SUMMARY
Rhinitis and rhinosinusitis (with/without polyposis), either allergic or non-allergic, represent a major medical problem. Their associated comorbidities and relationship with family history have so far been poorly investigated. We assessed these aspects in a large population of patients suffering from rhinosinusal diseases. Clinical history, nasal cytology, allergy testing and direct nasal examination were performed in all patients referred for rhinitis/rhinosinusitis. Fibre optic nasal endoscopy, CT scan and nasal challenge were used for diagnosis, when indicated. A total of 455 patients (60.7% male, age range 4-84 years) were studied; 108 (23.7%) had allergic rhinitis, 128 (28.1%) rhinosinusitis with polyposis, 107 (23.5%) non-allergic rhinitis (negative skin test); 112 patients had associated allergic and non-allergic rhinitis, the majority with eosinophilia. There was a significant association between non-allergic rhinitis and family history of nasal polyposis (OR = 4.45; 95%CI = 1.70-11.61; p = 0.0019), whereas this association was no longer present when allergic rhinitis was also included. Asthma was equally frequent in non-allergic and allergic rhinitis, but more frequent in patients with polyposis. Aspirin sensitivity was more frequent in nasal polyposis, independent of the allergic (p = 0.03) or non-allergic (p = 0.01) nature of rhinitis. Nasal polyposis is significantly associated with asthma and positive family history of asthma, partially independent of the allergic aetiology of rhinitis.
KEY WORDS: Allergic rhinitis, Non-allergic rhinitis, Nasal polyposis, Nasal cytology, Family history, Atopy
RIASSUNTO
Le riniti allergiche e non allergiche, le rinosinusiti (con / senza poliposi), rappresentano un importante problema medico. La loro comorbilità, e il rapporto con la familiarità sono stati finora poco studiati. Abbiamo valutato questi aspetti in un'ampia popolazione di pazienti affetti da patologie rino-sinusali. Tutti i soggetti sono stati sottoposti ad indagine anamnestica, citologia nasale, test allergologici, rinomanometria anteriore attiva (RAA) ed endoscopia nasale a fibre ottiche. Quando indicata è stata eseguita TC naso-sinusale. Sono stati studiati 455 pazienti (60,7% maschi, di età compresa tra i 4 e 84 anni). 108 di essi (23,7%) presentavano rinite allergica, 128 (28,1%) avevano rinosinusite con poliposi, 107 (23,5%) rinite non allergica. 112 pazienti avevano una rinite allergica associata ad una forma non allergica (riniti "sovrapposte"), prevalentemente a cellularità eosinofila. Abbiamo riscontrato una significativa associazione tra le forme di riniti non allergiche "cellulari" e la familiarità per poliposi nasale (OR = 4,45, 95%CI = 1,70-11,61, p = 0,0019), mentre questa associazione non è stata rilevata nelle forme allergiche. L'asma era ugualmente frequente nella rinite allergica e non allergica, maggiore nei pazienti con poliposi. La sensibilità all'aspirina era più frequente nella poliposi nasale, indipendente dalla presenza di associate allergie (p = 0,03) o riniti non allergiche (p = 0,01). Infine, la poliposi nasale è significativamente associata ad asma e familiarità per asma, indipendentemente dalla rinite allergica.
Introduction
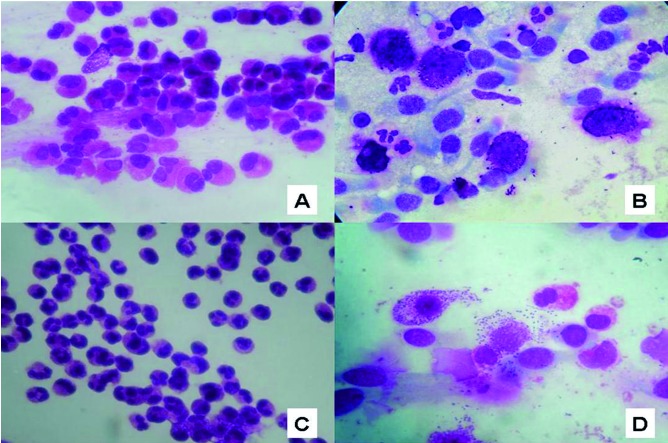
Allergic and non-allergic rhinitis, rhinosinusitis (with/ without nasal polyposis, NP) and other associated diseases (i.e. asthma, hearing disorders, sleep disorders) severely impair the quality of life of patients, and represent important challenges to the physician both from diagnostic and therapeutic points of view 1-4. For years, scientific efforts have been made to identify and distinguish the pathophysiology of these diseases, but to date many aspects still remain unclear. In fact, if for allergic rhinitis (AR) and chronic rhino-sinusitis without nasal polyposis, the epidemiology and immunological mechanisms are relatively well known 5-10, but few data are available for non-allergic rhinitis (NAR), in particular for the forms called "cellular", which include non-allergic rhinitis with neutrophils (NARNE), non-allergic rhinitis with eosinophils (NARES), non-allergic rhinitis with mast cells (NARMA), nonallergic rhinitis with eosinophils and mast cells (NARESMA) (Fig. 1A-D) 11-14. and the overlapping forms 15.
