Abstract
Disordered cancer metabolism was described almost a century ago as an abnormal adaptation of cancer cells to glucose utilization especially under hypoxic conditions; the so-called Warburg effect. Greater research interest in this area in the last several decades has led to the recognition of the critical coupling of specific malignant phenotypes such as increased proliferation and resistance to programmed cell death (apoptosis) with altered metabolic handling of key molecules that are essential for normal cellular metabolism. The altered glucose metabolism frequently encountered in cancer cells has been exploited for cancer diagnosis and treatment. More recently, the role of other glycolytic pathway intermediates as well as alternative pathways for energy generation and macromolecular synthesis in cancer cells has become recognized. Especially, the important role of altered glutamine metabolism in the malignant behavior of cancer cells and the potential exploitation of this cellular adaptation for therapeutic targeting has emerged as an important area of cancer research in the last decade. Expectedly, attempts to exploit this understanding for diagnostic and therapeutic ends are running apace with the elucidation of the complex metabolic alterations that accompany neoplastic transformation. Because lung cancer is a leading cause of cancer death with limited curative therapy options, careful elucidation of the mechanism and consequences of disordered cancer metabolism in lung cancer is warranted. This review provides a concise, systematic overview of the current understanding of the role of altered glutamine metabolism in cancer and how these findings intersect with current and future approaches to lung cancer management.
Introduction
Lung cancer is the most common cancer worldwide, and the leading cause of cancer associated deaths in the United States.1 Similar to other cancer types, altered glucose metabolism by cancer cells has been exploited in the diagnosis and staging of lung cancer mainly through the use of 18fluorodeoxyglucose (FDG)-PET imaging of patients with various stages of lung cancer.2 However, major new advances in exploiting abnormal cancer cell metabolism for the management of lung cancer are unlikely to arise from the already established utility of FDG-PET. Indeed, an increasing body of evidence suggests that the dysregulated metabolism of other enzymes and substrates beyond glucose are common accompaniments of neoplastic transformation, progression and resistance to therapy of cancer cells. Furthermore, recent findings highlighting the frequent mutations of genes encoding for metabolizing enzymes such as isocitrate dehydrogenase 1 and 2 (IDH1, IDH2), pyruvate kinase M2 (PKM2), fumarate hydratase (FH) and succinate dehydrogenase (SDH) have further encouraged the research interest in cancer metabolism and how such findings can be translated into therapeutic interventions.3 Importantly, the activation of alternative glycolytic pathway intermediate enzymes such as PKM2 and phosphoglycerate mutase 1 (PGAM1) has been directly linked to tumor growth.4,5 However, recognition of the role of glutamine metabolic pathway as an alternative source of energy and anabolic building block offers one of the most promising targets for anticancer strategies.6 The recognition of the important role of altered glutamine metabolism in cancer has led to the focus on this pathway as an actionable therapeutic target and the development of pharmacological agents that inhibit key enzymes involved in glutamine metabolism.7 Given the overall poor prognosis of lung cancer and the need for unconventional therapeutic strategies, it is reasonable to anticipate that translational and eventual clinical evaluation of this therapeutic approach will soon be extended to lung cancer patients. This review summarizes the current state of the literature regarding the altered metabolism of glutamine in cancers with a contextual discussion of the relevance and potential application of such findings to the comprehensive management of lung cancer.
