Abstract
Schisandra chinensis Baill is a Chinese traditional medicine with multiple pharmacological activities. In this study, chicanine, one of the major lignan compounds of Schiandra chinesis, was investigated for suppressive effects on lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages (RAW 264.7 cells). Chicanine was found to have anti-infammatory properties with the inhibition of nitric oxide (NO) and Prostaglandin E (2) (PGE2) production and nuclear factor-κB (NF-κB) signaling in LPS-stimulated RAW 264.7 cells with no cytotoxic effects. Treatment of RAW 264.7 cells with chicanine down-regulated LPS-induced expression of pro-inflammatory cytokines including TNFα, IL-1β, MCP-1, G-CSF, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS). These inhibitory effects were found with the blockage of p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinases 1 and 2 (ERK 1/2), and also IκB-α phosphorylation. These results indicated that anti-inflammatory actions of chicanine in macrophages involved inhibition of LPS-induced TLR4-IκBα/MAPK/ERK signaling pathways.
Keywords: Chicanine, inflammatory cytokines, IκBα, MAPK, ERK
1. Introduction
Inflammation was one of the complex biological responses of vascular tissues to harmful stimuli, such as pathogens, damaged cells, or irritants (Ferrero-Miliani et al., 2007). Chronic inflammations were also associated with malignancies, so it was important to prevent inflammation-mediated neoplastic formation, promotion and/or progression (Saw et al., 2010). Many substances could regulate the inflammatory response, which included vascular amines, metabolites of arachidonic acid (prostaglandin, leukotriene and lipoxin), cytokines (interleukin-1β, interleukin-6β and tumor necrosis factor-α), platelet activating factor, neuropeptides and nitric oxide (Guo et al., 2008). Nitric oxide (NO) was one of the important mediators of the inflammatory response. Large amounts of NO could stimulate the expression of many proteins and enzymes crucial to inflammatory reactions, such as the mitogen-activated protein kinases (MAPKs) and the nuclear factor-κB (NF-κB) pathways. NF-κB was the major transcriptional factor in the production of pro-inflammatory cytokines and mediators and toll-like receptors (TLRs) were the major pattern recognition receptors in the innate immune system to detect conserved microbial products (Akira et al., 2006). The extracellular regions of TLRs contained many leucine-rich repeat (LRR) motifs that could recognize activators such as lipopolysaccharides (LPS) from Gram-negative bacteria and peptidoglycan from Gram-positive bacteria (Lin et al., 2010). The particular LRR motifs of TLR were functionally asS.E.M.bled and triggered downstream signal transduction when ligand recognition occured (Takada et al., 2007). In Lu’s study, the stimulation of toll-like receptor 4 (TLR4) by LPS induced the release of critical proinflammatory cytokines that were necessary to activate potent immune responses (Lu et al., 2008). LPS/TLR4 signaling pathway had been intensively studied in recent years. Many of the cytokines in the pathway could be the targets in the treatment of inflammatory diseases.
Schisandra chinensis Baill (Wuweizi in Chinese) was a well-known Chinese traditional medicine with multiple pharmacological functions in treating chronic cough and dyspnea, nocturnal emission, spermatorrhea, enuresis, frequent urination, protracted diarrhea, spontaneous sweating, night sweating, shortness of breath and feeble pulse, diabetes and wasting-thirst (Lu & Chen, 2010). Components of fruits of Schisandra chinensis mainly included the volatile oil, organic acids, vitamins, lignins, sesquiterpenes, triterpenoids, amino acids, and polysaccharides (Wang et al., 2011). Recent studies had demonstrated that some lignans of Schisandra chinensis exhibit anti-oxidative and anti-inflammatory effects in vivo and in vitro (Oh et al., 2010; Guo et al., 2008). Chicanine was one of the main lignans of Schisandra chinensis. However, the anti-inflammatory effects and the mechanism of chicanine action have not been reported.
In the present paper, we reported the activities of chicanine on LPS-induced inflammatory responses in murine macrophages (RAW 264.7 cells).
2. Materials and methods
2.1. Materials and cell cultures
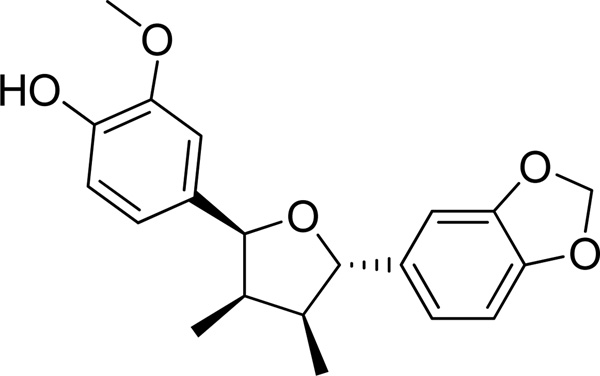
Chicanine was isolated from Schisandra chinensis and the chemical structures were confirmed by UV, IR, ESI-MS, 1H NMR data compared with the literature. The chemical structure of chicanine is shown in Fig. 1. The purity of chicanine was detected by HPLC at four wavelengths (210, 240, 254 and 280 nm), and the results suggested a purity of above 98%. RAW264.7, murine macrophage-like cells, was obtained from the American Type Culture Collection (Rockville, MD, U.S.A.). RPMI 1640, phosphate buffered saline (PBS), lipopolysaccharide (E. coli, serotype 0127: B8; LPS), celastrol and dimethyl sulfoxide were acquired from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Geneticin (antibiotic G-418) was purchased from Gibco BRL (Grand Island, NY, U.S.A.). All of the samples, solutions and buffers were prepared with deionized water. Primary antibodies for COX-2 (Cat.No. sc-376861), iNOS (Cat.No. sc-7271), IκBα (sc-52900), p-IκBα(cat. no. sc8404), p-p38(sc-7973), p38 (sc-136210), ERK(sc-292838) and p-ERK(1/2) sc-23759-R and secondary antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). PVDF membrane was obtained from Whatman GmbH (Germany).
Fig. 1.
Chemical structure of chicanine.
2.2. Cell culture and cell viability assay
Murine leukemic monocytic macrophage cell line, RAW 264.7 cells were maintained and cultured at 37 °C under humidified air, with 5% CO2 atmosphere in RPMI1640 (GIBCO Invitrogen Corporation, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 mg/mL streptomycin and 1.176 g/L sodium bicarbonate. The cells were seeded into 96-well plates at the density of 1×104 cells/well and allowed to adhere for 24 h, also at 37 °C under 5% CO2. After 18 h treatment with chicanine (6.25, 12.5, 25 and 50 µM) in the presence or absence of LPS (100 ng/ml), MTT solution was added to each well and incubated for another 4 h at 37 °C. After incubation, media were removed and DMSO was added to dissolve purple precipitates. Then plates were read at 570 nm using an emaxmicroplate reader (Molecular Devices, Sunnyvale, CA, U.S.A.).
