Abstract
As a predictor of potential clinical outcome, we performed a systematic review of controlled studies that assessed experimental stroke outcome in rodents maintained on special diets (calorie restriction, ketogenic diet) or following the direct administration of ketone bodies. Pre-clinical studies were identified by searching web databases and the reference lists of relevant original articles and reviews. Sixteen published studies (a total of 733 experimental animals) met specific criteria and were analyzed using Cochrane Review Manager software. This resulted in objective evidence to suggest beneficial effects of the ketogenic pathway on pathologic and functional outcomes following experimental stroke.
Keywords: meta-analysis, systematic review, caloric restriction, ketogenic diet, stroke
For decades we have known that caloric restriction and intermittent fasting extend the lifespan of rodent species (and probably primates) by reducing free radical production, inflammation, and/or increasing resistance to stress. Such strategies would, therefore, be predicted to benefit both cardio- and cerebro-vasculature (Mattson and Wan, 2005; Yamada, 2008). Other dietary regimens can also be beneficial to neurologic function. For instance, the ketogenic diet, in which fat replaces carbohydrate, is clinically useful in the treatment of epilepsy and there is growing interest in extending use of this diet to other neurological disorders (http://www.clinicaltrials.gov/ct2/results?term=ketogenic+diet&pg=1), including acute injury such as stroke and trauma (Gasior et al., 2006). To provide a prediction of potential clinical outcome we performed a systematic review and meta-analysis of controlled rodent studies that assessed stroke outcome where the use of ketone bodies as an energy source had been promoted. These include ketogenic diets, as well as calorie restriction, which promote the use of triacylglycerols and fatty acids. In addition, ketone bodies can be directly administered. All three dietary interventions are evaluated here.
Methods
Study Identification
Experimental animal studies assessing the effect of dietary interventions related to ketone body utilization on outcomes following experimental stroke were identified from Embase, PubMed, Web of Science and Google Scholar by searching for all published articles up to the end of April 2012. The earliest study for analysis was that of Go et al. (1988). Search keywords included combinations of caloric restriction, cerebral ischemia, ketogenic diet, rodent. Additional publications were identified from reference lists of all identified articles and narrative reviews. Pre-specified exclusion criteria were used to aid selection and prevent bias, and studies were only included if (i) experimental ischemia was induced, (ii) animals were exposed to a ketogenic state, (iii) there was a control group, and, (iv) an appropriate outcome was measured following stroke.
Data extraction
The term ‘intervention’ refers to any intervention that induces a ketogenic state in animals such that the brain is deprived of its usual energy source, i.e. glucose, and instead uses ketones as an alternative. A ketogenic state, experimentally, can be induced by; (i) restriction of caloric intake, (ii) ketogenic diet or (iii) exogenous administration of ketones. From the relevant studies data were extracted on animal species, number, type of brain injury, type of intervention, timing of intervention relative to onset of experimental injury, and outcome post-stroke. Outcomes included lesion volume, brain water content, neuron counts, survival rate and functional measures. Data for functional outcomes included: (i) open field activity (distance travelled), (ii) 8-arm radial maze (working memory errors) and, (iii) novel object recognition task (discrimination index = time spent exploring novel object/total exploration time).
A comparison (C) was defined as the assessment of outcome in intervention and control groups, whereby intervention constituted either a dietary intervention (caloric restriction, ketogenic diet) or administration of a ketone body at a stated time point relative to the induction of ischemia. For each comparison, data were extracted for mean outcome, standard deviation and the number of animals per group. Data were not extracted if mean values were not reported, i.e. if only median and confidence intervals were given. If published studies (S) used multiple groups, for example to assess dose-response relationships, then data were individually extracted. If numerical data were not reported in the text, they were extracted from enlarged versions of the graphs. The methodological quality of each study was determined by minor modification to the ten-point scale of O'Collins et al. (2012).