Fig. 1.
Nasal cytology: A) non-allergic rhinitis with eosinophils (NARES); B) non-allergic rhinitis with mast cells (NARMA); C) non-allergic rhinitis with neutrophils (NARNE); D) non-allergic rhinitis with eosinophils and mast cells (NARESMA) May-Grünwald- Giemsa staining, original magnification ×1,000.
To date, no precise pathogenic link among these diseases has been demonstrated, although it would be of enormous benefit in establishing appropriate medical/surgical management, and to prevent the onset of complications.
In this study, we evaluated a large number of patients suffering from nasal disorders (AR, NAR, NP, overlapping rhinitis), assessing the occurrence of selected comorbidities (allergy, asthma, aspirin sensitivity), and their possible correlation with family history of atopy, asthma and nasal polyposis.
Materials and methods
Patients referred to our ENT unit for nasal diseases between January 2010 and December 2012 were evaluated. Thorough medical history was always obtained, and all patients underwent skin prick test, flexible nasal endoscopy and nasal cytology.
The family history primarily focused on asthma, aspirin sensitivity, nasal polyposis and atopy, investigating at least four degrees of relationship (1st degree: parents and children; 2nd degree: siblings, grandparents and grandchildren, 3rd degree: aunts and uncles; 4th degree: cousins). This aspect was detailed as best as possible, since the usual response to the question "Do any of your family members and/or relatives suffer from nasal polyposis, asthma or atopy?" was vague or negative.
Nasal endoscopy was carried out by mean of a flexible fiberscope (Vision Science ENT 2000, diameter 3.4 mm). None of the patients received local anaesthesia or nasal decongestion. Allergic sensitization was assessed by a skin prick test that was carried out and read according to guidelines. Results were considered positive if the major wheel diameter was 3 mm or greater 16. The panel of commercial allergens used (Stallergenes, Milan, Italy) included: house dust mite (Dermatophagoides farinae and pteronyssinus), cat, dog, grass mix, Compositae mix, Parietaria judaica, birch, hazelnut, olive, Alternaria tenuis, Cladosporium and Aspergilli mix. A CAP-RAST assay (Phadia, Uppsala, Sweden) was carried out as a second line test when indicated, and a specific IgE concentration >; 0.35 kU/l was considered positive.
Nasal cytology was performed obtaining a nasal scraping from the middle portion of the inferior turbinate, using a Rhino-Probe® 17 18. Samples were placed on a glass slide, fixed by air drying and stained by May-Grunwald Giemsa method (Carlo Erba®, Milan, Italy). The slides were read under a Nikon E600 light microscope (Nikon, Canada) equipped with a digital camera. For the rhinocytogram analysis, 50 microscopic fields were read at a magnification of 1,000× to assess the presence of normal and abnormal cellular elements, or other pathologic microscopic features. Cell count was carried out by a semi-quantitative grading, as proposed by Meltzer and Jalowayski 19. In particular, bacteria and fungal spores were quantified as follows: Grade 0 (not visible); Grade 1+ (occasional groups); Grade 2+ (moderate number); Grade 3+ (easily visible); Grade 4+ (entire field).
Patients were classified as having AR or NAR based on skin prick test results. The cellular forms of rhinitis were further subdivided based on their cytotype into NARNE (neutrophils >; 50% with absent spores and bacteria); NARES (eosinophils >; 20%); NARMA (mast cells >; 10%); NARESMA (eosinophils >; 20% and mast cells >; 10%) 20.