2.3. NF-κB luciferase assay
Chicanine was studied in an NF-κB luciferase reporter assay in RAW264.7 cells to determine NF-κB activity according to the method ofWu et al. (2010). Briefly, RAW264.7 cells stably transfected with the NF-κB reporter gene were plated in 96-well plates. Following a 24 h recovery period, the cells were treated with chicanine (6.25, 12.5, 25 and 50 µM) for an additional 18 h in the presence of LPS (100 ng/ml). To determine NF-κB luciferase activity, the Luciferase Reporter Assay System purchased from Promega (Madison, WI) was used. Cell lysates (15 µL) from treated RAW264.7 cells were placed in opaque 96 well plates. Luciferase Assay Reagent (50 µL) was injected and samples were read by a fluorometer (LMAX 2, Molecular devices). Celastrol (250 nM) was used as the positive control, which is effective on the LPS-induced inflammatory responses in murine macrophages.
2.4. Nitrite and PGE2 assay
RAW264.7 cells (1×105 cells/well) were plated in 96-well plates. Following a 24 h recovery period, the cells were treated with chicanine (6, 12, 25 and 50 µM) for an additional 18 h in the presence or absence of LPS (100 ng/ml). After incubation, the nitrite concentrations of supernatants (50 µL/well) were measured by adding 50 µL of Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthyl ethylene diamine dihydrochloride in water). The optical density at 540 nm was measured using an emaxmicroplate reader (Molecular Devices, Sunnyvale, CA, USA). The nitrite concentration was calculated by comparison with the absorbance at 540 nm of standard solutions of nitrate sodium prepared in culture medium. Celastrol (250 nM) was used as the positive control, which is effective on the LPS-induced inflammatory responses in murine macrophages.
The level of PGE2 in RAW264.7 cell culture medium was measured by ELISA kits ( R&D Systems, Minneapolis, MN) according to the manufacturer's instruction.
2.5. RNA isolation and quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) assay
RAW 264.7 cells were treated with increasing concentrations of chicanine (6.25, 12.5, 25 and 50 µM) After 6h of treatment, total RNA was extracted using Aurum Total RNA Mini Kit(732-6820, Hercules, CA, USA). RNA concentrations were determined by Quant-iTTM RiboGreen1 RNA Reagent and Kit (Invitrogen, Grand Island, NY, USA). From each sample, 2.0 µg of total RNA was then reverse transcribed to single-stranded cDNA by Invitrogen Reverse Transcription Reagents (Applied Biosystems Inc., Foster City, CA, USA). Then qPCR analyses were performed on the aliquots of the cDNA preparations with SYBR Green PCR Master Mix (BioChain Institute, Newark, CA, USA) to detect quantitatively the gene expression of COX-2, iNOS, TNF-α, IL-1β, G-CSF, MCP-1 and glyceraldehydes 3-phosphate dehydrogenase (GAPDH) (as an internal standard) using Applied Biosystems 7500HT Fast Real-Time PCR System (Applied Biosystems Inc., Foster City, CA, USA). The primer pairs were designed using Primer Quest Oligo Design and Analysis Tool by Integrated DNA Technologies Inc. (Coralville, IA, USA) and the sequences are listed in Table 1. All assays were confirmed using melting curves to confirm the presence of single PCR products. At least three independent experiments were carried out for each treatment.
Table 1.
Oligonucleotide primers used for qualitative real-time RT-PCR (qRT-PCR).
| Gene | Sense | Antisense |
|---|---|---|
| GAPDH | TTGATGGCAACAATCTCCAC | CGTCCCGTAGACAAAATGGT |
| COX-2 | TGCCTGGTCTGATGATGTATG | AGTAGTCGCACACTCTGTTGT |
| iNOS | CCTGGTACGGGCATTGCT | GCTCATGCGGCCTCCTTT |
| TNF α | AGGGTCTGGGCCATAGAACT | CCACCACGCTCTTCTGTCTAC |
| IL-6 | ACCAGAGGAAATTTTCAATAGGC | TGATGCACTTGCAGAAAACA |
| IL-1 | GGTCAAAGGTTTGGAAGCAG | TGTGAAATGCCACCTTTTGA |
| MCP-1 | ATTGGGATCATCTTGCTGGT | CCTGCTGTTCACAGTTGCC |
| G-CSF | TGACACAGCTTGTAGGTGGC | TCCTGCTTAAGTCCCTGGAG |
2.6. Protein extraction and Western blotting
RAW 264.7 cells were treated with chicanine with increasing concentrations (6.25, 12.5, 25 and 50 µM). After 6 h of treatment, total cytoplasmic extracts were prepared. Protein extraction and Western blotting were carried out according toSaw et al. (2010). Protein samples (20 mg) were subjected to 10% gradient polyacrylamide gel (Criterion Tris-HCl gel, Bio-Rad Lab, Hercules, CA, USA) electrophoresis and the resolved proteins were then transferred to polyvinylidenedifluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, MA, USA) using a wet transfer system (Fisher Scientific, Pittsburgh, PA, USA). The nonspecific binding of antibodies was blocked with 5% bovine serum albumin (BSA) in TBST buffer (0.1% Tween 20 in PBS). Antibodies were purchased from Cell signaling Biotechnology, Inc. (CA, USA). Immunodetection of COX-2, iNOS, p38, p-p38, IKBα, p- IKBα, ERK, p-ERK and β-actin proteins was carried out using the respective primary antibodies (1:1000 in 5% BSA in TBST buffer) and horseradish peroxidase (HRP) conjugated secondary antibodies (1: 3000 in 5% BSA in TBST buffer). The immunocomplexes were determined by using the enhanced chemiluminescent system for detecting HRP on immunoblots (Thermo Scientific, Rockford, IL, USA) and the bands were visualized and captured by Bio-Rad ChemiDoc XRS system (Hercules, CA, USA). At least three independent experiments were carried out for each treatment.
2.7. Statistical analysis
The results are presented as means ± standard error of the mean (S.E.M.). Statistically significant differences between groups were calculated by the application of an analysis of variance (ANOVA) followed by the Newman-Keuls test. P-values less than 0.05 (P<0.05) were considered significant.
3. Results
3.1. Non-toxic effects of chicanine on RAW 264.7 cells treated with LPS
To determine the effects of chicanine on cell viability, RAW 264.7 cells were initially seeded in microplates followed by different treatments. Results of the MTT assay after 18 h treatment indicated that, with LPS stimulation, none of the treatments with chicanine at the four concentrations (6.25, 12.5, 25 and 50 µM) was toxic as compared to the untreated cells (p>0.05). The results of the treatments with chicanine at the four concentrations on RAW 264.7 cells without LPS stimulation were similar to those with LPS stimulation. However, when the concentration of chicanine was increased to 100 µM, cell viability rate was 75%.The concentrations (6.25, 12.5, 25 and 50 µM) were used in further studies of the potential anti-inflammatory properties of chicanine.