Data analysis
The data were analysed using Cochrane Review Manager (version 5), as in a previous animal meta-analysis (White & Murphy, 2011). The effect of ketogenic state, as compared with control, on post-stroke outcomes was assessed using the standardized mean difference, whereby the difference in effect between intervention and control treatment is divided by the total standard deviation. A standardised mean difference of zero represents a lack of intervention effect, whereas a positive value indicates a beneficial effect and a negative value demonstrates a detrimental effect of the intervention. This allows comparisons to be made even though different methods of measurement and/or different animal models are used. Statistical heterogeneity was accounted for through the use of the DerSimonian and Laird (1986) pooling model of random effects. The data were grouped and stratified meta-analyses based on: (i) type of intervention used to induce ketogenic state, (ii) quality score, (iii) type of outcome assessed following ischemia, (iv) quality score, and (iv) duration (chronic or acute) of intervention. For those tests in which an increase in nominal value represented an improvement in outcome (e.g. neuron counts following FluoroJade labelling) the inverse of the extracted data was used for data comparisons. Studies were weighted by sample size and the results are expressed as standardised mean difference with 95% confidence intervals. The significance was set at α = 0.05 and P values of <0.05 from meta-analyses were considered to be significant.
Results
Design of studies
Based on the stated search criteria, we identified 19 studies that investigated the effect of a ketogenic state on outcome following cerebral ischemia. However, three of these (Chiba et al., 2010, Combs et al., 1987; Marie et al., 1990) were excluded, as mean values and the distribution of data (standard error or standard deviation) were not reported. The main characteristics of the 16 included studies are reported in Table 1. All of these reported the effect of inducing a ketogenic state, versus control, on one or more outcomes following cerebral ischemia. The 16 included studies represent published data from 12 research groups; four research groups published two studies each with the remaining 8 studies coming from separate research groups. Data from a total of 733 experimental subjects were included for analysis.
Table 1.
Characteristics of the studies included in the review
| Study | Parameters assessed | Intervention | First dose timing* | Species | Model of ischemia |
|---|---|---|---|---|---|
| Arumugam et al., 2010 | lesion volume, neurological score | calorie restriction | −3m | C57 mouse | MCAO |
| Bobyn et al., 2005 | open field, neuronal viability | calorie restriction | −28d | gerbil | 2VO |
| Go et al., 1988 | survival, edema | calorie restriction | −4d | rat | global |
| Gueldry et al., 1990 | edema | ketone administration | −0.5h | SD rat | microspheres |
| Massieu et al., 2003 | lesion volume | ketone administration | −14d, −7d, 0h | Wistar rat | iodoacetate |
| McEwen and Paterson, 2010 | open field, neuronal count | calorie restriction | +1h | Gerbil | 2VO |
| Puchowicz et al., 2008 | lesion volume, edema | ketogenic diet | −21d | Wistar rat | MCAO |
| Roberge et al., 2008a | radial maze, neuronal count | calorie restriction | −3m | Wistar rat | 4VO |
| Roberge et al., 2008b | elevated maze, open field, neuronal count | calorie restriction | −3m | Wistar rat | 4VO |
| Robertson et al., 1992 | lesion volume | calorie restriction, ketone administration | −12h | LE rat | 2VO |
| Suzuki et al., 2001 | survival, edema | ketone administration | −0.5h | ddY mouse | global |
| Suzuki et al., 2002 | lesion volume, edema | ketone administration | +0.5h | Wistar rat | MCAO |
| Tai et al., 2008 | neuronal count | ketogenic diet | −25d | SD rat | 4VO |
| Xu et al., 2010 | NOR | ketogenic diet | −21d | F344 rat | hypoxia |
| Yoon et al., 2011 | lesion volume | calorie restriction | −3m | C57 mouse | MCAO |
| Yu and Mattson, 1999 | lesion volume, neurological score | calorie restriction | −3m | SD rat | MCAO |
First dose timing in relation to onset of experimental stroke.
MCAO = middle cerebral artery occlusion; m = months; d = days; h = hours; VO = vessel occlusion; SD = Sprague-Dawley rat; LE = Long Evans rat; NOR = novel object recognition.
