SUMMARY
The paradigm for the management of epistaxis, specifically posterior epistaxis, has undergone significant changes in the recent past. Recent prospective and retrospective data has shown that the endonasal surgical management of posterior epistaxis is superior to posterior nasal packing and angiography/embolization with regards to various factors including pain, cost-effectiveness, risk and overall control of bleeding. Endonasal endoscopic surgical techniques for posterior epistaxis include direct cauterization and transnasal endoscopic sphenopalatine/ posterior nasal artery ligation or cauterization with or without control of the anterior ethmoidal artery. Despite the evidence provided by the current literature, a universal treatment protocol has not yet been established. This review article provides an up-to-date assessment of the available literature, and presents a structured paradigm for the management of posterior epistaxis.
KEY WORDS: Epistaxis, Endoscopic sphenopalatine artery ligation, Posterior epistaxis, Sphenopalatine artery
RIASSUNTO
Il trattamento delle epistassi posteriori ha subito significativi cambiamenti negli ultimi anni. I recenti dati prospettici e retrospettivi hanno dimostrato che il trattamento chirurgico endoscopico delle epistassi posteriori presenta dei vantaggi rispetto al tamponamento nasale e/o all'embolizzazione previa angiografia ed in particolare in termini di dolore, rapporto costo-beneficio, effetti collaterali, e infine in termini di controllo di sanguinamento. Il trattamento endoscopico chirurgico delle epistassi posteriori include la cauterizzazione diretta e la legatura dell'arteria sfeno-palatina e/o cauterizzazione dell'arteria etmoidale anteriore. Nonostante le evidenze presenti in letteratura un protocollo universale non è stato ancora realizzato. Questa revisione della letteratura offre un aggiornamento sui dati attuali sull'argomento, proponendo un algoritmo per il trattamento delle epistassi posteriori.
Introduction
Epistaxis is a very common presenting complaint and the most common emergency for the Otolaryngologist- Head and Neck Surgeon. The distribution of epistaxis is bimodal. It is most common before age 10, and then peaks again between ages 45 and 65 years of age 1 2. Many factors including seasonal variation, concomitant inhalational allergy, oestrogens (extraneous and endogenous), environmental humidity and upper respiratory tract infections affect the incidence of epistaxis.
Epistaxis can be divided into anterior and posterior based upon the arterial supply and location of the offending vessel. Most cases of epistaxis are anteriorly located (90-95%), and are usually treated effectively after visual localization with local chemical or electrical cauterization via anterior rhinoscopy 3. Approximately 5-10% of epistaxis arises posteriorly 3, and requires more aggressive measures for control. It has been prospectively shown that patients with posterior epistaxis are more likely to require hospitalization, are twice as likely to require nasal packing and require a longer hospital stay 4.
The sphenopalatine (SPA) and posterior nasal (PNA) arteries, terminal branches of the internal maxillary artery (IMA), provide blood supply to the lateral nasal wall below the middle turbinate, rostrum of the sphenoid sinus and posterior nasal septum. Therefore, a majority of posterior epistaxes arise from these two vessels. Measures to control posterior epistaxis include direct cauterization, posterior nasal packing, embolization or surgery. Many studies have shown surgical control to be superior to angiography/ embolization 3 5 as well as posterior packing 3. Intervention for posterior epistaxis is direct endonasal endoscopic cauterization of the offending site. However, in a significant number of patients, if not most, with posterior epistaxis, the site will remain undefined. Recent reports suggest that ligation of the sphenopalatine and posterior nasal arteries seems to be the best option for patients with posterior epistaxis and without comorbidities that would preclude a surgical intervention 3 5.
The surgical management of epistaxis has undergone significant transitions and changes. Carnochan 6 described the first surgical technique to the pterygopalatine fossa (PTPF) in 1858, using a transfacial-transantral approach to the pterygopalatine fossa (PTPF). In 1890, Segond introduced a lateral transfacial approach to the pterygopalatine fossa 6. Subsequently, Hide introduced the ligation of the external carotid artery for the management of epistaxis. In 1948, Silverblatt first described the ligation of the anterior ethmoid artery. These techniques are still utilized around the world to manage patients with refractory epistaxis. In 1929, Seiffert described the sublabial-transantral approach for ligation of the maxillary artery, which was subsequently standardized and popularized by Chandler (1956); and, further improved upon by Simpson (1982) by focusing on its terminal branches (i.e. sphenopalatine and posterior nasal arteries) 6. In 1976, Prades described an endonasal microscopic ligation of the sphenopalatine artery, which was emulated by Borgstein who introduced the endoscope as a visualization tool in 1987 6.
A thorough knowledge of the anatomy of the posterior nasal cavity and pterygopalatine fossa is essential for the proper surgical management of posterior epistaxis. Arterial supply to the nasal cavity is both robust and variable, with contributing arteries deriving from both the internal and external carotid arteries. Contributions from the external carotid artery include the sphenopalatine, posterior nasal, superior labial, greater palatine, angular and ascending pharyngeal arteries. The internal carotid artery furnishes the anterior ethmoid and posterior ethmoid arteries via the ophthalmic artery. Branches of the ethmoidal arteries supply the lateral nasal wall above the level of the middle turbinate. It must be noted that there is ample communication between the two systems. The vidian artery and the artery that accompanies V2 (artery of the foramen rotundum) are robust examples of these communications that can lead to collateral blood flow, thus causing re-bleeding and which must be considered when embolizing the internal maxillary artery.