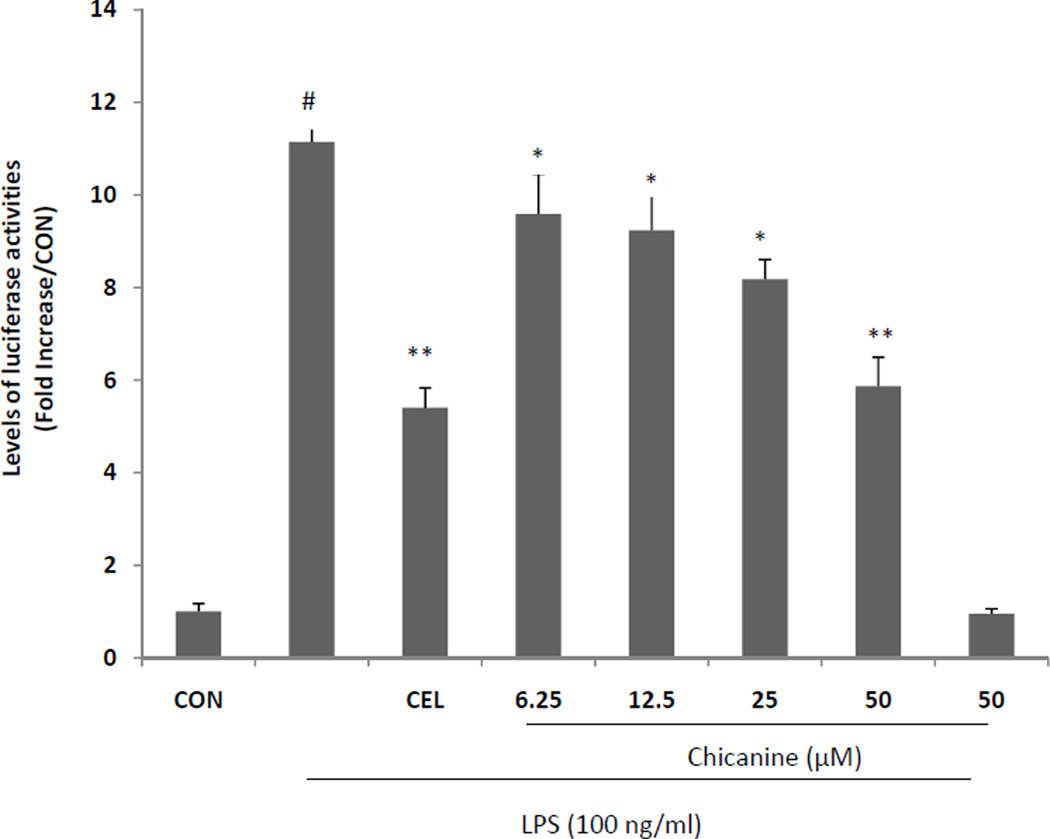
3.2. Chicanine inhibition of NF-κB induced luciferase activity
To analyze the potential anti-inflammatory properties of chicanine, we used RAW 264.7 murine macrophage cells that express a NF-κB responsive luciferase reporter gene. Cells were incubated with chicanine (6.25, 12.5, 25, 50 µM) and 100 ng/ml LPS for 18 h. Because chicanine was one main constituent from Chinese traditional medicine Schisandra chinensis, celastrol, a quinone methide triterpenoid from Chinese folk medicine Celastrus orbiculatus was used as positive control in this study. Celastrol was reported to suppress nitric oxide (NO) production and LPS-induced NF-κB activation with the IC50 values of 230 nM and 270 nM, respectively (Jin et al., 2002). Therefore, the concentration of celastrol tested in this study was set at 250 nM.
Celastrol was a potent anti-inflammatory drug in many references (Kannaiyan et al., 2011; Lee et al., 2006). The results indicated that chicanine reduced the expression of NF-κB luciferase in a concentration-dependent manner (Fig. 2), with a maximum effect of a 47% reductionwhen the cells were treated with 50 µM chicanine, which was similar to the results of the positive control celastrol at the concentration of 250 nM. Treatment with chicanine without LPS stimulation had a mild inhibitory effect on luciferase activity only at the highest concentration (50 µM).
Fig. 2. Effects of chicanine on the activity of NF-κB luciferase.
RAW 264.7 cells were cultured with chicanine (6.25, 12.5, 25, 50 µM ) with or without stimulation of 100 ng/ml LPS for 18 h. The Luciferase Reporter Assay System was used to measure luciferase activity. CEL (celastrol 250 nM) was used as assay positive control. Data are presented as means ±S.E.M. of three independent experiments. #P<0.05 vs. the control group; *P<0.05, **P<0.01 vs. LPS-stimulated group.
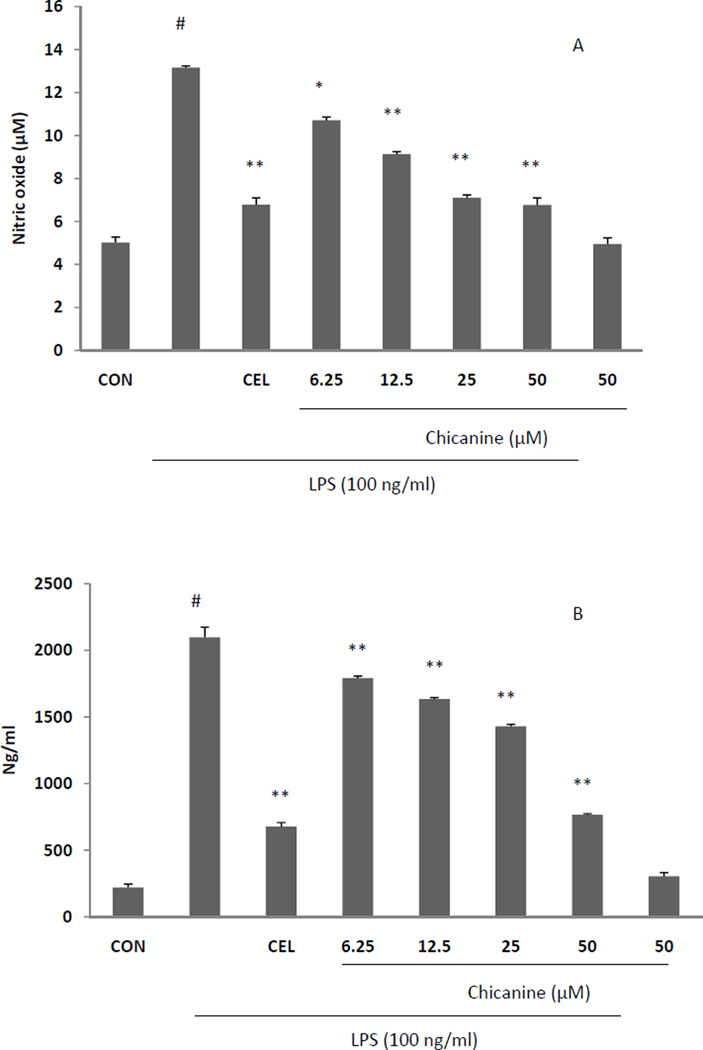
3.3. Chicanine suppressed NO and PGE2 production in RAW 264.7 cells
When RAW 264.7 cells were stimulated with LPS, nitrite was produced as a biomarker of NO. To examine whether chicanine had effects in suppressing the NO production in LPS-stimulated RAW 264.7 cells, various concentration of chicanine (6.25, 12.5, 25, 50 µM) were tested on RAW 264.7 cells with and without stimulation of LPS. Treatments with chicanine without LPS stimulation had a mild inhibitory effect on NO production only at the highest concentration (50 µM). Chicanine reduced NO and PGE2 production in a concentration-dependent manner after treatment of 18h (Fig. 3A and B), with a maximum effects of 50% NO reduction with 50 µM chicanine, which was similar to the results of the positive control celastrol at the concentration of 250 nM.
