh and older hemorrhages in both arms, two intravenous catheters secured in each wrist, and pulmonary edema (39). Propofol was detected in the femoral blood (2.4 µg/mL) by HPLC (39).
Seven other accidental propofol related deaths have been reported in members of anesthesiology training programs (3). Six of the victims were anesthesiology residents and one was an anesthesia technician (3). All were propofol abusers (3).
Suicide
In the first published report of propofol related death, a 29 year old female radiologist committed suicide with a self-administered dose of propofol (40). Propofol was detected in femoral blood (0.22 µg/mL) and liver (1.4 µg/g) using HPLC (40).
A 37 year old male medical doctor committed suicide with propofol following a recent break up from an extra-marital affair (41). He was found dead on the bed of a rented room in the supine position (41). Two needles were inserted into the dorsum of his left hand and one inserted into his right hand and each was attached to intravenous fluid (41). Eight empty propofol vials were found at the scene (41). His body was moderately decomposed and autopsy finding were fairly unremarkable except for extensive endocrine system decomposition (41). Propofol was detected in femoral blood (2.5 µg/mL), liver (22 µg/g), kidney (3.6 µg/g), and brain (11.3 µg/g) using head space GC and GC/MS (41). It was calculated that he self-administered 1600 mg of propofol via infusion (41).
Murder
A 24 year old woman was found dead in her house in 2005 near Gainesville, Florida (3). Syringes, needles, and 2 empty propofol vials were found in grocery store bags on the ground outside of her house next to garbage cans (3). Autopsy demonstrated a pinpoint puncture wound in the left antecubital fossa with subcutaneous hemorrhage directly over a subcutaneous vein (3). Toxocological analysis detected propofol in the blood (4.3 µg/mL) (3). The medical examiner concluded that the woman’s death was a homicide caused by a lethal dose of propofol administered by a person skilled in intravenous injections (3).
Investigation of the propofol bottle lot numbers found at the crime scene indicated that the vials were obtained from a University of Florida hospital automated medication dispensing system that had been removed by a male ICU nurse (3). He had acquired the propofol vials approximately 5 to 6 days prior to the murder (3). Thorough questioning of the suspect’s former roommate revealed important details and a motive.
The suspect had been previously introduced to the victim, a University of Florida student, by the former roommate. The suspect soon became infatuated and obsessed with the victim. After learning that the victim became engaged to her boyfriend of 4 years, the suspect became enraged and planned to kill her.
Detectives learned that the victim suffered from chronic migraine headaches. She apparently trusted the suspect to relieve her symptoms. One week after her engagement, the suspect injected her with a lethal dose of propofol.
A few weeks after the incident, the suspect left the country and was subsequently apprehended in the West African Republic of Senegal (3). He was transported to the Alachua County Department of the Jail by the US Marshall’s Service and was ultimately tried and convicted of first degree murder (3). He was sentenced to life in prison without parole (3).
Limitations and Future Directions
In all of the reported cases of propofol related accidental death and suicide, vials of propofol along with syringes and needles were found next to or near the victims. In the homicide case, propofol vials, syringes, and needles were discovered outside of the house in a remote location from the body. Thus, investigators concluded that another person injected the drug and removed the paraphernalia from the crime scene (3). However, it is possible that a crime scene can be “staged”. Thus, finding empty vials along with needles and syringes next to or near the victim may not necessarily rule out homicide.
Certainly, a known history of propofol abuse and confirmatory hair analysis can aide investigators in identifying an accidental propofol related death. Furthermore, there are likely to be instances where propofol related deaths will be clear cases of suicide where the victim is causally responsible and intends that their actions result in their own death. However, ambivalent-life threatening behavior associated with psychological propofol dependence could result in non-accidental, non-intentional self-killing (42). Thus, determination of propofol related accidental versus suicidal death may challenge investigators.