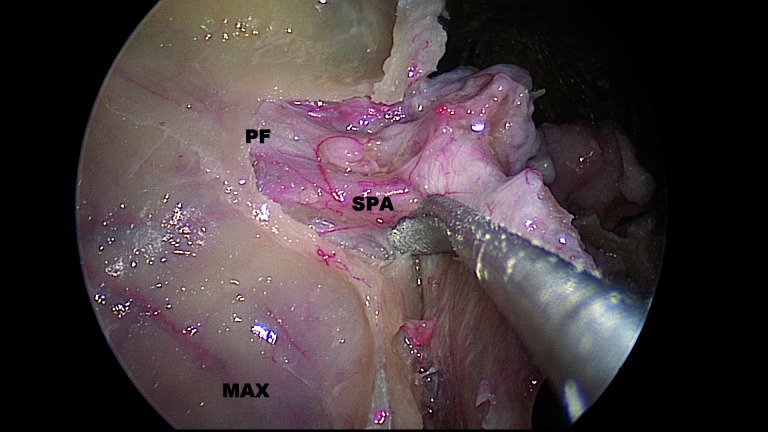
The sphenopalatine foramen (SPF), which is a notch between the orbital and sphenoidal processes of the ascending aspect of the palatine bone (Fig. 1), usually lies at the posterior end of the middle turbinate, in the lower part of the superior meatus, and at the junction between the palatine and sphenoid bone on the lateral nasal wall. Simmen reported that the mean vertical and horizontal diameters of the SPF are 6.2 and 5.1 mm, respectively 7. From an endoscopic standpoint, the foramen can be found just posterior to the superior one-third of the posterior wall of the antrum. Another reliable landmark is the crista ethmoidalis, which is a small spur of bone just anterior to the sphenopalatine foramen (Fig. 2).
Fig. 1.
Right pterygopalatine fossa.
PF: pterygopalatine fossa; SPA: sphenopalatine artery; MAX: posterior wall of maxillary sinus.
Fig. 2.
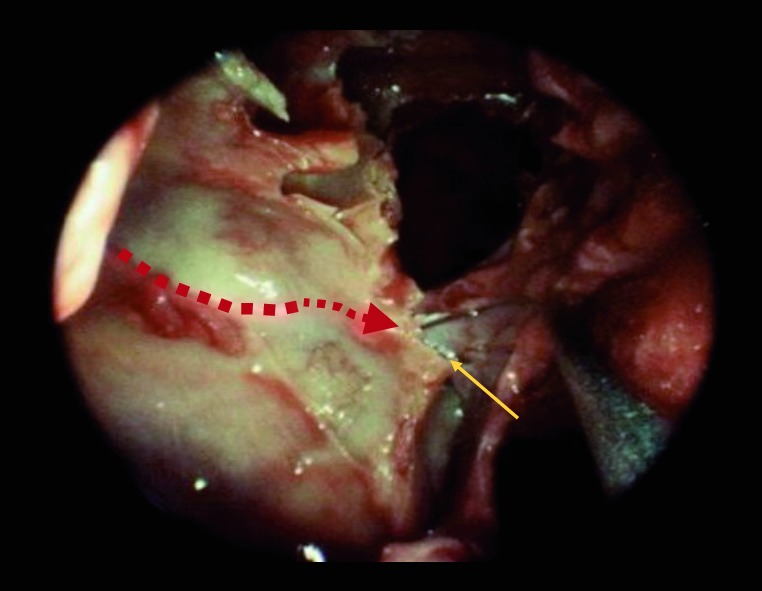
Right ethmoid crest. Yellow arrow pointing to crista ethmoidalis. Red dashed arrow showing the path of the internal maxillary before it divides in to the sphenopalatine and posterior nasal arteries.
The arterial configuration within the pterygopalatine fossa is also highly variable and complex. In a cadaveric study of 128 tissue blocks by Chiu 8, it was found that the internal maxillary artery bifurcates before reaching the sphenopalatine foramen in 89% of cases, splitting off into two (69%), three (19%) or four branches (2%). In a similar anatomical study, Simmen documented that there may even be up to 10 arterial branches 7. This study also demonstrated that in 58% of cases the SPF lies in both the superior and middle meati.
Variability of the vascular anatomy within the pterygopalatine fossa is remarkable and ranges from a relatively simple or "classic" pattern to one that is highly complex 9. In turn, the branching pattern of the sphenopalatine artery is also striking. Schartzbauer showed that in fresh cadavers, approximately 16% of the terminal branches split off from the maxillary artery distal to the sphenopalatine foramen, 42% branch proximally and 42% branch through separate foramina 10. In 75 cadaveric specimens, Simmen showed that in 97% of the samples the sphenopalatine artery had 2 or more branches exiting the lateral nasal wall, 67% had 3 or more branches, 35% had 4 or more branches, 3% had 1 single trunk and 1% had 10 branches 7. A representative example of the sphenopalatine artery is shown in Fig. 1.