In terms of the type of intervention used to induce a ketogenic state, three studies utilised a ketogenic diet (Puchowicz et al., 2008; Tai et al., 2008; Xu et al., 2010), five studies exogenously administered ketone bodies (Gueldry et al., 1990; Massieu et al., 2003; Robertson et al., 1992; Suzuki et al., 2001, 2002), and the remaining 8 studies along with the study by Robertson et al., 1992 used calorie restriction. Of the five studies that exogenously administered ketone bodies, two administered β-hydroxybutyrate (Suzuki et al., 2001, 2002), two administered 1,3 butanediol (Gueldry et al., 1990; Robertson et al., 1992), one administered acetoacetate (Massieu et al., 2003), and the study by Robertson et al. (1992) also administered triacetin. When applying an intervention to induce a ketogenic state, the majority of studies did so prior to the onset of cerebral ischemia. Ten of the included studies introduced the intervention at least 7 days prior to the onset of cerebral ischemia whereas 4 studies (Gueldry et al., 1990; Go et al., 1988; Robertson et al., 1992; Suzuki et al., 2001) introduced the intervention less than 7 days prior to the onset of cerebral ischemia. The remaining two studies (McEwen & Paterson 2010; Suzuki et al., 2002), along with the study by Massieu et al. (2003), examined the effects of inducing a ketogenic state following the induction of ischemia.
In order to assess the effects of a ketogenic state on outcome following cerebral ischemia, the majority of studies utilised various models to induce cerebral ischemia; thirteen used vessel occlusion (filament occlusion of the middle cerebral artery, or clip occlusion of a number of vessels including the middle cerebral artery), one study used microspheres (Gueldry et al., 1990), one study used a model of glutamate excitotoxicity via application of iodoacetate (Massieu et al., 2003), and one study used a hypoxic chamber (Xu et al., 2010). Male rat models (F344, Long Evans, Sprague-Dawley, Wistar) were used in 12 of the 16 studies; the remaining 4 used either a mouse strain (Arumugam et al., 2010; Suzuki et al., 2001; Yoon et al., 2011) or gerbils (McEwen & Paterson, 2010).
In terms of assessing outcome following cerebral ischemia, the majority of studies used lesion volume (see Table 1), five used % water content (Go et al.,1998; Gueldry et al., 1990; Puchowicz et al., 2008;Suzuki et al., 2001, 2002), three used neuron count (McEwen & Paterson 2010; Roberge et al., 2008a, 2008b), two studies used survival rate (Go et al., 1988; Suzuki et al., 2001), and one study used neurological score (Arumugam et al., 2010). A number of studies also used measures of functional outcome, including the elevated maze (Roberge et al., 2008b), open field activity (Bobyn et al., 2005; McEwen & Paterson 2010), radial maze (Roberge et al., 2008a), and novel object recognition (Xu et al., 2010).
Overall efficacy of inducing a ketogenic state
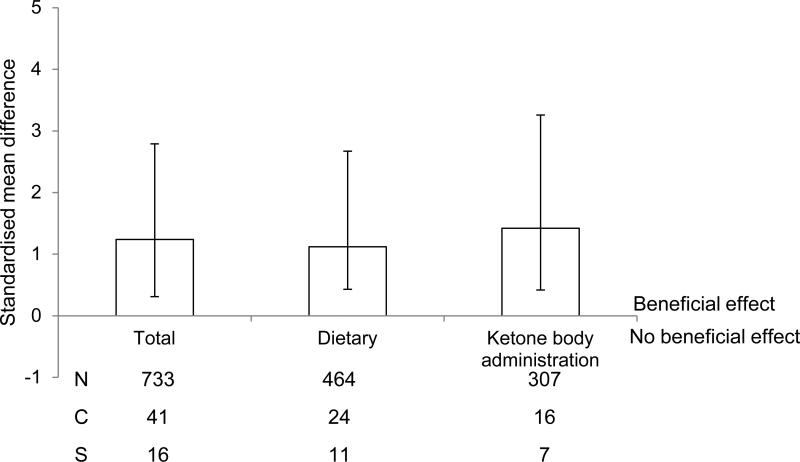
We determined initially that there was an overall significant protective effect of a ketogenic state on outcome following cerebral ischemia (1.24, 1.55 - 0.93, P < 0.001), regardless of individual study characteristics. Our literature search indicated that studies tended to induce a ketogenic state either by intervention (calorie restriction or a ketogenic diet) or through the exogenous administration of ketones. Thus, we also determined if a beneficial effect was seen following either intervention (1.12, 1.55 – 0.69, P < 0.001) or exogenous ketone administration (1.42, 1.84 – 1.0, P < 0.001; Fig. 1).