Fig. 3. Effects of chicanine on NO and PGE2 production in RAW 264.7 cells.
Cells were cultured with chicanine (6.25, 12.5, 25, 50 µM ) with or without stimulation of 100 ng/ml LPS for 18 h. NO levels in culture media were determined using Griess assays. The level of PGE2 in RAW264.7 cell culture medium was measured by ELISA kits .CEL (celastrol 250 nM) was used as assay positive control. Data are presented as means ±S.E.M. of three independent experiments. #P<0.05 vs. the control group; *P<0.05, **P<0.01 vs. LPS-stimulated group.
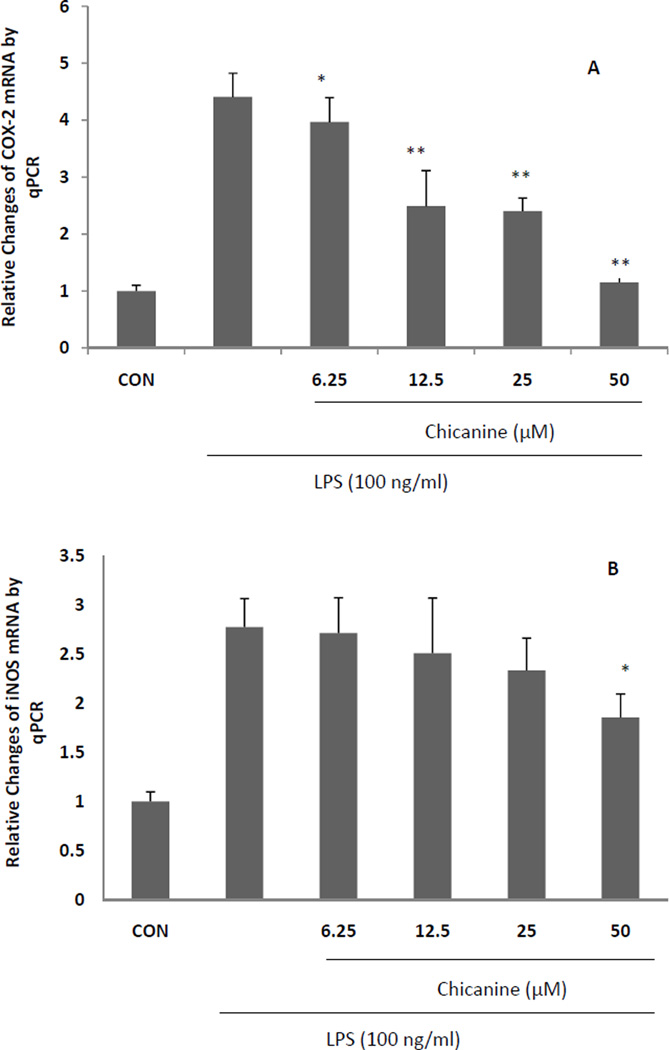
3.4. Chicanine inhibited the expression of pro-inflammatory cytokines
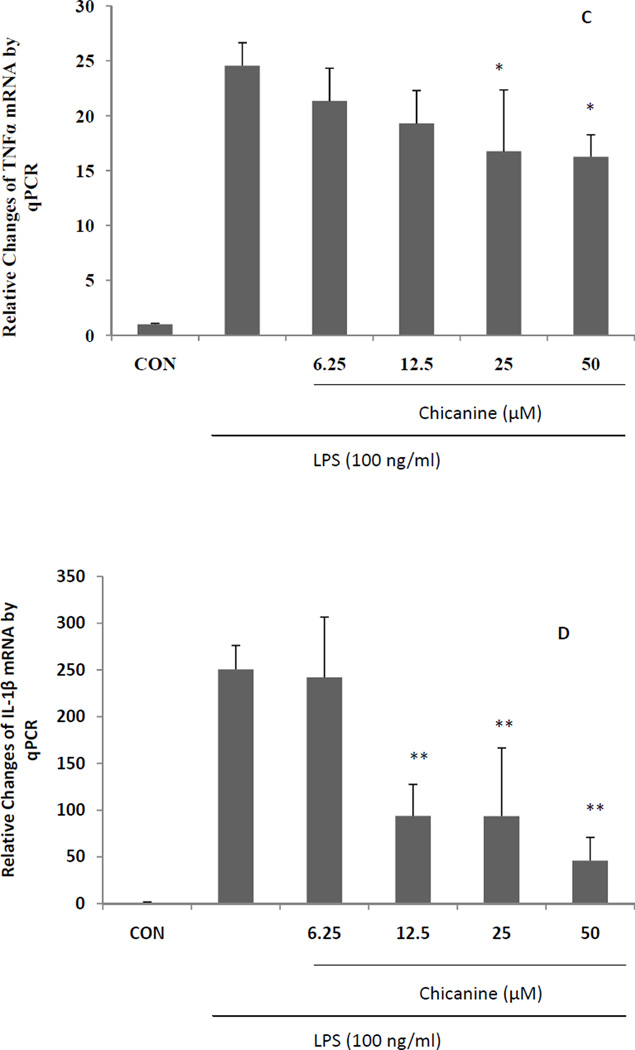
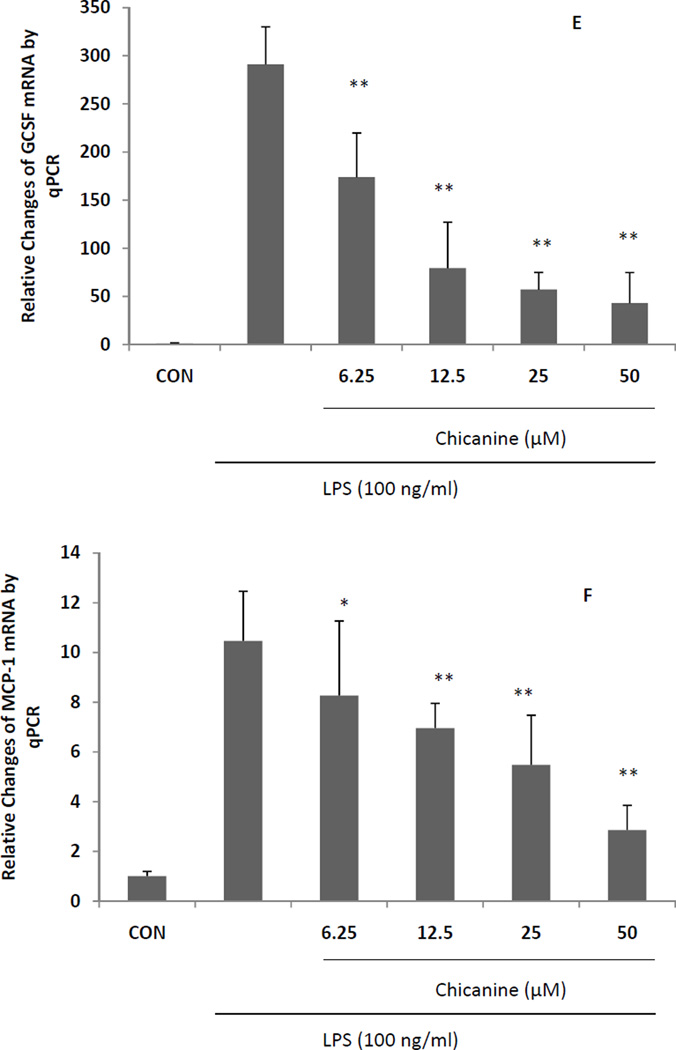
The effects of chicanine on the expression of pro-inflammatory cytokines were investigated by RT-PCR. Under stimulation of LPS for 6 h, the mRNA levels from pro-inflammatory genes, including COX-2,iNOS, TNF-α, IL-1β, MCP-1 and G-CSF were highly induced (Fig. 4). Co-treatment of cells with chicanine for 6h significantly decreased the expression of these pro-inflammatory mediators in concentration-dependent manner. The strongest effects were observed in expression of IL-1β, G-CSF and MCP-1 with 82%, 85% and 73% inhibition, respectively. Chicanine was found to decrease the levels of COX-2 mRNA and PGE2 in a dose-dependent manner. These results suggested that chicanine might significantly suppress LPS-induced PGE2 via inhibiting COX-2 expression at the transcriptional level.