Another common and useful landmark for the identification of the sphenopalatine foramen is the ethmoid crest or crista ethmoidalis (CE), (Fig. 2). In a study by Rezende, the crista ethmoidalis was found in 96% of cadaveric specimens, and was located just anterior to the SPF in most cases 11. Similar to other aforementioned studies, they found that 43% of specimens had accessory foramina. Similarly, Padua showed that the crista ethmoidalis was anterior to the sphenopalatine foramen in 98% of specimens 12. In this latter study, the SPF was located between the middle meatus and the superior meatus in 87% of specimens, and at the superior meatus in 13%. In Padua's study accessory foramina were present in 10% of specimens. These studies suggest that, in fact, the most reliable localizer for the SPF is the crista ethmoidalis; however, the frequency of multiple foramina and branches is significant; thus, the surgeon should anticipate their presence 12.
Initial management
The initial evaluation of the patient presenting with epistaxis should focus on evaluating the stability of the airway, initial control of the bleeding and stabilization of vital signs with fluid replacement or blood transfusions. In a patient with severe uncontrolled epistaxis, the need for intubation or even a surgical airway, albeit rare, must be considered. If the airway is deemed stable, nonsurgical approaches for haemostasis should first be attempted. Local vasoconstrictors (i.e. epinephrine), either topical and/or injected, may assist with haemostasis. If the site of bleeding is visible, its cauterization may be possible. Posterior epistaxis is usually quite brisk, and also due to its inherent position in the posterior nasal cavity, it is often difficult to pinpoint the location of bleeding. In the surgical theatre, however, Thornton et al. 13 were able to identify the location of posterior epistaxis in 36 of 43 cases. Of these, 20% were located on the posterior nasal septum, and 80% of those were located on the lateral aspect of the middle or inferior turbinates or the lateral aspect of the middle or inferior meatus. It should be noted that all identified sites were located within the distribution of the sphenopalatine artery. Intraoperatively, anaesthesia-induced hypotension and/or elevation of the head of the bed may decrease bleeding; thus, potentially facilitating the identification and control of the bleeding site.
Any surgical intervention requires prior stabilization of the patient and control of bleeding. If the patient had previously been treated (commonly by a non-otolaryngologist), the patient may present with posterior packing already in place. In the hands of a non-specialist, posterior packing is efficacious in approximately 70% of cases 14. In the untreated patient, a posterior nasal pack using traditional tonsil packs with ribbon-gauze coated with antibiotic ointment, expandable sponges such as the Merocel Pope nasal packing (Medtronic ENT, Jacksonville, FL, USA), Rapid Rhino (Applied Therapeutics, Tampa, FL, USA) Epistat, Postpac (Medtronic ENT, Jacksonville, FL, USA) or any of the multiple available balloon-packing devices may be effective. Topical haemostatic compounds such as a mixture of gelatin and thrombin (e.g. Floseal, Baxter Healthcare Corp., Deerfield, IL, USA) has been shown to be effective in anterior epistaxis 15, but it has not been properly assessed for posterior bleeds; thus, it is not advocated in the setting of posterior epistaxis where a specific site of bleeding cannot be identified.
There have been multiple studies comparing the various types of nasal packing for anterior bleeds, but unfortunately, there are relatively few studies looking at comparisons between various posterior nasal packings. In a comparison study by Callejo 16, classic tetracaine-coated gauze packing was compared to a bi-chambered pneumatic packing device. They found that the classic packing was less expedient, and less comfortable, but was associated with fewer episodes of re-bleeding (17% against 28%, respectively) and less expensive (€ 1327 vs. € 1648).
Posterior packing is associated with its own set of specific complications, such as the naso-vagal reflex that can trigger cardiac dysfunction or respiratory arrest 17. In addition, packing may be inadvertently swallowed or aspirated if not adequately secured 18. Conversely, if the packing is secured too tightly, it may lead to alar, columellar or septal necrosis 19. Due to the possible compressive ischaemia of nasal structures, we advocate to avoid bilateral posterior packing whenever possible, and to routinely deflate the cuff of balloon occlusive devices to allow septal blood flow.
If a Foley balloon-type device (i.e. any inflatable balloon) is used for posterior packing, air is not suitable for inflating the balloon. Rashid showed that Foley catheters inflated with air deflated within approximately 48 hr 20; therefore, saline or sterile water should be utilized for balloon inflation.
We recommend utilizing antibiotics while the posterior packing is in place, even though prophylactic antibiotics have not been shown to decrease infectious complications 21. However, rare complications such as infective endocarditis and spondylodiscitis have been reported in patients with posterior nasal packing who were not covered with systemic antibiotic prophylaxis 22.