Discussion
Glutamine structure and function
Glutamine is the most abundant, naturally occurring, non-essential amino acid in the human body.8 It is synthesized through enzymatic action of glutamine synthetase (GS) that combines glutamate and ammonia.9,10 Glutamine has two nitrogen-containing side chains, an amino and an amide group. This property makes it one of the most important circulating nitrogen shuttles, accounting for 30% to 35% of all amino acid nitrogen transported in the blood.11 It serves as a vehicle for transporting ammonia in a nontoxic form from the peripheral tissues to visceral organs where it is cleared and excreted either as ammonium in urine or as urea through the liver. Glutamine is also central to a variety of biochemical functions such as protein synthesis, cellular energy homeostasis, purine synthesis and the citric or tricarboxylic acid (TCA) cycle through its capacity to serve as a nitrogen or carbon donor. Glutamine exists in a free circulating form in the blood and in storage forms mainly in skeletal muscles and in smaller amounts in other organs such as the lung and brain.12 Physiologically, glutamine is utilized by the small intestine and the renal epithelial cells for acid-base balance.13 However, other metabolically active cells including activated immune cells and cancer cells can become major glutamine users.14 Hepatocytes serve both as a glutamine producer and consumer depending on the overall metabolic needs of the body. As such, hepatocytes play a regulatory role in glutamine metabolism by taking up large amounts of glutamine derived from the gut.13
Glutamine metabolism in normal and neoplastic cells
Glutamine is one of the most abundant amino acids in the body and is especially abundant in the liver, kidney, skeletal muscle and brain.8,12 It is the precursor for the synthesis of many amino acids, proteins, nucleotides and other biologically important molecules.13 It is also required for the removal of alpha amino nitrogen from other amino acids through transdeamination and is considered the main precursor for ammoniagenesis and urea formation in the kidney.13,15 Hepatic glutamine metabolism has a vital role in the stimulation of glycogen synthesis and is one of the major end products of ammonia trapping pathways.16,17 Moreover, glutamine metabolism plays a critical role in gluconeogenesis and is an oxidative fuel in rapidly proliferating cells and tissues.12 It is required for synthesis of glutathione; a key component in the body's scavenging defense mechanism against oxidative stress.18,19 Because of the varied roles of glutamine in normal cell physiology and metabolism, it is expected that altered handling of glutamine metabolism will contribute to neoplastic transformation and cancer progression. Careful study and elucidation of potential alteration in glutamine metabolism in cancer cells is currently one of the most active areas of cancer metabolism research. Recent findings have shown that aberrant energy metabolism and the associated alterations in intracellular handling of glutamine by cancer cells form an adaptive mechanism that contributes directly to the malignant phenotype. Thus, proliferating cancer cells compete with normal cells for circulating glutamine.10 As a consequence, marked changes in glutamine metabolism may occur with progressive tumor growth. A careful elucidation of glutamine metabolism in cancer patients and its impact on cancer prognosis may inform optimal patient management.
The stiff competition between neoplastic and normal cells for glutamine and the requirement for glutamine supplementation for optimal growth of cancer cells in in vitro culture constitute empiric evidence in support of the role of glutamine as a major energy source for cancer cell proliferation.20-23In vivo studies in hepatomas and fibrosarcomas24,25 showed a five- to ten-fold higher rate of glutamine consumption by cancer cells compared to normal hepatocytes.26 Also, the dependence of lung cancer cells on sufficient availability of glutamine for short-term proliferation and long-term survival was previously demonstrated in studies of human-derived lung cancer cell lines and tissue grafts.27-29 Metabolic reprogramming in cancer cells facilitates glutamine uptake and utilization for anabolism.21 One of the earliest steps in the generation of metabolic intermediates required for cell growth and replication is the oxidative action of mitochondrial phosphate-dependent glutaminase (GLS) which converts glutamine to glutamate and ammonia.22 Glutaminolysis mediated by GLS promotes the generation of metabolic intermediates required for macromolecular biosynthesis in proliferating cancer cells. A series of cellular adaptations ensures the availability of glutamine to cancer cells. This includes the development of efficient sodium ion-dependent membrane transporter systems, System A and System ASC, leading to enhanced transmembrane transfer of glutamine from the circulation in order to overcome the stiff competition with normal cells.30,31,10 The normal repression of the System A glutamine transporter in normal cells is de-repressed following neoplastic transformation, leading to augmented glutamine membrane transport into the cell.32 In addition, a carrier-mediated process determines the intracellular trafficking of glutamine into the mitochondria.33 However, this intracellular flow of glutamine is bidirectional depending on the dynamic balance between the need for optimal blood levels of glutamine and the intracellular metabolic requirements.