All data were recorded in a dedicated database (FileMaker Pro) and analyzed using STATA software MP11. For proportions, the chi-square test was used. To evaluate the association between the disease and the investigated determinants, the OR with confidence intervals was calculated and the chi-square test was performed. For all tests, a p < 0.05 was considered statistically significant.
Results
In the present study, 455 patients (60.7% male, mean age 38.7 ± 18.3 years, age range 4-84 years) were studied. Of these, 108 (23.7%) had AR (30.5% monosensitized), 128 (28.1%) had nasal polyposis (32.8% with concomitant allergy), 107 (23.5%) had cellular rhinitis, of which 53 (49.5%) NARES, 54 (50.5%) NARESMA. Finally, 112 (24.6%) patients were classified as "overlapping" AR+NAR, of whom 39 (34.8%) with NARES, 8 (7.1%) with NARMA and 65 (58%) with NARESMA. These overlapping patients had a positive skin test/IgE assay, but also positive cytology for eosinophils and/or mast cells outside the pollen season.
Within IgE positive patients, the most frequent sensitizations were for mite (57.4%) followed by cypress (46.3%), olive (44.4%), grasses (38.9%), Parietaria (36.1%), dog/cat epithelium (30.5%) and fungi (6.5%). The distribution of patients by gender was not different for any of the diseases (chi-square = 4.12, p = 0.38). Table I summarizes the occurrence of the various diseases, the frequency of positive family history for allergy/asthma, nasal polyps and acetylsalicylic acid sensitivity.
Table I.
Occurrence of family history of atopy, asthma, nasal polyps and clinical signs such as: allergies, asthma and acetylsalicylic acid (ASA) sensitivity.
| AR n = 108 |
NAR n = 107 |
AR+NAR n = 112 |
NP n = 128 |
|
|---|---|---|---|---|
| Family history of allergy | 9 (8.3%) |
10 (9.3%) |
22 (19.6%) |
8 (6.25%) |
| Family history of asthma | 21 (19.4%) |
26 (24.3%) |
43 (38.4%) |
21 (16.4%) |
| Family history of nasal polyposis | 5 (4.7%) |
19 (17.8%) |
22 (19.6%) |
21 (16.4%) |
| Allergy | - | - | - | 42 (32.8%) |
| Asthma | 16 (14.8%) |
8 (7.5%) |
29 (25.9%) |
42 (32.8%) |
| Sensitivity to acetylsalicylic acid (ASA syndrome) | 5 (4.6%) |
4 (3.7%) |
5 (4.5%) |
16 (12%) |
AR: allergic rhinitis; NAR: non-allergic rhinitis; NP: nasal polyposis.
Positive family history for allergy had the same prevalence in AR and NAR groups (p = 0.79), and in the AR and NP groups (p = 0.37). A positive family history of allergy was more frequent in the overlapping forms of rhinitis compared to the pure AR group (p = 0.03), and when compared with NAR (p = 0.02) and nasal polyposis patients (p = 0.002). Table II shows the frequencies for family history of allergy at different degrees of relationships.
Table II.
Frequency of family history of allergy, asthma and nasal polyposis in different degrees of relationship.