Once the bleeding is controlled, all contributing factors that may be exacerbating the epistaxis should be addressed. These may include co-morbidities such as coagulopathies (congenital or acquired), hypertension, maxillofacial trauma, recent endonasal or orthognathic surgery and history of hereditary hemorrhagic telangiectasia (HHT). Basic laboratory studies (including complete blood count, chemistry panel, platelet count, prothrombin time and partial thromboblastin time) should be obtained during initial workup. Consider blood transfusion if haemoglobin is noted to be significantly low (this varies according to patient's cardiovascular reserve, comorbidities, symptoms and regional practices). These contributing factors should be addressed prior to any operative intervention whenever feasible.
There are specific clinical scenarios that deserve special consideration. In patients with hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant disorder resulting in localized vascular malformations, these malformations may extend posteriorly, and their acute management includes surgical cauterization or angiography and embolization. Ligation of the arteries is rarely performed as this precludes the possibility of angiography and embolization and due to the nature of the disease, and the benefits of the surgery are short-lived. The possibility of a primary or metastatic tumour causing the epistaxis may also need to be addressed with thorough history and physical examination and possibly further imaging studies. In any adolescent male patient, the possibility of a juvenile nasopharyngeal angiofibroma should also be considered.
A history of recent maxillofacial trauma, or recent endonasal or orthognathic surgery, poses the possibility of an arterial injury or a pseudoaneurysm. This latter lesion results from an incomplete tear a major artery, causing bleeding from the artery into the arterial adventitia, resulting in a localized haematoma with a continued connection to the offending artery. Pseudoaneurysms are usually unresponsive to nasal packing (immediate re-bleeding upon packing removal). They may arise from any sinonasal artery, but the arteries most commonly involved after orthognathic surgery are the internal maxillary artery and the sphenopalatine artery 2. The treatment for a pseudoaneurysm is arterial selective embolization 2.
Surgery
After haemodynamic stabilization, the patient is taken to the operative suite. As previously discussed, anaesthesiacontrolled hypotension and/or elevation of the head of the bed decreases the bleeding, and potentially facilitates localization of the offending bleeding site. Any previous nasal packing is removed and a thorough nasal endoscopy is performed to identify the specific site of bleeding. Some common locations for bleeding include the spheno-ethmoid recess, turbinates, middle meatus and the septum. If a bleeding site is identified, it may be directly cauterized. Local cauterization has the advantage of requiring no packing, and is associated with shorter hospital stay and greater patient comfort 23. Direct cauterization may also be conducted under topical or local anaesthesia. A potential disadvantage of this technique include a lower success rate than formal sphenopalatine artery ligation (mostly due to inadequate identification of the bleeding site).
Some propose attempting a local cauterization of bleeding sites in cases of posterior epistaxis 23 under general or local anaesthesia, by first visualizing the various sites of possible bleeding including the posterior aspect of the lateral wall of inferior meatus; posterior part of lateral nasal wall near the sphenopalatine foramen; posterior end of inferior turbinate; the middle turbinate and its medial surface; middle and posterior part of septum and floor of nose beneath the inferior turbinate 23. However, the preferred approach for surgical management of posterior epistaxis, in which a specific site is indisputably identified, is endonasal endoscopic ligation of the sphenopalatine and posterior nasal arteries. The efficacy of this technique is dependent on controlling the multiple, robust branches that the sphenopalatine and posterior nasal arteries give rise to. Indications for surgical ligation include the inability to place packing effectively due to an anatomical deformity, failure of non-surgical therapy, recurrent epistaxis, contraindications for embolization and patient preference. Contraindications for embolization include severe carotid atherosclerosis, prior external carotid or internal maxillary artery ligation or bleeding from the anterior ethmoid artery (which arises from the ophthalmic artery, a branch of the ICA).
A cost analysis study by Dedhia 24, showed that first-line endonasal endoscopic sphenopalatine/posterior nasal arteries ligation results in a significant overall cost savings if ≥ 3 days of posterior nasal packing were required ($ 6,450 vs. $ 8,246, respectively). Therefore, it is recommended that endonasal endoscopic sphenopalatine and posterior nasal artery ligation should be offered as an initial treatment option for medically stable patients diagnosed with posterior epistaxis 24.
Our preferred technique for endonasal endoscopic sphenopalatine and posterior nasal artery ligation 25 26 involves performing a standard uncinectomy, with identification of the natural maxillary sinus ostium and its enlargement inferiorly (to the level of the inferior turbinate), superiorly (to the level of the orbit) and posteriorly (to be flush with the back wall of the antrum). Next, the sphenopalatine foramen is identified using all the previously discussed anatomical landmarks (posterior wall of the antrum, middle turbinate root, and crista ethmoidalis). Using a Freer or Cottle periosteal elevator, the mucoperiosteum over the ascending process of the palatine bone is widely elevated to expose the sphenopalatine foramen and the sphenopalatine and posterior nasal arteries. Wide elevation is important to identify anatomical variants such as multiple foramina and/or multiple vessels traversing the lateral nasal wall from the pterygopalatine fossa. The vessels can frequently be controlled at this point either with haemostatic clips or bipolar electrocautery. If necessary a longer segment of the arteries can be exposed by removing the anterior aspect of the sphenopalatine foramen (i.e. posterior nasal wall) using a Kerrison or Citelli rongeur; thus, following the arteries into the pterygopalatine fossa. It is important to dissect the sphenopalatine and posterior nasal arteries free from the posterior aspect of the SPF, as this will allow a complete clipping or cauterization of the arteries.