Fig. 1.
Standardised mean difference and 95% CI of all outcome measures by type of intervention i.e. all, dietary restriction only, exogenous administration of ketones. A beneficial effect following any type of intervention was observed. N, number of animals; C, number of comparisons; S, number of studies.
Type of parameter used to assess outcome
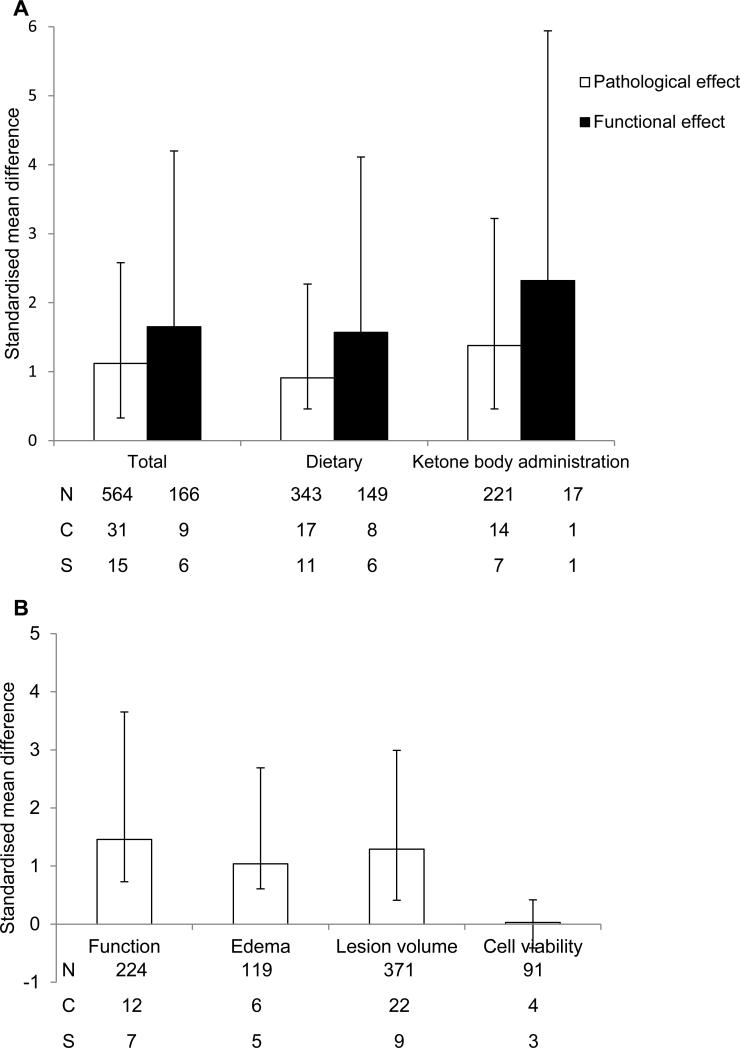
The effect of intervention on cerebral ischemia outcome was analysed according to type (Fig 2). First, data were grouped according to intervention, i.e. all, intervention, or exogenous administration, and then analysed to determine if they had a significant beneficial effect on either pathology or function (Fig. 2A). Pathological outcomes included lesion volume, brain water content and neuronal counts, whereas functional outcomes included all measures of behaviour. Regardless of whether a dietary intervention or administration had been used to induce a ketogenic state, a significant beneficial effect was seen on both outcomes (P < 0.01). However, a variety of outcomes were used in the included studies and we went on to analyse each separately (Fig. 2B). Induction of a ketogenic state was found to have a beneficial effect on functional tests (1.46, 2.19 – 0.73 P < 0.001), lesion volume (1.29, 1.7 – 0.88, P <0.001) and brain water content (1.04, 1.65 – 0.43 P < 0.001). However, a ketogenic state did not have a beneficial effect on neuron count, as assessed by FluoroJade labelling (−0.03, 0.39 - −0.44, P = 0.9), which included data from 3 studies, 4 comparisons and 91 experimental subjects.