Fig. 4. Real-time PCR (qRT-PCR) results expressed in fold changes of mRNA over the control, using GAPDH as endogenous housekeeping gene.
(A) COX-2; (B) iNOS; (C) TNF-α; (D) IL-1β; (E) G-CSF. (F) MCP-1. Results are expressed as means ± S.E.M. *P <0.05, **P<0.01 compared with the LPS treated cells.
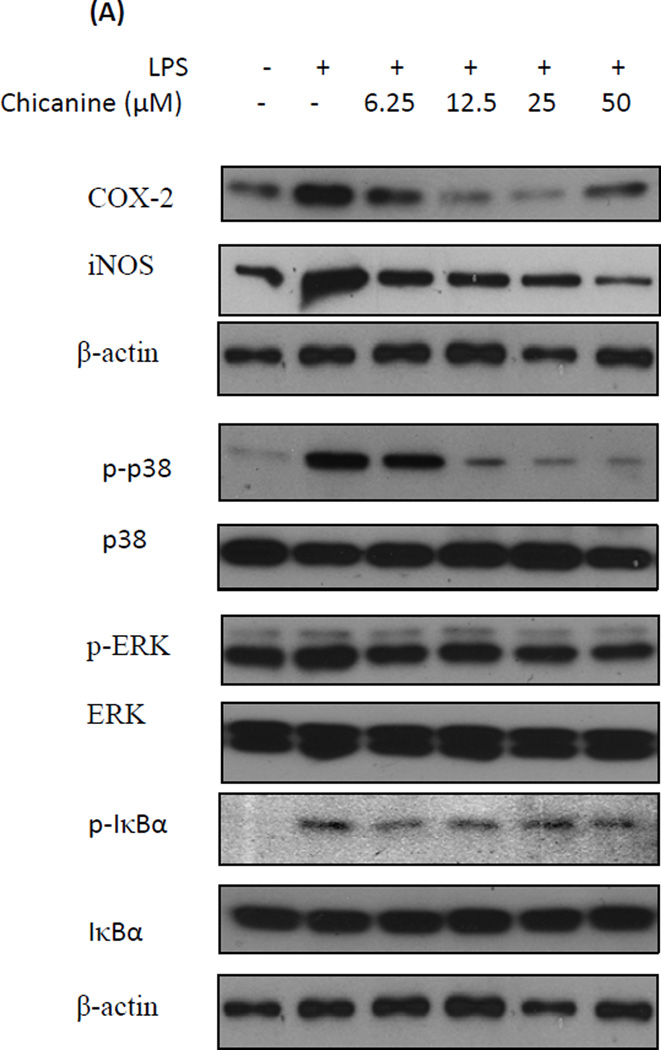
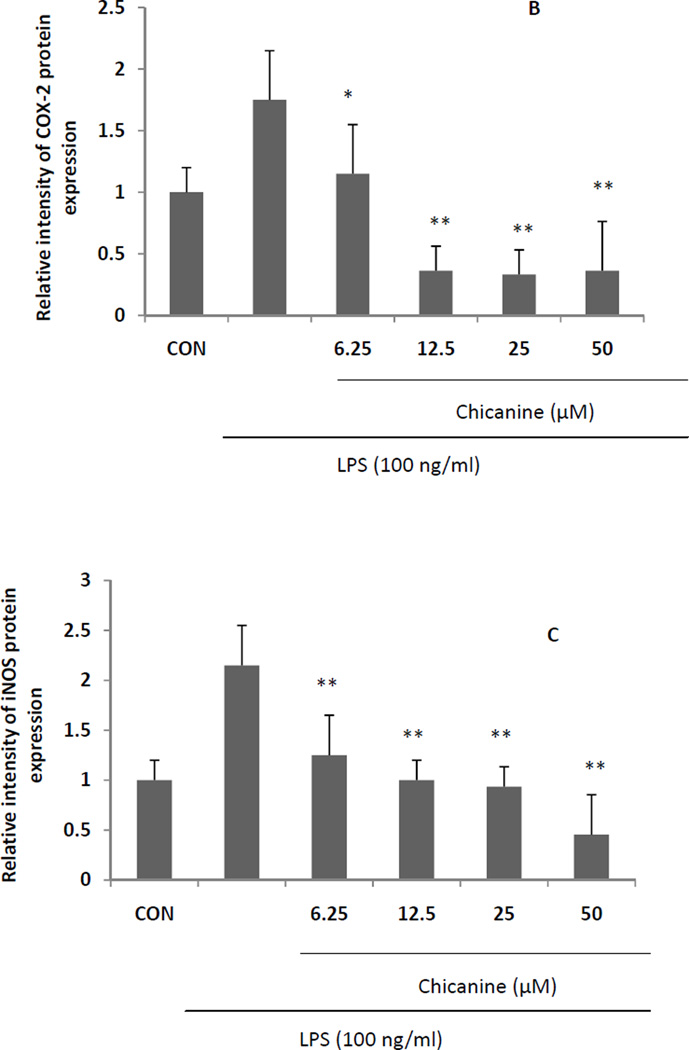
Further studies of cytokine protein expression were consistent with our studies of mRNA expression. The results (Fig. 5) showed that with chicanine co-treatment, LPS-induced expressions of COX-2 and iNOS protein were reduced in concentration-dependent manners in the concentration from 6.25 to 25 µM by up to 81% and 77%, respectively. The results of iNOS protein and mRNA expression levels were not completely in agreement with the measurement of nitrite. This might be due to the different treated time of chicanine on RAW264.7 cells and chicanine might suppress the LPS-mediated induction of the cytokines at the different levels. On the other hand, chicanine might not only potently inhibit the generation of NO in macrophages, but also effectively scavenged it.