| DEGREE OF RELATIONSHIP | |||
|---|---|---|---|
| 1st DEGREE Parents and offspring | 2nd DEGREE Brothers, grandparents/ grandchildren | 3rd/4th DEGREE Aunts, uncles and cousins | |
| FAMILIARITY FOR ALLERGY | |||
| Allergic rhinitis (n = 12) | 7 (58.3%) | 4 (33.3%) | 1 (8.3%) |
| Non-allergic rhinitis (n = 11) | |||
| NARES | 2 (18.1%) | 2 (18.1%) | - |
| NARMA | - | - | - |
| NARESMA | 6 (54.5%) | - | 1 (9%) |
| Allergic rhinitis + non-allergic rhinitis (c.n. 24) | |||
| NARES | 5 (20.8%) | 2 (8.3%) | 3 (12.5%) |
| NARMA | 1 (4.1%) | 1 (4.1%) | - |
| NARESMA | 6 (24%) | 4 (16.7%) | 2 (8.3%) |
| Nasal polyps (n = 9) | 4 (44.4%%) | 4 (44.4%%) | 1(11.1%) |
| FAMILIARITY FOR ASTHMA | |||
| Allergic rhinitis (n = 21) | 10 (47.6%) | 6 (28.6%) | 5 (23.8%) |
| Non-allergic rhinitis (n = 30) | |||
| NARES | 8 (26.7%) | 8 (26.7%) | 2 (6.7%) |
| NARMA | - | - | - |
| NARESMA | 5 (16.7%) | 4 (13.3%) | 3 (10%) |
| Allergic rhinitis + non-allergic rhinitis (n = 45) | |||
| NARES | 4 (8.9%) | 2 (4.4%) | 4 (8.9%) |
| NARMA | 1 (2.2%) | 2 (4.4%) | - |
| NARESMA | 12 (26.7%) | 11 (24.4%) | 9 (20%) |
| Nasal polyps (n = 22) | 12 (54.5%) | 6 (27.3%) | 4 (18.1%) |
| FAMILIARITY FOR NASAL POLYPS | |||
| Allergic rhinitis (n = 6) | 2 (33.3%) | 3 (50%) | 1 (16.7%) |
| Non-allergic rhinitis (n = 20) | |||
| NARES | - | 1 (5%) | 3 (15%) |
| NARMA | - | - | - |
| NARESMA | 9 (45%) | 4 (20%) | 3 (15%) |
| Allergic rhinitis + non-allergic rhinitis (n = 25) | |||
| NARES | 2 (8%) | 3 (12%) | 5 (20%) |
| NARMA | - | 1 (4%) | - |
| NARESMA | 5 (20%) | 4 (16%) | 5 (20%) |
| Nasal polyps (n = 22) | 10 (45.5%) | 7 (31.8%) | 5 (22.7%) |
| TOTAL (n = 247) | 111 (44.9%) | 79 (31.9%) | 57 (23%) |
A positive family history for asthma was found with the same prevalence in the AR group and NP (p = 0.54), in AR and NAR (p = 0.41) and in NAR and NP (p = 0.68). In contrast, overlapping rhinitis showed a higher frequency of familiarity both for AR (p = 0.002) and for NP (p = 0.02). Comparing NAR and overlapping rhinitis, asthma was associated with a greater risk for the overlapping forms (OR = 1.96, 95%CI = 1.06-3.68, p = 0.02) (Table II).
Comparing the frequency of family history for nasal polyps in NAR and AR patients, a statistically significant association emerged in favour of NAR (OR = 4.45, 95%CI = 1.70-11.61, p = 0.0019). No significant difference in the frequency of family history of nasal polyps between NAR and NP (p = 0.46), and between overlapping rhinitis and NP (p = 0.31) was found (Table II).
The difference in the frequency of asthma in the AR and NAR groups remained borderline (p = 0.06). In addition, asthma had a comparable frequency between overlapping rhinitis and NP groups (p = 0.15), but asthma was more common in the NP group compared with the AR group (p = 0.001) and the NAR group (p < 0.0001).
ASA sensitivity had the same prevalence in the AR vs. NAR group (p = 0.5), in the AR group vs. overlapping rhinitis group (p = 0.6) and in the NAR vs. overlapping group (p = 0.5). ASA sensitivity resulted more frequent in the NP group than in AR (p = 0.03), as compared to NAR (p = 0.01) and overlapping rhinitis (p = 0.02).
Discussion
In daily clinical practice of an ENT-Allergology centre, different forms of rhinitis, such as allergic, non-allergic, rhinosinusitis with or without nasal polyposis are seen. Therefore, the specialist must adopt increasingly complex diagnostic and instrumental methods for diagnosis and management. In fact, only a detailed diagnosis allows to characterize and optimally treat nasal diseases. Nonetheless, the identification of more complex and less characterized entities still poses diagnostic and therapeutic challenges 15 17 21.