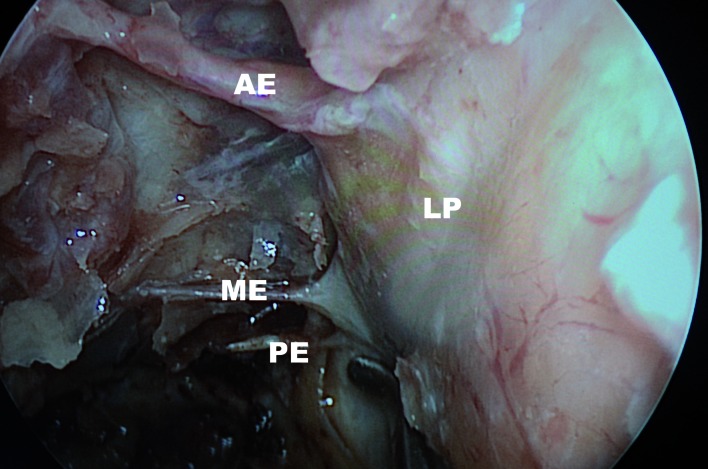
A concurrent anterior ethmoid artery (AEA) ligation along with the endonasal endoscopic ligation of the sphenopalatine and posterior nasal arteries should be considered, if the site of bleeding is not known pre-operatively, if the patient's history is unreliable, if packing was placed at an outside institution, or if there is no evidence of bleeding at the time of surgery (unidentified site of bleeding). AEA ligation has a low morbidity, and should be strongly considered if the patient has been referred for definitive treatment from a region distant from the hospital. Approaches for AEA ligation include an external incision and dissection between the lamina papyracea and the periorbita with endoscopic assistance, and endonasal approach with bipolar cauterization of the AEA (Fig. 3). Identification of the anterior ethmoidal artery on coronal computed tomography is assisted with its location at the retro-bulbar level, or by utilizing the "nipple or pyramidal sign" (a triangular evagination of the lamina papyracea between the superior oblique and medial rectus muscles) (Fig. 4). It has been shown that 36% of anterior ethmoidal arteries were located in a mesentery, and 20% could be clipped endoscopically 27. However, an external approach is safer to access the AEA. A small naso-orbital incision provides access to the periorbita, which is incised and elevated under endoscopic visualization. Following the frontoethmoidal suture leads to the ethmoidal foramina, located an average of 24 mm from the lacrimal crest. In turn, the posterior ethmoid artery (PEA) is located 12 mm posterior to the anterior ethmoidal artery, and the optic canal is located 6 mm posterior to the posterior ethmoid artery. After surgical control is achieved, silicone septal splints are placed if there was excessive trauma to the mucosa or if there is a possibility of post-operative nasal synechiae.
Fig. 3.
Endoscopic view of left nasal cavity. Orbital decompression with lamina papyracea bone (LP) removed. This specimen shows a middle ethmoidal artery.
AE: anterior ethmoid artery; ME: middle ethmoid artery; PE: posterior ethmoid artery/
Fig. 4.
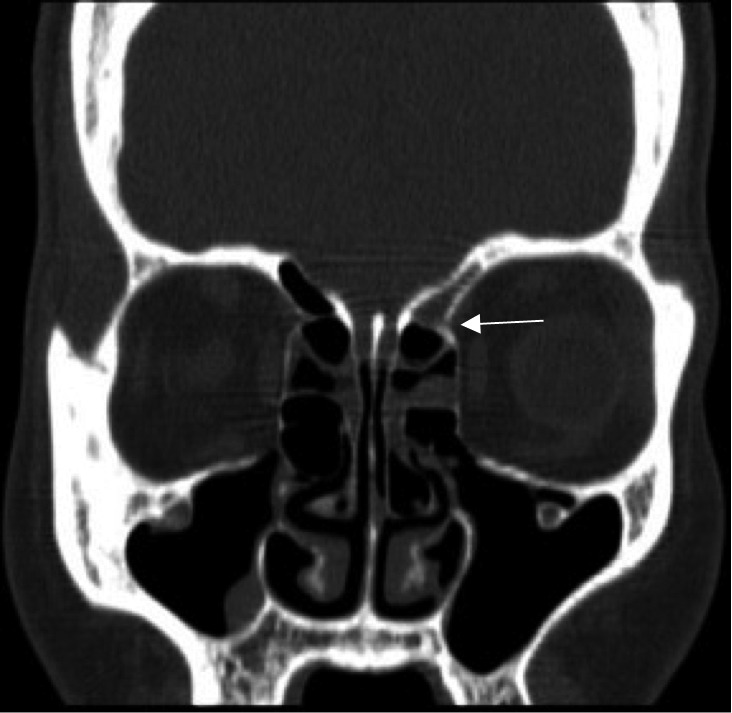
Coronal cut of CT image showing "nipple sign" at the level of entry (white arrow) of the anterior ethmoid artery into the nasal cavity.