In animal experiments, an inverse relationship exists between rapid tumor cell growth and proliferation and a fall in blood glutamine concentration.34-36 Furthermore, a series of inter-related adaptive changes occurs in different organs in response to the disproportionate use of glutamine by the proliferating cancer cells. For instance, low blood glutamine levels induce reciprocal adaptive changes in organs involved in body glutamine balance such as skeletal muscles, intestine, liver and lung. As such, low blood glutamine levels resulting from increased tumor burden induces an increase in the activity of GS enzyme and a consequent increase in intracellular glutamine stores in muscle cells.37 Failure of this compensatory mechanism results in significant depletion of glutamine in skeletal muscle and may contribute to the commonly observed tumor-associated cachexia of advanced cancer.38,39 Similarly, in response to the diminished extractable fraction of circulating glutamine due to the reduced total body glutamine that results from high utilization by cancer cells, intestinal epithelial cells preserve their glutamine transport activity and metabolism by decreasing mucosal GLS activity while at the same time increasing the number of brush border transporters.24,35,40 Metabolic changes induced by altered glutamine metabolism in the liver are more complicated. In the early stages of cancer, the liver serves as a net producer of glutamine by releasing glutamine into the circulation through an Na(+)-independent carrier-mediated glutamine transporter system.41 This is facilitated by an increase in the gradient between intracellular and circulating glutamine concentration.38 However, with advanced stage cancer, the release of tumor necrosis factor (TNF) and other cytokines by cancer cells induces membrane expression of System N, an influx carrier.42 This leads to increased intracellular glutamine uptake by hepatocytes, which compete with cancer cells as the main glutamine consumers. The elevated levels of tissue and circulating cytokines observed in cancer patients suggest a contributory role for these cytokines in the altered glutamine metabolism associated with cancer development and progression.43
Glutamine metabolism and lung cancer
There is an increased level of glutamine expression in lung cancer tissue especially in non-small lung cancer (NSCLC) when compared to other cancer types such as colon or stomach cancer.44 Also, cigarette smoking impairs the metabolic conversion of glutamine and therefore promotes a high level of glutamine in lung cancer cells.45 While high GS and high glutamine levels have been shown to enhance the metastatic potential and rate of tumor recurrence in hepatocellular carcinoma,46 the prognostic significance of glutamine and its metabolizing enzymes in lung cancer has not been well elucidated. Nonetheless, glutamine is an important molecule required for the normal function of non-neoplastic lungs47 and plays a significant role in cancer cell growth, protein translation, anaplerosis and macromolecule synthesis.44 The action of GLS on glutamine generates glutamic acid, a key nitrogen donor for amino acid synthesis, thereby placing glutamine as a key ingredient required for protein synthesis in rapidly growing cancer cells.48 Given the central role of glutamine in normal cell physiology, alterations in the handling of this important molecule as well as its transporter and metabolizing enzymes are expected to also impact the biology of lung cancer. The bidirectional L-type amino acid transporter 1 (LAT1) also known as SLC7A5/SLC3A2 promotes glutamine efflux in exchange for the influx of L-leucine and other essential amino acids across the cell membrane. This action of LAT1 is an important mechanism that maintains intracellular availability of essential amino acids, which activates the mammalian target of rapamycin (mTOR) pathway, promotes protein synthesis and inhibits autophagy for orderly cell proliferation and growth.49 LAT1 expression has been shown to be of prognostic implication in neuroendocrine lung tumors.50 Furthermore, in patients with recurrent NSCLC treated with platinum based chemotherapy, LAT1 positive tumors had a lower rate of response of 16% in comparison to 45% response rate for LAT1 negative tumors (p=0.002). High expression of LAT1 was also positively associated with poor overall survival of 16.5 vs. 31.5 months, respectively (p=0.045).51,52 Furthermore, LAT1 expression showed positive correlation with markers of intratumoral hypoxia, angiogenesis and energy deprivation in adenocarcinoma and non-adenocarcinoma types of NSCLC suggesting it to be a marker of aggressive tumor biology. Interestingly, LAT1 expression is especially high in lung cancer with activated EGFR and PI3K/AKT pathway as measured by phosphorylated isoforms of AKT, mTOR and S6.53 Inhibition of LAT1 using 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) in the H1395 lung cancer cell line reduced cellular uptake of L-leucine, and consequently inhibited mTOR pathway activity, leading to reduced cell proliferation and viability. BCH in combination with gefitinib induced an additive antiproliferative effect in the same lung cancer cell line.52
The Na(+)-dependent glutamine transporter, SLC1A5, mediates intracellular influx of glutamine and was shown to be responsible for more than 50% of the glutamine influx across the cell membrane. A pharmacologic SLC1A5 inhibitor, γ-L-glutamyl-p-nitroanilide (GPNA), as well as an siRNA gene silencing approach in lung cancer cell lines led to G1 cell growth arrest and impaired cell viability mediated through the abrogation of mTOR signaling.54 Furthermore, this glutamine flux, which is a dynamic balance between uptake and subsequent export out of the cell, is coupled with intracellular influx of essential amino acids and thus serves as a strong regulatory signal for the mTORC1 complex, the master regulator of cancer cell growth.