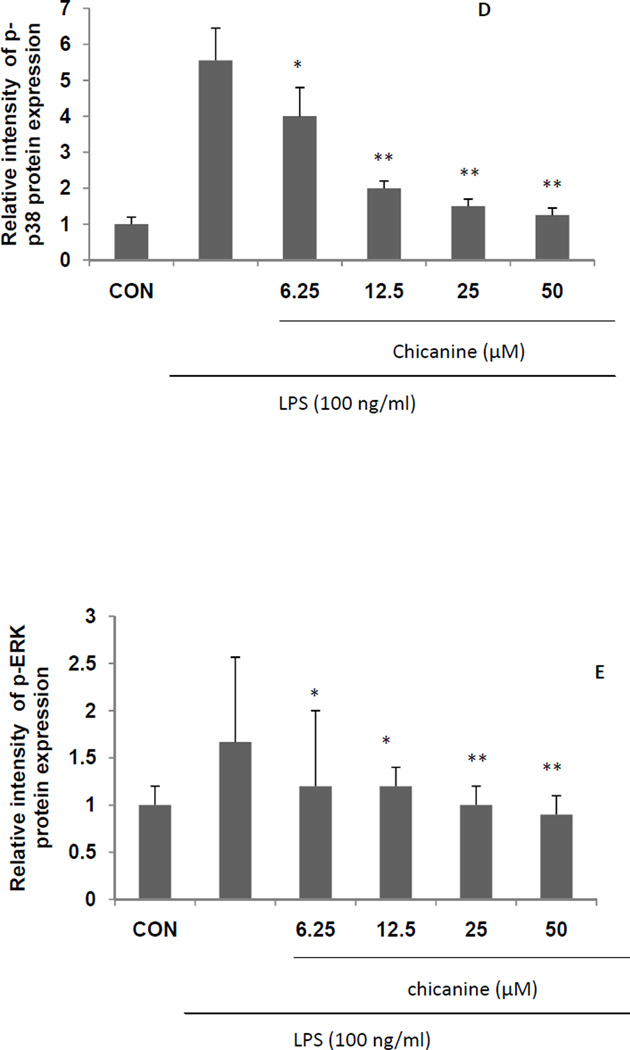
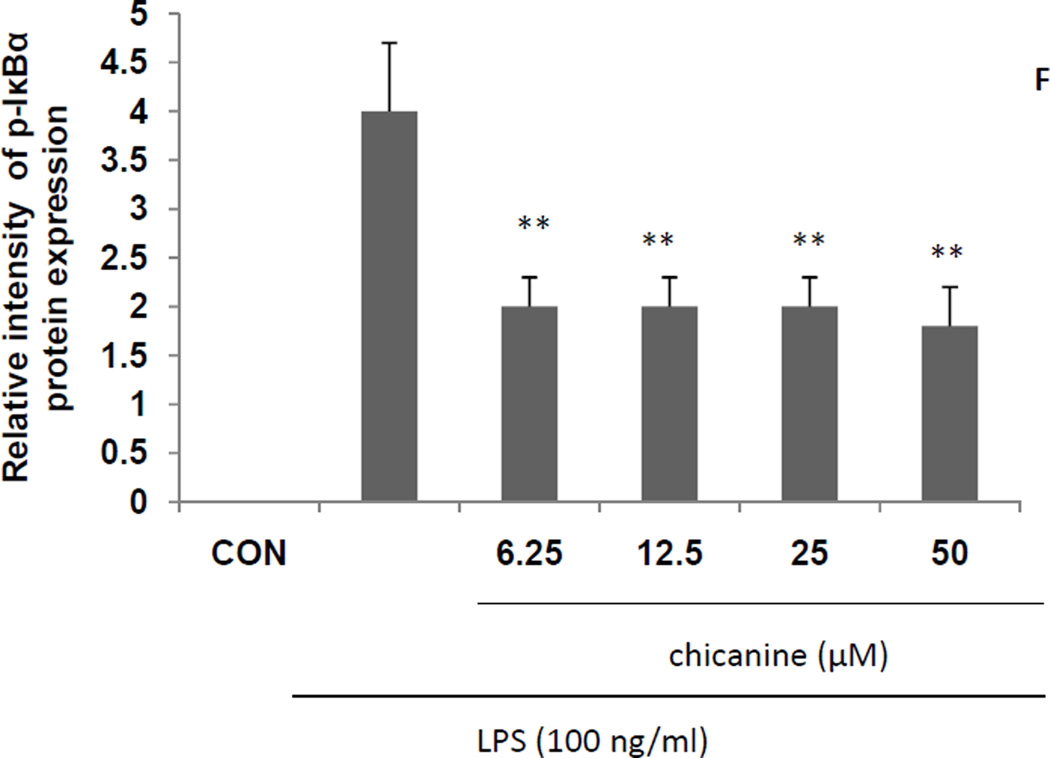
Fig. 5. Effects of chicanine on the LPS-induced cell signaling pathway cytokines in RAW 264.7 cells by Western blotting.
Figures show the representative images of three independent experiments. The relative intensity of protein expression is expressed as mean ± S.E.M. *P <0.05, **P<0.01 compared with the LPS treated cells.
3.5. Effect of chicanine on P38 and ERK1/2 phosphorylation
LPS induction of cytokine expression occurs via activation of MAPK and ERK phosphorylation following binding to TLR4 (Guo et al., 2011). To understand whether the anti-inflammatory activities of chicanine are mediated through the MAPK pathway, we examined the effects of chicanine on LPS-induced phosphorylations of p38 MAPK and ERK 1/2 by Western-blot analysis. RAW 264.7 cells were treated with increasing concentrations of chicanine and stimulated with 100 ng/ml of LPS for 6h. As indicated in Fig. 5, activation of MAPK(P38 and ERK) signaling did occur in RAW264.7 cells compared with control group. LPS induced p38and ERK phosephorylation was attenuated by chicanine. Co-treatment with increasing concentrations of chicanine suppressed LPS-induced phosphorylation of p38 and ERK 1/2 in concentration-dependent manners by as much as 77% and 46%, respectively, at the highest concentration. The amounts of non-phosphorylated p38 and ERK 1/2 were apparently unaffected by LPS or LPS plus chicanine treatment. These results suggested that the anti-inflammatory activity of chicanine was mediated by inhibition of the LPS-induced phosphorylation of p38 and ERK 1/2.