Fig. 2.
Standardised mean difference and 95% CI of all outcome measures by type of outcome following cerebral ischemia. A beneficial effect of any type of intervention was observed when assessing pathologic or functional outcomes (A). When data were analysed according to the different types of outcome, i.e. function, edema, lesion volume, and cell viability, a beneficial effect of a ketogenic state was observed for all outcomes, apart from the last. N, number of animals; C, number of comparisons; S, number of studies.
Reported study quality
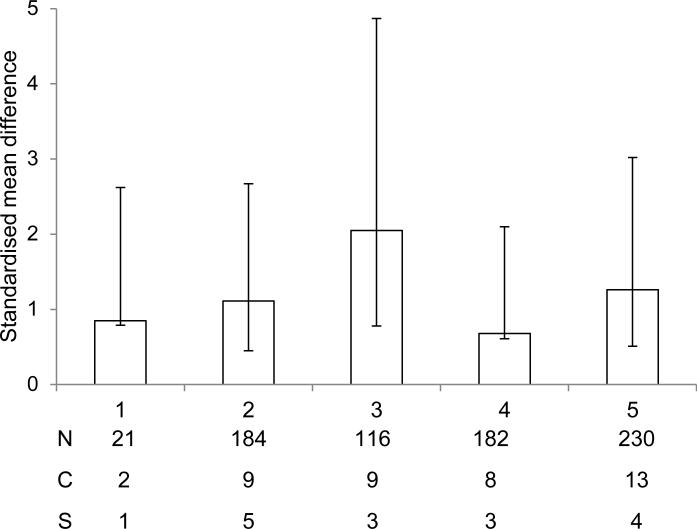
The quality scores of the included studies ranged from 1 (low) to 5 with a median score of 3. In order to determine whether the quality score of an individual study had any impact on whether or not the intervention had a beneficial effect following cerebral ischemia, included studies were analysed according to quality score (Fig.3). Only those with a score of 2, 3 or 5 demonstrated a significant (P < 0.01) beneficial effect of the intervention. Studies that had the lowest quality score had no significant beneficial effect (P = 0.07). In addition, three studies received a quality score of 4 and these showed no beneficial effect (P = 0.08).
Fig. 3.
Standardised mean difference and 95% CI for all outcome measures when data are grouped according to study quality. A beneficial effect was only observed in those studies that received a quality score of 2, 3 or 5. N, number of animals; C, number of comparisons; S, number of studies.
Duration of intervention
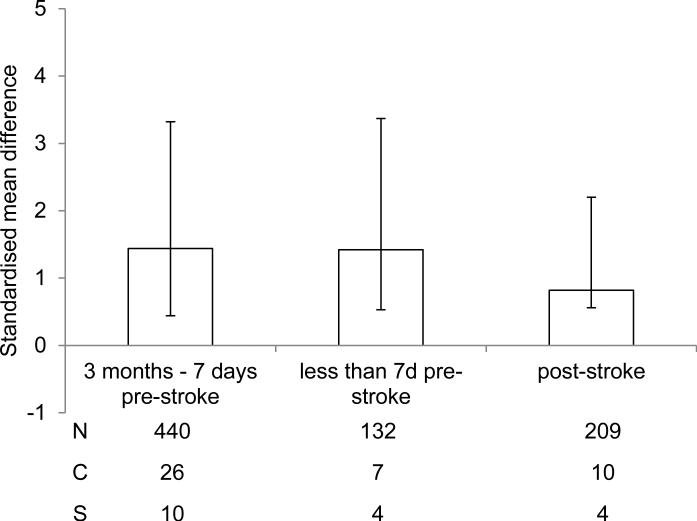
When comparing data across all studies it was determined that there was a beneficial effect of intervention to induce a ketogenic state, regardless of whether this occurred pre- or post-experimental ischemia (Fig. 4). A beneficial effect was observed when the intervention began more than 7 days prior to the onset of experimental ischemia (1.44, 1.88 – 1.0 P < 0.001) or less than 7 days prior to the onset of experimental ischemia (1.42, 1.95 – 0.89 P < 0.001). In addition, a significant beneficial effect was observed if the intervention began following the onset of experimental ischemia (0.82, 1.38 – 0.26 P = 0.004).