The metabotropic glutamate receptor 1 (GRM1) is normally expressed in the central nervous system where it is involved in neuronal proliferation and migration during development and with the normal function of mature neuronal cells.55 Ectopic expression of GRM1 has however, been demonstrated in various cancer cell lines and tumor tissues including lung cancer. The autocrine activation of this receptor by glutamate is a driver molecular event in the development of melanoma. GRM1 activation drives cellular progression and cancer growth through the MAPK and AKT/mTOR pathways.56,57 Expectedly, GRM1 antagonist or inhibitors of extracellular glutamate release inhibit cancer cell growth and induce tumor regression in vivo thus supporting a critical role for this glutamic acid-dependent signaling pathway in tumorigenesis.56-59
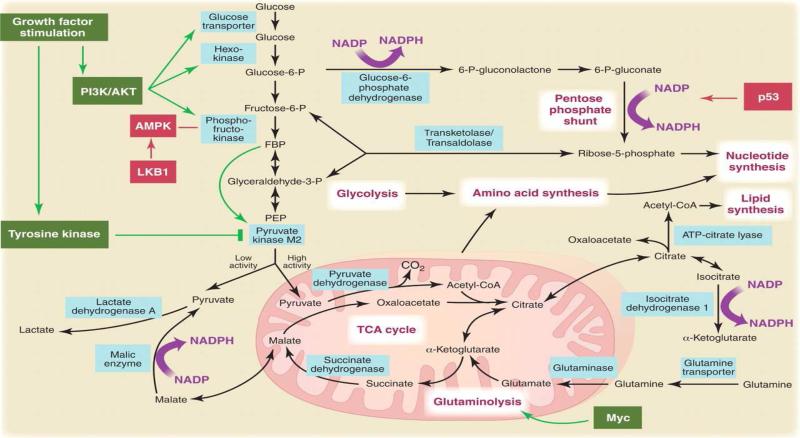
The recent finding that the myc gene upregulates GLS gene promoter activity thereby facilitating glutamine utilization in cancer cells through its conversion by GLS into glutamic acid, and finally into lactic acid may be of significance in small cell lung cancer (SCLC) where myc amplification is a poor prognostic indicator.60-62 Other frequently observed genetic alterations in lung cancer such as the loss of tumor suppressor function of p53 and LKB1 genes and the growth promoting function of oncogenes such as K-Ras and PI3K carry metabolic consequences that directly or indirectly impact glutamine metabolism (Figure 1).63,64 For instance, the anchorage-independent growth characteristic of K-Ras mutant cancer cell lines requires the availability of reactive oxygen species derived from mitochondrial metabolism. Glutamine conversion to glutamic acid mediated by GLS is an early step in the entry of glutamine intermediates into the TCA cycle and is thus critical for mitochondrial metabolism. In this regard, the K-Ras subset of lung cancer, which constitutes approximately 30% of all NSCLC cases, may be particularly susceptible to glutamine-directed therapy.65
Figure 1.
The Warburg effect in cancer cells and the interaction between metabolic reprogramming and oncogenic genetic alterations relevant to lung cancer. Reprinted with permission from Vander Heiden et al. Science, 2009 May 22;324(5930):1029-33.64
The expression of the KGA splice variant of GLS was found to be lower in lung cancer tissue samples relative to adjacent normal lung. Moreover, transient knockdown of the GAC splice variant of GLS inhibited lung cancer cell line growth highlighting additional opportunities to exploit the glutamine-dependence of some subsets of lung cancer for therapeutic intervention.66 Finally, using the EGFR T790M mutant model of lung cancer, Weaver et al. showed a close link between altered glutamine metabolism and tumor response to combined EGFR and mTOR targeted therapy in this resistant tumor model.67
The preceding body of data highlights the strong intersection of glutamine metabolism and trafficking and the mTOR serine/threonine signaling pathway, which mediates cellular responses to energy sufficiency and extracellular growth signals and is one of the most frequently activated signaling pathways in lung cancer.68 Nutritional sufficiency and or growth factor activation of mTOR kinase results in protein translation and inhibition of macroautophagy. Contrarily, during periods of nutrient deficiency, especially amino acid starvation or lack of growth factor signals, mTOR orchestrates a metabolic switch to promote cell survival by inhibiting ribosome biogenesis and protein translation while inducing cell cycle arrest, and autophagy.69 L-glutamine plays a critical role in this metabolic switch through its efflux in exchange for the influx of leucine and other essential amino acids influx into the cell.49 In the absence of these amino acids, growth signals from the two main activation pathways for mTOR, namely the insulin/insulin-like growth factor (IGF)/phosphatidyl inositol-3-OH kinase (PI3K) and the MAP kinase/extra-cellular signal regulated kinase (ERK) pathways, cannot be relayed to the mTOR kinase complex.70 The tight regulatory control that glutamine and other essential amino acids exert on mTOR pathway signaling may therefore inform the optimal approach for the therapeutic targeting of aberrant activation of the mTOR pathway including its upstream modulators in lung cancer. It is reasonable to anticipate that future studies will successfully exploit the unique reprograming of glutamine metabolism in molecular subsets of lung cancer for therapeutic intervention. Ongoing clinical trials and potential opportunities for targeting the glutamine pathway are detailed in Table 1.
Table 1.