3.6. Effects of chicanine on IκBα phosphorylation
To further examine the effects of chicanine on up-stream regulators of immune signaling, effects were determined on IκBα phosphorylation by Western blot. As shown in Fig. 5, LPS induced the phosphorylation of IκBα, which was inhibited by up to 55% by the treatment of chicanine. These results were consistent with an anti-inflammatory effect of chicanine that was mediated by inhibition of IκBα phosphorylation. However, there was no dose-dependent effect found. The reason might be due to the other mode of action by other controversy pathways at the high concentration, which will need further investigation.
4. Discussion
Macrophages play important roles in both innate and adaptive immunity in vertebrate animals. Some phagocytic activities of macrophages are involved in many inflammatory diseases. Therefore, development of a drug that can suppress macrophage activity is a valuable approach to the treatment of inflammatory disease (Oh et al., 2010). Macrophages play a crucial role in the inflammatory response through the release of a variety of factors, such as nitric oxide (NO), prostaglandin mediators and pro-inflammatory cytokines such as TNF-α, IL-1β, MCP-1 and G-CSF, in response to an activating stimulus, e.g. lipopolysaccharide (LPS). The stimulation of TLR4 by LPS induces the release of critical pro-inflammatory cytokines. Production of these mediators has been demonstrated in several inflamed tissues, along with enhanced expression of their mRNAs (Moro et al., 2012). In this study, chicanine was found to inhibit the mRNAs expression of the pro-inflammatory cytokines in LPS-induced inflammatory responses in murine macrophages.
Schinsandra chinensis is a Chinese traditional medicine for the treatment of hepatic disease. Some studies found that lignans from Schinsandra chinensis have anti-inflammatory activities in the RAW 264.7 macrophage cell line (Oh et al., 2010; Guo et al., 2008). Chicanine is one of the major lignans of Schinsandra chinensis, but there is no report on the anti-inflammatory effects of chicanine. In this study, chicanine significantly inhibited NO production and NF-κB luciferase activity. Therefore, we conducted a further, mRNA and protein-level study, and found that chicanine could decrease the mRNA and protein expression of proinflammatory mediators COX-2 and iNOS in concentration-dependent manners. Thus, chicanine reduces the expression of these pro-inflammatory factors at the level of transcription likely by modulation of upstream regulators.
Chicanine was found to inhibit the mRNA expression of the pro-inflammatory cytokines, including TNF-α, IL-1β, MCP-1 and G-CSF, which are mainly produced in the LPS-induced TLR4 mediated pathway and play critical roles in inflammatory and autoimmune diseases. To further elucidate the anti-inflammatory mechanism, the phosphorylation of IκB-α protein was examined and found to be inhibited by treatment of chicanine. The results of this study showed that the phosphorylation of p38 and ERK1/2 were also inhibited by chicanine in concentration-dependent manner.
Cells recognize and respond to extracellular stimuli by engaging specific intracellular programs, such as the signaling cascade that leads to activation of the mitogen-activated protein kinases (MAPKs). All eukaryotic cells possess multiple MAPK pathways, which coordinately regulate diverse cellular activities running the gamut from gene expression, mitosis, and metabolism to motility, survival and apoptosis, and differentiation. To date, five distinct groups of MAPKs have been characterized in mammals: extracellular signal-regulated kinases (ERKs) 1 and 2 (ERK1/2), c-Jun amino-terminal kinases (JNKs) 1, 2, and 3, p38 isoforms α, β, γ, andδ , ERKs 3 and 4, and ERK5 (Philippe&Blenis, 2004). Generally, phosphorylation of these kinases is induced by stress and bacterial endotoxin and they are involved in cell differentiation and proliferation of mammalian cells (Johnson &Lapadat, 2002). Several studies have reported that MAP kinase is involved in the production of LPS-induced inflammatory mediators, the expression of various inflammatory inducers such as NO, COX and cytokines. Therefore, some MAP kinases such as p38and ERK has become the target molecules in anti-inflammatory medicine (Oh et al., 2010).
NF-κB, the mammalian transcription factor, is involved in regulating many aspects of cellular activity, in stress, injury and especially in pathways of the immune response. Some examples are many aspects of the inflammatory response, e.g. induction of IL-1 (alpha and beta), TNF-alpha and leukoyte adhesion molecules (E-selectin, VCAM-1 and ICAM-1). NF-κB itself is induced by stimuli such as pro-inflammatory cytokines and bacterial toxins (e.g. LPS, exotoxin B) and a number of viruses/viral products (e.g. HIV-1, HTLV-I, HBV, EBV, Herpes simplex) as well as pro-apoptotic and necrotic stimuli (oxygen free radicals, UV light, gamma-irradiation) (May et al., 1998).
IκBα is one member of a family of cellular proteins that function to inhibit the NF-κBtranscription factor. IκBα inhibits NF-κB by masking the nuclear localization signals (NLS) of NF-κB proteins and keeping them sequestered in an inactive state in the cytoplasm (Jacobs & Harrison, 1998). In addition, IκBα blocks the ability of NF-κB transcription factors to bind to DNA, which is required for NF-κB's proper functioning (Verma et al., 1995).In this study, the LPS-induced phosphorylation of p38, ERK1/2 and IκB-α were reduced by chicanine, which implied the anti-inflammatory activities.