Another limitation in the investigation of propofol related death is the toxicological analysis. In the majority of reported deaths, propofol levels in blood and tissue were within or below the therapeutic range. Thus, victims did not die from propofol toxicity, per se. Instead, examiners concluded that propofol induced respiratory depression, apnea, and hypoxia as a mechanism of death. So, detection of propofol in biological samples may be more important than the actual concentrations.
It is clear that in a very short period of time, propofol has become a drug for recreational use and abuse with the potential for psychological dependency. Because of the lethal and forensic aspects of propofol, medical institutions should focus their efforts on developing systems to prevent and detect diversion of this potential drug of abuse and forensic scientists should become familiar with these aspects. Furthermore, it is likely that propofol will soon become a controlled substance similar to other intravenous anesthetic agents with the potential for abuse.
Footnotes
Supported by NIH/NIGMS 5K08GM074117-04.
References
- 1.Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10:3639–3649. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 2.Abad-Santos F, Gálvez-Múgica MA, Santos MA, Novalbos J, Gallego-Sandín S, Méndez P, et al. Pharmacokinetics and pharmacodynamics of a single bolus of propofol 2% in healthy volunteers. J Clin Pharmacol. 2003;43:397–405. doi: 10.1177/0091270003251391. [DOI] [PubMed] [Google Scholar]
- 3.Kirby RR, Colaw JM, Douglas MM. Death from propofol: accident, suicide, or murder? Anesth Analg. 2009;108:1182–1184. doi: 10.1213/ane.0b013e318198d45e. [DOI] [PubMed] [Google Scholar]
- 4. [accessed December 16, 2009]. http://www.nytimes.com/2009/08/29/us/29jackson.html?_r=1.
- 5.Roussin A, Montastruc JL, Lapeyre-Mestre M. Pharmacological and clinical evidences on the potential for abuse and dependence of propofol: a review of the literature. Fundam Clin Pharmacol. 2007;21:459–466. doi: 10.1111/j.1472-8206.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- 6.Schneider U, Rada D, Rollnik JD, Passie T, Emrich HM. Propofol dependency after treatment of tension headache. Addict Biol. 2001;6:263–265. doi: 10.1080/13556210120056607. [DOI] [PubMed] [Google Scholar]
- 7.Weetman DB, Mascardo LA, Ross MB, Abramowitz PW. Propofol as a drug of diversion. Am J Health Syst Pharm. 2004;61:1185–1186. doi: 10.1093/ajhp/61.11.1185. [DOI] [PubMed] [Google Scholar]
- 8.Bennett SN, McNeil MM, Bland LA, Arduino MJ, Villarino ME, Perrotta DM, et al. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N Engl J Med. 1995;333:147–154. doi: 10.1056/NEJM199507203330303. [DOI] [PubMed] [Google Scholar]
- 9.Fong JJ, Sylvia L, Ruthazer R, Schumaker G, Kcomt M, Devlin JW. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008;36:2281–2287. doi: 10.1097/CCM.0b013e318180c1eb. [DOI] [PubMed] [Google Scholar]
- 10.Parke TJ, Stevens JE, Rice AS, Greenaway CL, Bray RJ, Smith PJ, et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992;305:613–616. doi: 10.1136/bmj.305.6854.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fudickar A, Bein B. Propofol infusion syndrome: update of clinical manifestation and pathophysiology. Minerva Anestesiol. 2009;75:339–344. [PubMed] [Google Scholar]
- 12.Rinaldi RC, Steindler EM, Wilford BB, Goodwin D. Clarification and standardization of substance abuse terminology. JAMA. 1988;259:555–557. [PubMed] [Google Scholar]