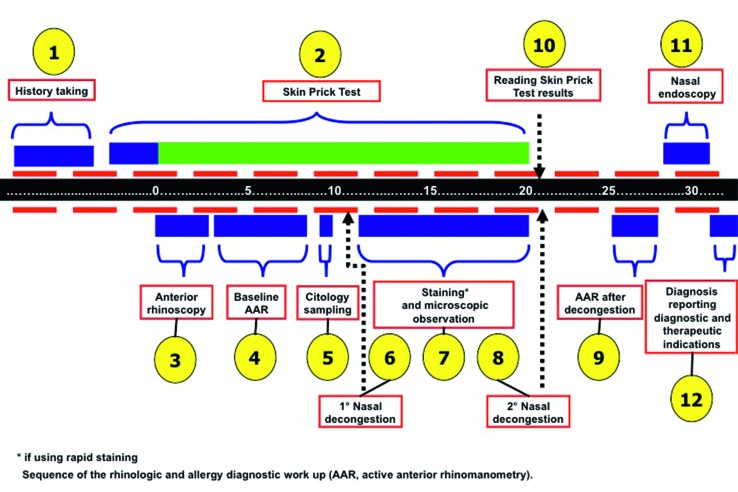
To receive the most accurate diagnosis, the patient should undergo thorough diagnostic work-up, where family history must not be excluded, and accompanied by imaging, functional and immunological evaluations (Fig. 2).By performing a broad investigational procedure, in the present study we observed that there was a high "global" familial incidence of allergy, asthma and nasal polyposis, not only between first and second degree relatives (44.9 and 31.9%), but also in third and fourth degree ones (23%). These data confirm the fact that, for some diseases, genetic background plays a crucial role and should be taken into consideration.
Fig. 2.
For more accurate diagnosis, the rhinological patient must be able to follow a precise diagnostic work-up, where family history must not be excluded, in addition to careful and thorough clinical history, and at least four levels of analysis should be provided: "macroscopic" investigation (by anterior rhinoscopy and nasal endoscopy); "microscopic" investigation (by nasal cytology); allergy investigation (by skin prick tests) and "functional" investigation (by basic active anterior rhinomanometry and after decongestion).
In particular, "cellular" rhinitis such as NARES, NARMA and NARESMA had a high percentage of positive family history for asthma and nasal polyposis, similar to patients with NP (24.3 vs. 16.4% and 17.8 vs. 16.4%); while allergic rhinitis (although with a familial incidence of asthma slightly higher than in patients with NP [19.4 vs. 16.4%]) presented a lower positive family history for nasal polyposis (4.7 vs. 16.4%), approaching the epidemiological data of the general population 22.
Patients with "overlapping" rhinitis (AR+NAR) showed a familial incidence of asthma and nasal polyps greater than the NAR forms, of 38.4 and 19.6%, respectively. The data describing the familial incidence of NP, both in "cellular" and "overlapping rhinitis" (17.8 and 19.6%, respectively) demonstrate a clear link between these diseases and NP (OR = 4.45; 95%CI = 1.70-11.61, p = 0.0019), further confirming the confusion of numerous aetiopathogenetic theories on the formation of nasal polyps described in the last 20 years 23, and from time to time the denial of the theory of "epithelial breaking" 24, fungal aetiology 25, superantigens, etc. 26 We propose a hypothesis based on "familial-inheritance" that leads to the formation of nasal polyps as the "hyperplastic" evolution of a vasomotor cellular type rhinopathy. If this occurs in association with allergy, the IgE component would seem not determinant to lead to hyperplasia. Nonetheless, longitudinal studies of patients with NARES, NARMA and NARESMA are necessary to confirm this hypothesis.
Concerning AR, our data confirm those of the available literature 29 that exclude a common aetiology with NP, with an incidence of 32.8% in the group of patients with NP, similar to that found in the general population. Concerning the prevalence of asthma in rhinitis, our data are also consistent with that reported in epidemiological studies for both AR (14.8%) and NP (32.8%) 16. On the other hand, our finding of a large difference in incidence of asthma between cellular NAR (7.5%) and "overlapping" rhinitis (22.3%) is more difficult to interpret. Undoubtedly, the presence of two diseases (AR + NAR) in the same patient could justify an increased damage to respiratory epithelium 31 32 34-36.
Remaining within the rhino-allergic diagnostic realm, we suggest that "overlapping" rhinitis should always be diagnosed, since it has a different clinical evolution from the "pure" forms of AR. Its diagnosis would also avoid erroneous epidemiological estimates, such as: i) indicating that AR increases the incidence of asthma, or increases comorbidities such as sinusitis, otological diseases, sleep disorders and nasal polyps; ii) treatment failures (e.g. the failure of specific immunotherapy), and iii) preventing complications such as asthma, nasal polyps, etc.).