Summary of ongoing preclinical and clinical testing of agents targeting different aspects of glutamine metabolism and their potential for cancer therapy or imaging.
| Agent | Target | Mechanism | Stage in Development | Remarks |
|---|---|---|---|---|
| Therapeutic Intervention | ||||
| Glutaminase | Glutamine | Degradation of glutamine | Phase I | Clinical development limited by toxicity72 |
| PEGylated-Glutaminase | Glutamine | Glutamine degradation | Phase I and II studies92-94 | Better tolerated than non PEGylated glutaminase with clinical activity demonstrated in advanced lung cancer patients73-76 |
| Riluzole | G-protein–coupled metabotropic glutamate receptor 1 (GRM1) | Glutamate release inhibition and disruption of autocrine glutamic signaling | Phase 0, I and II studies92-94 | Identifiers of ongoing clinical trials in advanced cancers: NCT01303341; NCT01018836; NCT00866840; |
| Peptide specific Cytotoxic T Lymphocyte | Glutamine synthetase I | Anti Lengsin specific immunity | Preclinical testing | Lengsin is a member of the GS family of protein with restricted expression in the lens and ectopic expression in lung cancer89,107 |
| Acivicin | Glutamine metabolizing enzymes | L-glutamine analogue; inhibition through poisoning of glutamine utilizing enzymes | Phase II | No activity observed in lung cancer108,109 |
| L-DON (6-diazo-5-oxo-l-norleucine) | Glutamine utilizing enzymes | L-glutamine analogue/antagonist; inhibition through poisoning of glutamine utilizing enzymes | Phase I and II | Evaluated in combination with PEGylated-glutaminase73-75 |
| Talampanel | AMPA glutamate receptor | Interruption of the autocrine loop between glutamic acid and AMPA receptors | Phase II | No activity in unselected gliomas patients110 |
| Glutamine | Prevention of chemotherapy-induced toxicity | Enhanced ROS scavenging | Phase II | |
| Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) | GLS | Allosteric inhibitor of kidney isoform of GLS | Preclinical90 | |
| 5-(3-bromo-4-(dimethylamino)phenyl)-2,2-dimethyl-2,3,5,6-tetrahydrobenzo[a]phenanthridin-4(1H)-one, (compound 968)111Imaging application | GLS | Allosteric inhibitor of kidney isoform of GLS | Preclinical | |
| 18F-(2S, 4R) 4-fluoroglutamine | Glutamine uptake into cells | Glutamine-dependent cancer will show greater tracer uptake | Preclinical testing only100,112 | Clinical application supported by the long half-life of 18F |
| L-[5-11C]-glutamine | Glutamine uptake into cells | Higher tracer uptake in glutaminolytic tumors | Preclinical testing only105 | |
| 18F-labeled (2S,4R)-4-fluoro-l-glutamate | Glutamine uptake into cells | Glutamine-dependent cancer will show greater tracer uptake112 | Preclinical testing only100 |
Exploiting glutamine dependence for cancer therapy
The recognition that altered glutamine metabolism plays significant roles in cancer development and progression has fueled ongoing efforts to exploit this metabolic change for cancer treatment. Initial evaluation of glutamine analogues as potential chemotherapeutics in preclinical animal models and in early phase human studies have shown some promise while highlighting critical challenges that must be overcome for this approach to work. One approach to infuse GLS into the bloodstream in order to induce low blood glutamine levels and thereby decrease the availability of glutamine to the cancer cells was tested several decades ago.71 While this strategy successfully reduced the blood glutamine levels to near undetectable levels in large animal experiments, it also resulted in intolerable and fatal gastrointestinal side effects such as diarrhea, villous atrophy and intestinal necrosis.72 The PEGylated formulation of GLS was better tolerated and has now entered clinical development with encouraging anti-tumor effects observed in lung and colorectal cancer patients.73-76
Rho-GTPases induce oncogenic transformation and are overexpressed and hyperactivated in cancer cells.77,78 Because GLS mediates the activation of Rho-GTPases, a strategy using GLS inhibitor was evaluated in a preclinical model of breast cancer.79 Intriguingly, the use of small molecule inhibitors of GLS to uncouple this interaction reduced the rate of growth and invasive activity of transformed fibroblasts and human cancer cells.79,80 The precise mechanism by which altered glutamine metabolism resulted in an antitumor effect is still not fully elucidated and is the focus of ongoing research work by different groups.