- 13.Wischmeyer PE, Johnson BR, Wilson JE, Dingmann C, Bachman HM, Roller E, et al. A survey of propofol abuse in academic anesthesia programs. Anesth Analg. 2007;105:1066–1071. doi: 10.1213/01.ane.0000270215.86253.30. [DOI] [PubMed] [Google Scholar]
- 14.Follette JW, Farley WJ. Anesthesiologist addicted to propofol. Anesthesiology. 1992;77:817–818. doi: 10.1097/00000542-199210000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Soyka M, Schutz CG. Propofol dependency. Addiction. 1997;92:1369–1370. doi: 10.1111/j.1360-0443.1997.tb02856.x. [DOI] [PubMed] [Google Scholar]
- 16.Pain L, Oberling P, Sandner G, Di Scala G. Effect of propofol on affective state as assessed by place conditioning paradigm in rats. Anesthesiology. 1996;85:121–128. doi: 10.1097/00000542-199607000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Pain L, Oberling P, Sandner G, Di Scala G. Effect of midazolam on propofol-induced positive affective state assessed by place conditioning in rats. Anesthesiology. 1997;87:935–943. doi: 10.1097/00000542-199710000-00029. [DOI] [PubMed] [Google Scholar]
- 18.Le Sage MG, Stafford D, Glowa JR. Abuse liability of the anaesthetic propofol: self-administration of propofol in rats under fixed-ratio schedules of drug delivery. Psychopharmacology (Berl.) 2000;153:148–154. doi: 10.1007/s002130000430. [DOI] [PubMed] [Google Scholar]
- 19.Weerts EM, Ator NA, Griffiths RR. Comparison of the intravenous reinforcing effects of propofol and methohexital in baboons. Drug Alcohol Depend. 1999;57:51–60. doi: 10.1016/s0376-8716(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 20.Zacny JP, Lichtor JL, Thompson W, Apfelbaum JL. Propofol at subanaesthetic doses may have abuse potential in healthy volunteers. Anesth Analg. 1993;77:544–552. doi: 10.1213/00000539-199309000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Uniform Controlled Substances Act: National Conference of Commissioners on Uniform State Laws; Section 309, Diversion Prevention and Control; 1994. www.NCCUSL.org. [Google Scholar]
- 22.Dexter F. Detecting diversion of anesthetic drugs by providers. Anesth Analg. 2007;105:897–898. doi: 10.1213/01.ane.0000282022.75007.f7. [DOI] [PubMed] [Google Scholar]
- 23.Guitton J, Desage M, Lepape A, Degoute CS, Manchon M, Brazier JL. Quantitation of propofol in whole blood by gas chromatography-mass spectrometry. J Chromatogr B Biomed Appl. 1995;669:358–365. doi: 10.1016/0378-4347(95)00105-r. [DOI] [PubMed] [Google Scholar]
- 24.Fujita A, Higuchi J, Nagai T, Tokudome S, Sakio H. A simple method for detecting plasma propofol. Anesth Analg. 2000;90:1452–1454. doi: 10.1097/00000539-200006000-00038. [DOI] [PubMed] [Google Scholar]
- 25.Teshima D, Nagahama H, Makino K, Kataoka Y, Oishi R. Microanalysis of propofol in human serum by semi-microcolumn high-performance liquid chromatography with UV detection and solid-phase extraction. J Clin Pharm Ther. 2001;26:381–385. doi: 10.1046/j.1365-2710.2001.00375.x. [DOI] [PubMed] [Google Scholar]
- 26.Dowrie RH, Ebling WF, Mandema JW, Stanski DR. High-performance liquid chromatographic assay of propofol in human and rat plasma and fourteen rat tissues using electrochemical detection. J Chromatogr B Biomed Appl. 1996;678:279–288. doi: 10.1016/0378-4347(95)00475-0. [DOI] [PubMed] [Google Scholar]
- 27.Favetta P, Guitton J, Degoute CS, Van Daele L, Boulieu R. High-performance liquid chromatographic assay to detect hydroxylate and conjugate metabolites of propofol in human urine. J Chromatogr B Biomed Sci Appl. 2000;742:25–35. doi: 10.1016/s0378-4347(00)00097-9. [DOI] [PubMed] [Google Scholar]