In this regard, the important role of nasal cytology is paramount, as it is the only laboratory investigation capable of diagnosing "cellular" NAR, to expose "overlapping" rhinitis (Table III), and to confirm the IgE-mediated rhinitis and monitor treatment. Therefore, although little used and valued, in our opinion this very useful method should systematically be used as a tool for diagnosis of rhinitis.
Table III.
When to suspect "overlapping" of different rhinopathies (allergic rhinitis+NARES, NARMA or NARESMA).
| When to suspect "overlapping" of different rhinopathies (allergic rhinitis + NARES, NARMA or NARESMA) |
|---|
| Clinical criteria |
|
| Cytologic criteria |
|
Rhino-allergic diagnosis is an articulate and complex procedure, and detailed patient history is mandatory. The instrumental diagnostic tools play a crucial role in clinical diagnosis, especially in the "cellular" forms of nonallergic vasomotor rhinitis and "overlapping" rhinitis, diseases that are not always diagnosed correctly and for many still unknown. Our results reasonably suggest the existence of a direct link between non-allergic rhinitis and nasal polyposis, based on hereditary-familial aspects. More systematic and larger cohort studies will be of help in understanding the pathogenetic mechanisms of both nasal polyposis and its comorbidities.
References
- 1.Bousquet PJ, Demoly P, Devillier P, et al. Impact of allergic rhinitis symptoms on quality of life in primary care. Int Arch Allergy Immunol. 2012;160:393–400. doi: 10.1159/000342991. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. Functional limitations and workdays lost associated with chronic rhinosinusitis and allergic rhinitis. Am J Rhinol Allergy. 2012;26:120–122. doi: 10.2500/ajra.2012.26.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelardi M, Maselli Del Giudice A, Fiorella ML, et al. Quality of life in non-allergic rhinitis depends on the predominant inflammatory cell type. J Biol Regul Homeost Agents. 2008;22:73–81. [PubMed] [Google Scholar]
- 4.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 5.Osguthorpe JD. Pathophysiology of and potential new therapies for allergic rhinitis. Int Forum Allergy Rhinol. 2013;3:384–392. doi: 10.1002/alr.21120. [DOI] [PubMed] [Google Scholar]
- 6.Kavut AB, Kalpaklıoğlu F, Atasoy P. Contribution of neurogenic and allergic ways to the pathophysiology of nonallergic rhinitis. Int Arch Allergy Immunol. 2012;160:184–191. doi: 10.1159/000339739. [DOI] [PubMed] [Google Scholar]
- 7.Sedaghat AR, Gray ST, Wilke CO, et al. Risk factors for development of chronic rhinosinusitis in patients with allergic rhinitis. Int Forum Allergy Rhinol. 2012;2:370–375. doi: 10.1002/alr.21055. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy DW. The role of biofilms in the pathogenesis of chronic rhinosinusitis. Editorial. Int Forum Allergy Rhinol. 2011 Sep-Oct;1(5) doi: 10.1002/alr.20099. Fmx. doi: 10.1002/alr.20099. [DOI] [PubMed] [Google Scholar]
- 9.Seiberling KA, Church CA, Herring JL, et al. Epigenetics of chronic rhinosinusitis and the role of the eosinophil. Int Forum Allergy Rhinol. 2012;2:80–84. doi: 10.1002/alr.20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crombruggen K, Zhang N, Gevaert P, et al. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–732. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs RL, Freedman PM, Boswell RN. Non-allergic rhinitis with eosinophilia (NARES syndrome): clinical and immunologic presentation. J Allergy Clin Immunol. 1981;67:253–257. doi: 10.1016/0091-6749(81)90019-1. [DOI] [PubMed] [Google Scholar]
- 12.Connell JT. Nasal mastocytosis. J Allergy. 1969;43:182–189. [Google Scholar]
- 13.Gelardi M, Maselli Del Giudice A, Fiorella ML, et al. Nonallergic rhinitis with eosinophils and mast cells (NARESMA) constitutes a new severe nasal disorder. Int J Immunopathol Pharmacolol. 2008;23:325–331. doi: 10.1177/039463200802100209. [DOI] [PubMed] [Google Scholar]