In a retrospective review, Kumar showed that the overall mean success rate of sphenopalatine artery ligation in 11 case series including 127 patients was 98% (range 92- 100%) 28. In a retrospective study of 678 patients, Soyka 5 showed that the successful treatment in patients with posterior epistaxis could be achieved in 62% by packing (Foley + fat-gauze), and in 97% by surgery. Despite the high success rate of arterial ligation, there is still the possibility of failure. Possible reasons for recurrent bleeding include failure to ligate all terminal branches of the sphenopalatine artery, dislodged clips, bleeding diatheses, the development of collateral blood vessels or unrecognized AEA bleeding sites.
In a recent prospective study by Nikolaou 29, it was shown that surgery was the most cost effective and least painful treatment regimen for posterior epistaxis. Their treatment regimen consisted of placement of either a Rapid Rhino 7.5 cm packing or balloon packing for posterior epistaxis, followed by endonasal endoscopic sphenopalatine and posterior nasal artery ligation for patients that had further bleeding upon removal of the packing. In this study of 61 patients (45 with anterior epistaxis, 16 with posterior epistaxis), they showed that the median visual analogue scale for the evaluation of pain (VAS score) for Rapid Rhino packing, surgery and balloon packing was 6.0, 3.0 and 7.5, respectively. The median costs of treatment for 96 patients were calculated, and were found to be approximately the same for patients with Rapid Rhino packing and surgery (10,192 Swiss Francs), balloon packing and surgery (10,192 Swiss Francs) and surgery alone (10,269 Swiss Francs). Overall, their findings suggested that surgery is less troublesome to the patient, and does not increase the costs of treatment. This technique has also been shown to be efficacious and safe in the paediatric population 30.
After control of the posterior epistaxis is achieved, appropriate postoperative care is needed. Elevate the head of the bed, avoid hypertension, provide appropriate analgesia and promote aggressive nasal hygiene. Nasal hygiene includes saline nasal sprays, saline irrigations, use of oxymetazoline and/or a nasal sling. The patient should follow-up in clinic 5-7 days after surgical intervention for removal of silicone splints, if they were placed.
If endonasal endoscopic sphenopalatine and posterior nasal arteries ligation is not successful, a transantral internal maxillary artery (IMAX) ligation or angiography with embolization may be considered. Further, if IMAX ligation is not successful or angiography with embolization is not available, an external carotid artery ligation may be considered. It should be noted that this is a last resort, as a retrospective review conducted by Spafford 31 showed a high rate of re-bleeding with external carotid artery ligation (45%), but showed that IMAX ligation was successful in 90% of patients.
Embolization is an alternative option for posterior epistaxis, and is our preferred intervention for recurrent epistaxis after a seemingly adequate endonasal endoscopic sphenopalatine and posterior nasal artery ligation. Angiographic embolization was first described for posterior epistaxis in 1974 by Sokolof 32. Possible candidates for embolization include patients with HHT (Osler- Weber-Rendu) syndrome, bleeding tumours, poor surgical candidates or if the patient chooses it. Bleeding during transphenoidal or maxillofacial surgery should be considered for endovascular management due to possible internal carotid artery injury or pseudoaneurysm formation 33. Possible minor complications of angiography include trismus, facial pain, facial paresthesia or haematoma. Possible major complications include cerebrovascular accident, internal carotid artery dissection, blindness, necrosis or facial paralysis. In a retrospective review of 70 patients, Christensen found that 86% of their cases had minor or no complications after embolization, and were discharged within 24 hours 34. A major re-bleed, requiring surgical intervention, occurred within 6 weeks of embolization in 13% of these patients, and one patient had a significant cerebrovascular accident. Also of note, this study showed that the average cost of hospitalization in the respective institution was $ 18,000 per patient with epistaxis, and the cost of embolization was an average of $ 11,000 34. In a retrospective study by Cohen, 19 patients underwent endovascular embolization with no minor or major complications, and an average hospital stay of 11.1 days 35. As previously mentioned, in any adolescent male patient, the possibility of a juvenile nasopharyngeal angiofibroma should also be assessed. In these patients, embolization via angiography is usually utilized prior to surgical resection 33.
Given the various presentations and possible sources of bleeding in a patient with posterior epistaxis, we propose the following diagnostic workup and treatment (Fig. 5) to optimize the management of posterior epistaxis.
Fig. 5.