Fig. 4.
Standardised mean difference and 95% CI for all outcome measures when data are grouped according to onset of intervention. A beneficial effect was observed regardless of whether the ketogenic state was induced more than 7 days prior to onset, less than 7 days prior to onset, or at a time point following onset of experimental ischemia. N, number of animals; C, number of comparisons; S, number of studies.
Discussion
To restate the major findings from our analyses, we found beneficial effects on pathologic and functional outcomes of dietary intervention, or exogenous ketone administration, either prior to or following experimental stroke.
Neuropathologies, such as a stroke, cause a mismatch between energy demand and supply: blood flow goes awry, oxygen levels fall, and mitochondria malfunction. A period of fasting results in a short-term ketosis, and increased reliance on ketone bodies appears to be a form of cerebral metabolic adaptation (Manzanero et al., 2011). Ketone metabolism is enzymatically simpler and more efficient than glucose or pyruvate metabolism (Veech, 2004), and is reported to increase global cerebral blood flow (Gasior et al., 2006; Prins, 2008). Indeed, ketone bodies are the only circulating substrates in addition to glucose known to contribute significantly to cerebral metabolism. The precise mechanisms whereby caloric restriction, the ketogenic diet, and ketone bodies provide protection in ischemic stroke are not clear but there is notable improvement in mitochondrial function, a decrease in inflammation, and an increase in expression of neurotrophins such as BDNF and bFGF (Maalouf et al., 2009; Manzanero et al., 2011). Whether this relates to the higher level of potential energy available in the C-H bonds of beta hydroxybutyrate compared to pyruvate is unclear but potentially important to recovering ATP levels during reperfusion (Veech, 2004). More recently, the sirtuins have also been implicated. These histone deacetylases localize to different subcellular compartments and have a variety of substrates. Activity depends on nicotinamide adenine dinucleotide (NAD+), which links this family of enzymes to cellular energy levels. Indeed, some sirtuins (SIRT3) are located within mitochondria. Caloric restriction could activate and/or increase expression of sirtuins, which then modulate proteins involved in cell survival and apoptotic cell death (Maalouf et al., 2009; Morris et al., 2011).
Caloric restriction and the ketogenic diet appear to represent cost-effective and efficient strategies through which stroke incidence and/or subsequent pathology could be reduced.
Acknowledgements
Support for this study is provided by American Diabetes Association and NIH-NIDDK (A.N.M.), American Heart Association and NIH-NINDS (S.P.M.).
Footnotes
The authors declare that they have no conflicts of interest.