The potential utility of glutamine analogues as chemotherapeutic agents is also under evaluation.81 Two glutamine analogues L-DON (6-diazo-5-oxo-l-norleucine) and acivicin (α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid) have similar chemical structures and are able to compete with glutamine in replicating cells.71,82 Acivicin is a potent inhibitor of the glutamine-dependent rate limiting enzyme of de novo purine and pyrimidine biosynthesis,10,83 while L-DON and acivicin both inhibit enzymes that catalyze irreversible alkylation of L-cysteinyl residues, a glutamine-requiring process.84 Evaluation of these compounds as anticancer agents is currently ongoing with promising results observed in preclinical models of CD8F1 mammary tumor, L1210 leukemia and colon cancer.85,86 Limited clinical testing revealed potential toxicities, which are generally expected based on experience with other antimetabolite agents including pancytopenia, mucositis, nausea and vomiting.87 L-DON induced a 20-fold reduction in tumor growth and also reduced the incidence of distant metastasis to the liver, lung and kidney based on bioluminescence imaging and histologic examination of harvested tissue samples. The antimetastatic potential of this glutamine analogue has been tested in preclinical mouse models of lung cancer among other cancers.84,88 Clinical testing of L-DON in human subjects was pursued in combination with PEGylated-glutaminase and was well-tolerated leading to total depletion of glutamine in serum as well encouraging clinical activity with partial responses observed in patients with lung and colorectal cancers.73-75
Lengsin is a member of the GS I family of proteins that has restricted normal tissue expression in the vertebrate eye.89 It is also a novel tumor-associated antigen that is expressed aberrantly in lung cancer cells but not in normal lung tissue or other common cancers including melanoma, colorectal carcinoma, breast carcinoma and hepatocellular carcinoma. Lung cancer patients have detectable anti-Lengsin autoantibodies. In RNAi knockdown experiments, loss of Lengsin resulted in reduced cell viability and eventual cell death suggesting that this protein may play a critical role in cell survival especially in lung cancer where it is aberrantly expressed. Robust preclinical studies are warranted to establish this protein as a viable target of anti-cancer therapy in lung cancer patients. Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) is an allosteric inhibitor of GLS.90 It was tested for potency against the R132H mutation in isocitrate dehydrogenase-1 (IDH1), a frequently observed molecular aberration in low-grade gliomas and some subsets of leukemias. BPTES preferentially suppressed α-ketoglutarate levels while selectively inhibiting the growth of cells with mutant IDH1.91
The autocrine activation of the GRM1 by glutamate is also implicated in a variety of cancers including lung cancer.56,58,59 The required extracellular release of glutamate for autocrine receptor activation is targeted by the use of riluzole, an extracellular glutamate release inhibitor. This agent is currently being tested in the clinic with encouraging early results.92-94
A contrary approach of using increased glutamine availability to cells as a therapeutic intervention has been evaluated specifically in lung cancer patients. This involved the use of glutamine supplementation to ameliorate treatment-induced toxicity. A retrospective study of 41 patients with stage III lung cancer treated with radiation showed that glutamine supplementation was associated with a lower rate (7 of 21 patients who received glutamine versus 13 of 20 patients who did not get glutamine) and reduced severity (36.8% vs. 0% grade 3) of acute radiation-induced esophagitis.95 This finding was replicated in a larger study of 104 lung cancer patients treated with concurrent chemoradiation. Glutamine supplementation was associated with reduced incidence of grade 3 acute onset (7.2% vs. 16.7%; p=0.02) and later onset esophagitis (0% vs. 6.3%; p=0.06). Contrary to expectations, the possible deleterious effect of glutamine on therapeutic efficacy, given its role in tumor biology, did not materialize, with comparable median overall survival and progression free survival of 21.4 vs. 20.4 (p=0.35) and 10.2 vs. 9.0 months (p=0.11) in glutamine-treated and untreated patient groups respectively.96 A possible explanation for the preserved treatment efficacy with glutamine supplementation could be the retrospective nature of the studies since a modest effect may be too small for small retrospective studies to demonstrate. Additionally, whether the glutamine supplementation was sufficient to induce any meaningful alterations in the biologic behavior of glutamine-dependent tumors is unknown.