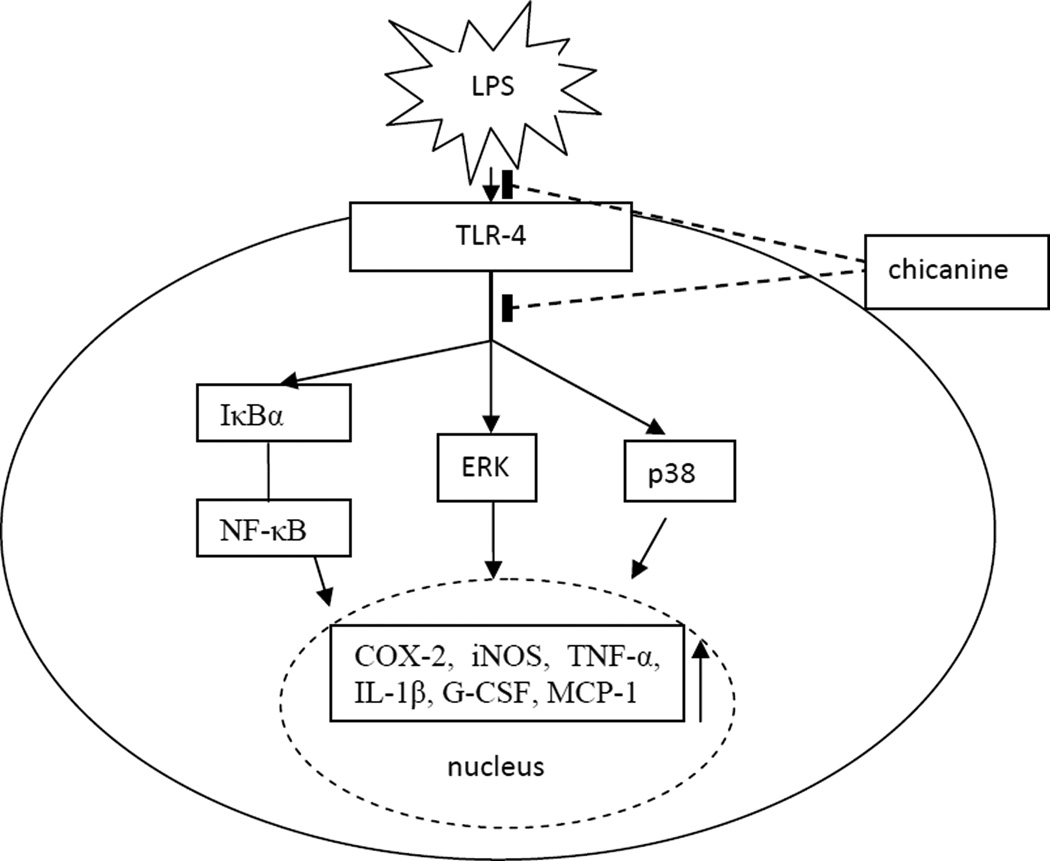
There were many compounds were investigated on the suppression of LPS-induced inflammatory responses in RAW 264.7 cells. Scott et al. found that cationic antimicrobial peptides block the binding of LPS to LPS binding protein, which was correlated with their ability to block LPS-induced TNF-α production by the RAW 264.7 macrophage cell line ( Scott et al., 2000). On the other hand, Matsunaga et al. reported that TAK-242 (resatorvid), a small-molecule–specific inhibitor of-TLR 4 signaling, inhibited the production of lipopolysaccharide-induced inflammatory mediators by binding to Cys747 in the intracellular domain of TLR4 (Matsunaga et al., 2011). In this study, it was indicated that chicanine reduced NO production, inhibited NF-κB luciferase activity and suppressed iNOS and COX-2 expression through inhibition of NF-κB activation and blocking of the p38 and ERK 1/2 pathways. These effects are similar to the anti-inflammatory mechanism of schisandrin(Guo et al., 2008). The proposed targets for the anti-inflammatory effects of chicanine in the RAW264.7 cells stimulated by LPS are shown in Fig. 6. Whether chicanine inhibits LPS binding to TLR4 or otherwise binds to TLR4 to inhibit signaling will need further study.
Fig. 6.
Schematic diagram of the proposed targets for the anti-inflammatory effects of chicanine in the RAW264.7 cells stimulated by LPS, potentially leading to the inhibition of the pro-inflammatory cytokines and related mediators.
In conclusion, this is the first investigation of the anti-inflammatory activity of chicanine and its functional mechanism in activated macrophages (RAW 264.7 cells). Chicanine was found to inhibit LPS-induced TLR4-IκB-α/MAPK/ERK signaling pathways, indicating that chicanine has a potential anti-inflammatory application.
Acknowledgement
This work was supported in part by a grant from the Natural Science Foundation of China (NSFC 3137)1879) and National High Technology Research and Development Program ("863"Program) of China (Grant No. SS2013AA100207).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunology. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY, Hong YN, Kang SS, Kim HP, Kim YS. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008;591:293–299. doi: 10.1016/j.ejphar.2008.06.074. [DOI] [PubMed] [Google Scholar]
- Guo YM, Zhang XT, Meng J, Wang ZY. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur. J. Pharmacol. 2011;658:114–122. doi: 10.1016/j.ejphar.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MD, Harrison SC. Structure of an IkappaBalspha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH, Lee JJ. Antiinflammatory constituents of Celastrus orbiculatus inhibits the NF-κB activation and NO production. J. Nat. Prod. 2002;65:89–91. doi: 10.1021/np010428r. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303:9–20. doi: 10.1016/j.canlet.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koo TH, Yoon HK, Jung HS, Jin HZ, Lee K, Hong YS, Lee JJ. Inhibition of NF-κB activation through targeting IκB kinase by celastrol, a quinone methide triterpenoid. Biochem. Pharm. 2006;72:1311–1321. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Lin YC, Huang DY, Chu CL, Lin WW. Anti-inflammatory actions of Syk inhibitors in macrophages involve non-specific inhibition of toll-like receptors-mediated JNK signaling pathway. Mol. Immunol. 2010;47:1569–1578. doi: 10.1016/j.molimm.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Lu Y, Chen DF. Analysis of Schisandra chinensis and Schisandra Sphenanthera. J Chromatogr. A. 2009;1216:1980–1990. doi: 10.1016/j.chroma.2008.09.070. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-Like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- May MJ, Ghosh S. Signal transducion through NF-κB, Immun. Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- Moro C, Palacios I, Lozano M, Arrigo MD, Guillamón E, Villares A, Martínez JA, García-Lafuente A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012;130:350–355. [Google Scholar]
- Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim CY, Lee HJ, Lee JK. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci. Biotechnol. Biochem. 2010;74:285–291. doi: 10.1271/bbb.90597. [DOI] [PubMed] [Google Scholar]
- Philippe PR, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. MolBiol. Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw CLL, Huang Y, Kong AN. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010;79:421–430. doi: 10.1016/j.bcp.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Scott MG, Vreugdenhil ACE, Buurman WA, Hancock REW, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 2000;164:549–553. doi: 10.4049/jimmunol.164.2.549. [DOI] [PubMed] [Google Scholar]
- Takada E, Okahira S, Sasai M, Funami K, Seya T, Matsumoto M. C-terminal LRRs of human Toll-like receptor 3 control receptor dimerization and signal transmission. Mol. Immunol. 2007;44:3633–3640. doi: 10.1016/j.molimm.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;99:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Wang ZS, Chen HX, Zhang WJ, Lan GS, Zhang LK. Comparative studies on the chemical composition and antioxidant activities of Schisandra chinensis and Schisandra sphenanthera fruits. J. Med. Plants Res. 2011;5:1207–1216. [Google Scholar]
- Wu QX, Crews MS, Draskovic M, Sohn J, Johnson TA, Tenney K, Valeriote FA, Yao XJ, Bjeldanes LF, Crews P. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola Org. Lett. 2010;12:4458–4461. doi: 10.1021/ol101396n. [DOI] [PMC free article] [PubMed] [Google Scholar]