References
- Arumugam TV, Philips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobyn PJ, Corbett D, Saucier DM, Noyan-Ashraf MH, Juurlink BHJ, Paterson PG. Protein-energy malnutrition impairs functional outcome in global ischemia. Exp. Neurol. 2005;196:308–315. doi: 10.1016/j.expneurol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Chiba T, Ezaki O. Dietary restriction suppresses inflammation and delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2010;399:98–103. doi: 10.1016/j.bbrc.2010.07.048. [DOI] [PubMed] [Google Scholar]
- Combs DJ, D'Alecy LG. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-Butanediol. Stroke. 1987;18:503–511. doi: 10.1161/01.str.18.2.503. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta analysis in clinical trials. Control Clin. Trials. 1986;7:177–178. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Benefits of histone deacetylase inhibitors for acute brain injury: a systematic review of animal studies. J. Neurochem. 2010;115:806–813. doi: 10.1111/j.1471-4159.2010.06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Gray L, Bath PMW, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- Go KG, Prenen GHM, Korf J. Protective effect of fasting upon cerebral hypoxicischemic injury. Metab. Br. Dis. 1988;3:257–262. doi: 10.1007/BF00999535. [DOI] [PubMed] [Google Scholar]
- Gueldry S, Marie C, Rochette L, Bralet J. Beneficial effect of 1,3-Butanediol on cerebral energy metabolism and edema following brain embolization in rats. Stroke. 1990;21:1458–1463. doi: 10.1161/01.str.21.10.1458. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Br. Res. Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Gelderblom M, Magnus T, Arumugam TV. Calorie restriction and stroke. Exp. Transl. Stroke Med. 2011;3:8. doi: 10.1186/2040-7378-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Bralet A-M, Gueldry S, Bralet J. Fasting prior to transient cerebral ischemia reduces delayed neuronal necrosis. Metab. Br. Dis. 1990;5:65–75. doi: 10.1007/BF01001047. [DOI] [PubMed] [Google Scholar]
- Massieu L, Haces ML, Montiel T, Hernandez-Fonseca K. Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience. 2003;120:365–378. doi: 10.1016/s0306-4522(03)00266-5. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- McEwen BR, Paterson PG. Caloric restriction provided after global ischemia does not reduce hippocampal cornu ammonis injury or improve functional recovery. Neuroscience. 2010;166:262–270. doi: 10.1016/j.neuroscience.2009.11.076. [DOI] [PubMed] [Google Scholar]
- Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J. Cereb. Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Howells DW. Evaluation of combination therapy in animal models of ischemia. J. Cereb. Blood Flow Metab. 2012;32:585–597. doi: 10.1038/jcbfm.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 2008;28:1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J. Cereb. Blood Flow Metab. 2008;28:1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge M-C, Messier C, Staines WA, Plamondon H. Food restriction induces long-lasting recovery of spatial memory deficits following global ischemia in delayed matching and non-matching-to-sample radial arm maze tasks. Neuroscience. 2008a;156:11–29. doi: 10.1016/j.neuroscience.2008.05.062. [DOI] [PubMed] [Google Scholar]
- Roberge M-C, Hotte-Bernard J, Messier C, Plamondon H. Food restriction attenuates ischemia-induced spatial learning and memory deficits despite extensive CA1 ischemic injury. Beh. Br. Res. 2008b;187:123–132. doi: 10.1016/j.bbr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Robertson C, Goodman C, Grossman RG, Claypool M, White A. Dietary non-protein calories and cerebral infarction size in rats. Stroke. 1992;23:564–568. doi: 10.1161/01.str.23.4.564. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki M, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. Effect of β-hydroxybutyrate; a cerebral function improving agent, on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn. J. Pharmacol. 2001;87:143–150. doi: 10.1254/jjp.87.143. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki M, Kitamura Y, Mori S, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. β-hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn. J. Pharmacol. 2002;89:36–43. doi: 10.1254/jjp.89.36. [DOI] [PubMed] [Google Scholar]
- Tai K-K, Nguyen N, Pham L, Truong DD. Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J. Neural. Transm. 2008;115:1011–1017. doi: 10.1007/s00702-008-0050-7. [DOI] [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox state, insulin resistance, and mitochondrial metabolism. Prost. Leuk. Ess. Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- White AT, Murphy AN. Administration of thiazolidinediones for neuroprotection in ischemic stroke: a pre-clinical systematic review. J. Neurochem. 2010;115:845–853. doi: 10.1111/j.1471-4159.2010.06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Sun X, Eroku BO, Tsipis CP, Puchowicz MA, LaManna JC. Diet-induced ketosis improves cognitive performance in aged rats. Adv. Exp. Med. Biol. 2010;662:71–76. doi: 10.1007/978-1-4419-1241-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KA. Calorie restriction and glucose regulation. Epilepsia. 2008;49(Suppl. 8):94–96. doi: 10.1111/j.1528-1167.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Mughal MR, Mattson MP. Energy restriction negates NMDA receptor antagonist efficacy in ischemic stroke. Neuromol. Med. 2011;13:175–178. doi: 10.1007/s12017-011-8145-y. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioural outcome: evidence for a preconditioning mechanism. J. Neurosci. Res. 1999;57:830–839. [PubMed] [Google Scholar]