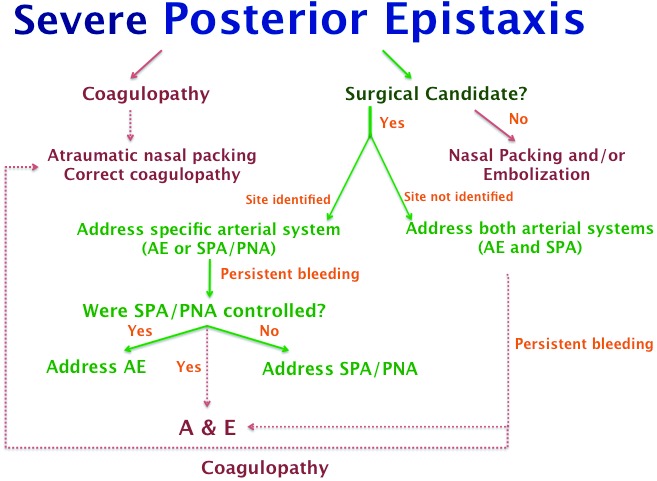
Flow diagram for management of posterior epistaxis. Note that this diagram should be used for patients that have failed conservative management and local cauterization in the non-operative setting. AE: anterior ethmoid artery; A&E: angiography and embolization; SPA: sphenopalatine artery; PNA: posterior nasal artery.
Conclusion
Ligation of the sphenopalatine and posterior nasal arteries is a very effective treatment for severe posterior epistaxis. Concomitant anterior ethmoidal artery ligation may be more effective than sphenopalatine artery/posterior nasal artery ligation alone. Surgical intervention of posterior epistaxis provides a low-morbidity and cost-effective treatment. We present a flow diagram for management of posterior epistaxis.
References
- 1.Tomkinson A, Roblin DG, Flanagan P, et al. Patterns of hospital attendance with epistaxis. Rhinology. 1997;35:129–131. [PubMed] [Google Scholar]
- 2.Nikoyan L, Matthews S. Epistaxis and hemostatic devices. Oral Maxillofac Surg Clin North Am. 2012;24:219–228. doi: 10.1016/j.coms.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180–183. doi: 10.1097/MOO.0b013e32814b06ed. [DOI] [PubMed] [Google Scholar]
- 4.Supriya M, Shakeel M, Veitch D, et al. Epistaxis: prospective evaluation of bleeding site and its impact on patient outcome. J Laryngol Otol. 2010;124:744–749. doi: 10.1017/S0022215110000411. [DOI] [PubMed] [Google Scholar]
- 5.Soyka MB, Nikolaou G, Rufibach K, et al. On the effectiveness of treatment options in epistaxis: an analysis of 678 interventions. Rhinology. 2011;49:474–478. doi: 10.4193/Rhino10.313. [DOI] [PubMed] [Google Scholar]
- 6.Wentges RT. Surgical anatomy of the pterygopalatine fossa. J Laryngol Otol. 1975;89:35–45. doi: 10.1017/s0022215100080051. [DOI] [PubMed] [Google Scholar]
- 7.Simmen DB, Raghavan U, Briner HR, et al. The anatomy of the sphenopalatine artery for the endoscopic sinus surgeon. Am J Rhinol. 2006;20:502–505. doi: 10.2500/ajr.2006.20.2928. [DOI] [PubMed] [Google Scholar]
- 8.Chiu T. A study of the maxillary and sphenopalatine arteries in the pterygopalatine fossa and at the sphenopalatine foramen. Rhinology. 2009;47:264–270. doi: 10.4193/Rhin08.153. [DOI] [PubMed] [Google Scholar]
- 9.Fortes FS, Sennes LU, Carrau RL, et al. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach. Laryngoscope. 2008;118:44–49. doi: 10.1097/MLG.0b013e318155a492. [DOI] [PubMed] [Google Scholar]
- 10.Schartzbauer HR, Shete M, Tami TA. Endoscopic Anatomy of the sphenopalatine and posterior nasal arteries: Implications for the endoscopic management of epistaxis. Am J Rhinol. 2003;17:63–66. [PubMed] [Google Scholar]
- 11.Rezende GL, Soares VY, Moraes WC, et al. The sphenopalatine artery: a surgical challenge in epistaxis. Braz J Otorhinolaryngol. 2012;78:42–47. doi: 10.1590/S1808-86942012000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padua FG, Voegels RL. Severe posterior epistaxis - endoscopic surgical anatomy. Laryngoscope. 2008;118:156–161. doi: 10.1097/MLG.0b013e31815708d0. [DOI] [PubMed] [Google Scholar]
- 13.Thornton MA, Mahesh BN, Lang J. Posterior epistaxis: identification of common bleeding sites. Laryngoscope. 2005;115:588–590. doi: 10.1097/01.mlg.0000161365.96685.6c. [DOI] [PubMed] [Google Scholar]
- 14.Schlosser RJ. Clinical practice. Epistaxis. N Engl J Med. 2009;360:784–789. doi: 10.1056/NEJMcp0807078. [DOI] [PubMed] [Google Scholar]
- 15.Mathiasen RA, Cruz RM. Prospective, randomized, controlled clinical trial of a novel matrix hemostaticsealant in patients with acute anterior epistaxis. Laryngoscope. 2005;115:899–902. doi: 10.1097/01.MLG.0000160528.50017.3C. [DOI] [PubMed] [Google Scholar]
- 16.García Callejo FJ, Muñoz Fernández N, Achiques Martínez MT, et al. Nasal packing in posterior epistaxis. Comparison of two methods. Acta Otorrinolaringol Esp. 2010;61:196–201. doi: 10.1016/j.otorri.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Fairbanks DN. Complications of nasal packing. Otolaryngol Head Neck Surg. 1986;94:412–415. doi: 10.1177/019459988609400337. [DOI] [PubMed] [Google Scholar]