- 28.Nitsun M, Szokol JW, Saleh HJ, Murphy GS, Vender JS, Luong L, et al. Pharmacokinetics of midazolam, propofol, and fentanyl transfer to human breast milk. Clin Pharmacol Ther. 2006;79:549–557. doi: 10.1016/j.clpt.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Cirimele V, Kintz P, Doray S, Ludes B. Determination of chronic abuse of the anaesthetic agents midazolam and propofol as demonstrated by hair analysis. Int J Legal Med. 2002;116:54–57. doi: 10.1007/s004140100240. [DOI] [PubMed] [Google Scholar]
- 30.Grossherr M, Hengstenberg A, Meier T, Dibbelt L, Igl BW, Ziegler A, et al. Propofol concentration in exhaled air and arterial plasma in mechanically ventilated patients undergoing cardiac surgery. Br J Anaesth. 2009;102:608–613. doi: 10.1093/bja/aep053. [DOI] [PubMed] [Google Scholar]
- 31.Bajpai L, Varshney M, Seubert CN, Dennis DM. A new method for the quantitation of propofol in human plasma: efficient solid-phase extraction and liquid chromatography/APCI-triple quadrupole mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:291–296. doi: 10.1016/j.jchromb.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Lhuillier F, Mouloua Y, Vignal B, Favetta P, Guitton J. Quantitative measurement of propofol and in main glucuroconjugate metabolites in human plasma using solid phase extraction-liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;854:165–172. doi: 10.1016/j.jchromb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Miekisch W, Fuchs P, Kamysek S, Neumann C, Schubert JK. Assessment of propofol concentrations in human breath and blood by means of HS-SPME-GC-MS. Clin Chim Acta. 2008;395:32–37. doi: 10.1016/j.cca.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Kranioti EF, Mavroforou A, Mylonakis P, Michalodimitrakis M. Lethal self administration of propofol (Diprivan): a case report and review of the literature. Forensic Sci Int. 2007;167:56–58. doi: 10.1016/j.forsciint.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Drummer OH. Post-mortem toxicology. Forensic Sci Int. 2007;165:199–203. doi: 10.1016/j.forsciint.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Iwersen-Bergmann S, Rösner P, Kühnau HC, Junge M, Schmoldt A. Death after excessive propofol abuse. Int J Legal Med. 2001;114:248–251. doi: 10.1007/s004149900129. [DOI] [PubMed] [Google Scholar]
- 37.Roussin A, Mirepoix M, Lassabe G, Dumestre-Toulet V, Gardette V, Montastruc JL, Lapeyre-Mestre M. Death related to a recreational abuse of propofol at therapeutic dose range. Br J Anaesth. 2006;97:268. doi: 10.1093/bja/ael168. [DOI] [PubMed] [Google Scholar]
- 38.Strehier M, Preuss J, Wollersen H, Madea B. Lethal mixed intoxication with propofol in a medical layman. Arch Kriminol. 2006;217:153–160. [PubMed] [Google Scholar]
- 39.Kranioti EF, Mavroforou A, Mylonakis P, Michalodimitrakis M. Lethal self administration of propofol (Diprivan). A case report and review of the literature. Forensic Sci Int. 2007;167:56–58. doi: 10.1016/j.forsciint.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Drummer OH. A fatality due to propofol poisoning. J Forensic Sci. 1992;37:1186–1189. [PubMed] [Google Scholar]
- 41.Chao TC, Lo DS, Chui PP, Koh TH. The first fatal 2,6-di-isopropylphenol (propofol) poisoning in Singapore: a case report. Forensic Sci Int. 1994;66:1–7. doi: 10.1016/0379-0738(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 42.Cholbi M. Self-manslaughter and the forensic classification of self-inflicted death. J Med Ethics. 2007;33:155–157. doi: 10.1136/jme.2005.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]