- 14.Hsu J, Peters AT. Pathophysiology of chronic rhinosinusitis with nasal polyp. Am J Rhinol Allergy. 2011;25:285–290. doi: 10.2500/ajra.2011.25.3680. [DOI] [PubMed] [Google Scholar]
- 15.Gelardi M, Russo C, Fiorella ML, et al. When allergic rhinitis is not only allergic. Am J Rhinol Allergy. 2009;23:312–315. doi: 10.2500/ajra.2009.23.3320. [DOI] [PubMed] [Google Scholar]
- 16.Bousquet J, Heinzerling L, Bachert C, et al. Global Allergy and Asthma European Network, author. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. Allergic Rhinitis and its Impact on Asthma. [DOI] [PubMed] [Google Scholar]
- 17.Gelardi M. Atlas of nasal cytology. Milano: Edi Ermes; 2012. [Google Scholar]
- 18.Gelardi M, Fiorella ML, Russo C, et al. Role of nasal cytology. Int J Immunopathol Pharmacol. 2010;23(1 Suppl):45–49. [PubMed] [Google Scholar]
- 19.Meltzer EO, Jalowayski AA. Nasal cytology in clinical practice. Am J Rhinol. 1988;2:47–54. [Google Scholar]
- 20.Gelardi M, Incorvaia C, Passalacqua G, et al. The classification of allergic rhinitis and its cytological correlate. Allergy. 2011;66:1624–1625. doi: 10.1111/j.1398-9995.2011.02741.x. [DOI] [PubMed] [Google Scholar]
- 21.Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol. 2001;86:494–507. doi: 10.1016/S1081-1206(10)62896-7. [DOI] [PubMed] [Google Scholar]
- 22.Fokkens WJ, Lund VJ, Mullol J, et al. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. PJ.EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. [DOI] [PubMed] [Google Scholar]
- 23.Pietruszewska W, Olejniczak I, Józefowicz-Korczyaska M, et al. Etiology of nasal polyps: an update. Otolaryngol Pol. 2006;60:551–557. [PubMed] [Google Scholar]
- 24.Tos M. The pathogenetic theories on formation of nasal polyps. Am J Rhinol. 1990;4:51–55. [Google Scholar]
- 25.Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 26.Bachert C, Zhang N, Patou J, et al. Role of staphylococcal superantigens in upper aiway disease. Curr Opin Allergy Clin immunol. 2008;8:34–38. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 27.Bürger J, Macek M, Jr, Stuhrmann M, et al. Genetic influences in the formation of nasal polyps. Lancet. 1991;337:974–974. doi: 10.1016/0140-6736(91)91603-r. [DOI] [PubMed] [Google Scholar]
- 28.Molnar-Gabor E, Endreffy E, Rozsari A. HLA-DRB1, DQA1 and DQB1 genotypes in patients with nasal polyposis. Laryngoscope. 2000;110:422–425. doi: 10.1097/00005537-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein JM, Gorfien J, Noble B. Role of allergy in nasal polyposis: a review. Otolaryngol Head Neck Surg. 1995;113:724–732. doi: 10.1016/s0194-5998(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 30.Gelardi M, Quaranta N, Passalacqua G. When sneezing indicates the cell type. Int Forum Allergy Rhinol. 2013;3:393–398. doi: 10.1002/alr.21119. [DOI] [PubMed] [Google Scholar]
- 31.Shaw JL, Ashoori F, Fakhri S, et al. Increased percentage of mast cells within sinonasal mucosa of chronic rhinosinusitis with nasal polyp patients independent of atopy. Int Forum Allergy Rhinol. 2012;2:233–240. doi: 10.1002/alr.21021. [DOI] [PubMed] [Google Scholar]
- 32.Tiddens H, Silverman M, Bush A. The role of inflammation in airway disease: remodeling. Am J Respir Crit Care Med. 2000;162(2 Pt 2):S7–S10. doi: 10.1164/ajrccm.162.supplement_1.maic-2. [DOI] [PubMed] [Google Scholar]
- 33.Amorim MM, Araruna A, Caetano LB, et al. Nasal eosinophilia: an indicator of eosinophilic inflammation in asthma. Clin Exp Allergy. 2010;40:867–874. doi: 10.1111/j.1365-2222.2009.03439.x. [DOI] [PubMed] [Google Scholar]
- 34.Pohunek P, Warner JO, Turzíková J, et al. Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol. 2005;16:43–51. doi: 10.1111/j.1399-3038.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 35.Mauad T, Bel EH, Sterk PJ. Asthma therapy and airway remodeling. J Allergy Clin Immunol. 2007;120:997–1009. doi: 10.1016/j.jaci.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 36.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–879. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]