- 18.Hashmi SM, Gopaul SR, Prinsley PR, et al. Swallowed nasal pack: a rare but serious complication of the management of epistaxis. J Laryngol Otol. 2004;118:372–373. doi: 10.1258/002221504323086589. [DOI] [PubMed] [Google Scholar]
- 19.Civelek B, Kargi AE, Sensöz O, et al. Rare complication of nasal packing: alar region necrosis. Otolaryngol Head Neck Surg. 2000;123:656–657. doi: 10.1067/mhn.2000.110619. [DOI] [PubMed] [Google Scholar]
- 20.Rashid M, Karagama Y. Inflation of Foley catheters for postnasal packing. J Laryngol Otol. 2010;124:997–998. doi: 10.1017/S0022215110000629. [DOI] [PubMed] [Google Scholar]
- 21.Pepper C, Lo S, Toma A. Prospective study of the risk of not using prophylactic antibiotics in nasal packing for epistaxis. J Laryngol Otol. 2012;126:257–259. doi: 10.1017/S0022215111003215. [DOI] [PubMed] [Google Scholar]
- 22.Gungor H, Ayik MF, Gul I, et al. Infective endocarditis and spondylodiscitis due to posterior nasal packing in a patient with a bioprosthetic aortic valve. Cardiovasc J Afr. 2012;23:e5–e7. doi: 10.5830/CVJA-2011-002. [DOI] [PubMed] [Google Scholar]
- 23.Paul J, Kanotra SP, Kanotra S. Endoscopic management of posterior epistaxis. Indian J Otolaryngol Head Neck Surg. 2011;63:141–144. doi: 10.1007/s12070-010-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedhia RC, Desai SS, Smith KJ, et al. Cost-effectiveness of endoscopic sphenopalatine artery ligation vs. nasal packing as first-line treatment for posterior epistaxis. Int Forum Allergy Rhinol. 2013;3:563–566. doi: 10.1002/alr.21137. [DOI] [PubMed] [Google Scholar]
- 25.Snyderman C, Carrau R. Endoscopic ligation of the sphenopalatine artery for epistaxis. Operative Techniques in Otolaryngology - Head and Neck Surgery. 1997;8:85–89. [Google Scholar]
- 26.Snyderman C, Goldman S, Carrau R, et al. Endoscopic sphenopalatine artery ligation is an effective method of treatment for posterior epistaxis. Am J of Rhinol. 1999;13:137–140. doi: 10.2500/105065899782106805. [DOI] [PubMed] [Google Scholar]
- 27.Floreani SR, Nair SB, Switajewski MC, et al. Endoscopic anterior ethmoidal artery ligation: a cadaver study. Laryngoscope. 2006;116:1263–1267. doi: 10.1097/01.mlg.0000221967.67003.1d. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Shetty A, Rocker J, et al. Contemporary surgical treatment of epistaxis. What is the evidence for SPA ligation? Clin Otolaryngol Allied Sci. 2003;28:360–363. doi: 10.1046/j.1365-2273.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 29.Nikolaou G, Holzmann D, Soyka MB. Discomfort and costs in epistaxis treatment. Eur Arch Otorhinolaryngol. 2013;270:2239–2244. doi: 10.1007/s00405-012-2339-2. [DOI] [PubMed] [Google Scholar]
- 30.Eladl HM, Khafagy YW, Abu-Samra M. Endoscopic cauterization of the sphenopalatine artery in pediatric intractable posterior epistaxis. Int J Pediatr Otorhinolaryngol. 2011;75:1545–1548. doi: 10.1016/j.ijporl.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Spafford P, Durham JS. Epistaxis: efficacy of arterial ligation and long-term outcome. J Otolaryngol. 1992;21:252–256. [PubMed] [Google Scholar]
- 32.Sokoloff J, Wickbom I, McDonald D, et al. Therapeutic percutaneous embolization in intractable epistaxis. Radiology. 1974;111:285–287. doi: 10.1148/111.2.285. [DOI] [PubMed] [Google Scholar]
- 33.Abruzzo TA, Heran MK. Neuroendovascular therapies in pediatric interventional radiology. Tech Vasc Interv Radiol. 2011;14:50–56. doi: 10.1053/j.tvir.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Christensen NP, Smith DS, Barnwell SL, et al. Arterial embolization in the management of posterior epistaxis. Otolaryngol Head Neck Surg. 2005;133:748–753. doi: 10.1016/j.otohns.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JE, Moscovici S, Gomori JM, et al. Selective endovascular embolization for refractory idiopathic epistaxis is a safe and effective therapeutic option: technique, complications, and outcomes. J Clin Neurosci. 2012;19:687–690. doi: 10.1016/j.jocn.2011.08.019. [DOI] [PubMed] [Google Scholar]