Targeting glutamine metabolism for cancer imaging
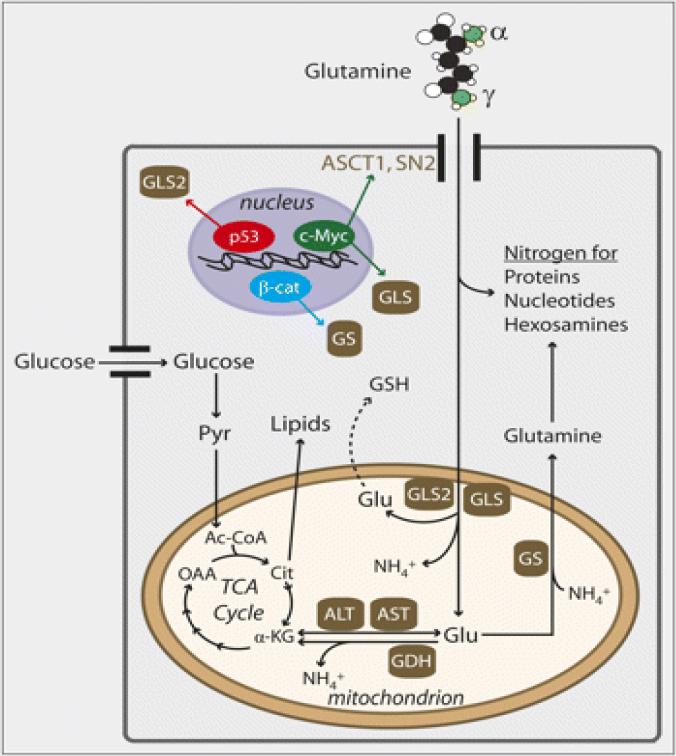
The disordered glucose metabolism of cancer cells has been successfully exploited in the care of cancer patients leading to the development of FDG-PET imaging as a clinical tool with resultant clinical benefit in terms of accurate staging and prognostication.97,98 The dependence of some cancer subsets on altered glutamine metabolism presents an opportunity for therapeutic targeting and prognostication similar to the development and incorporation of FDG-PET imaging into cancer management (Figure 2). Metabolic reprogramming in cancer cells may result in a switch from glucose to glutamine as the predominant source of energy and anabolic building block. This phenomenon may be responsible for the limited sensitivity of FDG-PET imaging in certain tumor types. Glutamine-directed metabolic imaging may therefore complement or even supplant FDG-PET imaging for accurate prognostic and predictive assessment of tumor behavior and response to therapy.99 While glutamine imaging is still in its early days, various approaches have been explored. One of the most advanced approaches is the use of glutamine analogs as PET imaging radiotracers in order to measure tumor-related glutaminolysis. 18F-labeled (2S,4R)-4-fluoro-l-glutamine (-18F-(2S,4R)4F-GLN) was developed as a PET radiotracer based on the hypothesis that glutamine-dependent tumors will have higher avidity for glutamine which will manifest as increased tumor uptake of the radio-labeled glutamine.100,101 This tracer was tested in experimental in vivo mouse models of c-myc amplified glioblastoma cell lines, F33 and L9. As expected, there was selective uptake and trapping of 18F-(2S, 4R) 4-fluoroglutamine by tumor cells resulting in successful imaging of the tumor.101-103 This approach may be especially relevant and worthy of future evaluation in SCLC where c-myc amplification is a frequently observed and prognostically relevant genomic alteration.
Figure 2.
Glutamine metabolism showing potential opportunities for cancer imaging including surface membrane transporters, intracellular glutamine and metabolic intermediates including glutamic acid, glutamine synthetase and glutaminase enzymes. Adapted from Rajagopalan and DeBerardinis, J Nucl Med July 1, 2011 vol. 52 no. 7 1005-1008.97
Preclinical in vitro and in vivo comparison of 18F-(2S,4R)4F-GLN and 18F-labeled (2S,4R)-4-fluoro-l-glutamate (18F-(2S,4R)4F-GLU) showed a high uptake of the glutamine-based radiotracer in highly proliferative tumor.104 The ASC transporter preferentially transferred 18F-(2S,4R)4F-GLN while system X(C)- transported the glutamic acid radiotracer, 18F-(2S,4R)4F-GLU, preferentially. An in vitro cellular assay revealed a differential intracellular handling of the two radiotracers. Whereas 18F-(2S,4R)4F-GLN became incorporated and trapped in protein macromolecules, 18F-(2S,4R)4F-GLU remained in its original free amino acid form. This differential handling resulted in a higher tumor uptake of 18F-(2S,4R)4F-GLN, while 18F-(2S,4R)4F-GLU, showed a superior tumor-to-background ratio indicating that these two radiotracers may be adapted to study the involvement of different aspects of glutamine metabolism in various cancer types, specifically lung cancer, in the clinical setting.
Because glutamine is very rich in carbon and nitrogen atoms, alternative approaches are under exploration to use 11C and 13N radioisotope labeling for imaging.105 The success of these approaches will depend on whether or not the imaging algorithm can overcome the challenges posed by the rapid extrusion of carbon and nitrogen atoms from glutaminolytic cells. The high activity of GS in such cells, however, is expected to render them susceptible to this type of imaging, which depends on incorporation of the radiolabeled substrate into intracellular glutamine stores.106 Readers are referred to a recent review by Rajagopalan and DeBerardinis for a comprehensive overview of potential applications of glutamine imaging in cancer therapy.97
Conclusions
Glutamine is the most abundant amino acid in circulation and plays important roles in normal cell metabolism and energy balance. The critical role of altered glutamine metabolism in supporting neoplastic transformation and cancer progression has garnered strong scientific and research interest. Glutamine together with its metabolizing enzymes offer promising targets for anticancer therapy because of their central role in energy generation and macromolecule synthesis in support of the disordered growth of cancer cells. Potential therapeutic opportunities in lung cancer include the application of dynamic changes in the level of glutamine within the tumor and host blood circulating compartments as biomarkers of disease activity and response to therapy. In this regard, metabolic imaging techniques based on glutamine analogues and radioisotope-labeled glutamine have produced encouraging results that may be ready for clinical application in the not too distant future. Although direct targeting of the glutamine metabolic pathway for cancer therapy is still in its infancy and has a number of hurdles to overcome, our ability to link specific molecular characteristics of lung cancer to glutamine dependence may offer a fast track for clinical evaluation of the emerging glutamine-targeting agents in lung cancer.
Acknowledgements
We thank Anthea Hammond, PhD for her editorial assistance in reviewing the draft manuscript.
Funding: Supported through the National Institute of Health grants (1K23CA164015, P01 CA116676) and Georgia Cancer Coalition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest declaration: All authors have no significant conflicts of interest to declare.
References
- 1.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA: a cancer journal for clinicians. 2011;61:91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 2.Vansteenkiste JF, Stroobants SS. PET scan in lung cancer: current recommendations and innovation. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2006;1:71–3. [PubMed] [Google Scholar]
- 3.Teicher BA, Linehan WM, Helman LJ. Targeting cancer metabolism. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5537–45. doi: 10.1158/1078-0432.CCR-12-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Locasale JW, Swanson KD, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–9. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hitosugi T, Zhou L, Elf S, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22:585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer research. 2010;70:859–62. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla K, Ferraris DV, Thomas AG, et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) analogs as glutaminase inhibitors. Journal of medicinal chemistry. 2012 doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergstrom J, Furst P, Noree LO, et al. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36:693–7. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- 9.Austgen TR, Chakrabarti R, Chen MK, et al. Adaptive regulation in skeletal muscle glutamine metabolism in endotoxin-treated rats. J Trauma. 1992;32:600–6. doi: 10.1097/00005373-199205000-00011. discussion 606-7. [DOI] [PubMed] [Google Scholar]
- 10.Medina MA, Sanchez-Jimenez F, Marquez J, et al. Relevance of glutamine metabolism to tumor cell growth. Mol Cell Biochem. 1992;113:1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- 11.Souba WW. Interorgan ammonia metabolism in health and disease: a surgeon's view. JPEN J Parenter Enteral Nutr. 1987;11:569–79. doi: 10.1177/0148607187011006569. [DOI] [PubMed] [Google Scholar]
- 12.Newsholme P, Lima MM, Procopio J, et al. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36:153–63. doi: 10.1590/s0100-879x2003000200002. [DOI] [PubMed] [Google Scholar]
- 13.Brosnan JT. Interorgan amino acid transport and its regulation. J Nutr. 2003;133:2068S–2072S. doi: 10.1093/jn/133.6.2068S. [DOI] [PubMed] [Google Scholar]
- 14.Yuneva M, Zamboni N, Oefner P, et al. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craan AG, Lemieux G, Vinay P, et al. The kidney of chicken adapts to chronic metabolic acidosis: in vivo and in vitro studies. Kidney Int. 1982;22:103–11. doi: 10.1038/ki.1982.142. [DOI] [PubMed] [Google Scholar]
- 16.Berman HK, O'Doherty RM, Anderson P, et al. Overexpression of protein targeting to glycogen (PTG) in rat hepatocytes causes profound activation of glycogen synthesis independent of normal hormone- and substrate-mediated regulatory mechanisms. J Biol Chem. 1998;273:26421–5. doi: 10.1074/jbc.273.41.26421. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–7. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- 18.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 19.Meister A. Glutathione, ascorbate, and cellular protection. Cancer Res. 1994;54:1969s–1975s. [PubMed] [Google Scholar]
- 20.Souba WW. Glutamine and cancer. Ann Surg. 1993;218:715–28. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–14. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 22.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–76. [PubMed] [Google Scholar]
- 23.Kovacevic Z, Morris HP. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972;32:326–33. [PubMed] [Google Scholar]
- 24.Fischer JE, Chance WT. Total parenteral nutrition, glutamine, and tumor growth. JPEN J Parenter Enteral Nutr. 1990;14:86S–89S. doi: 10.1177/0148607190014004101. [DOI] [PubMed] [Google Scholar]
- 25.Bode BP, Fuchs BC, Hurley BP, et al. Molecular and functional analysis of glutamine uptake in human hepatoma and liver-derived cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1062–73. doi: 10.1152/ajpgi.00031.2002. [DOI] [PubMed] [Google Scholar]
- 26.Bode BP, Kaminski DL, Souba WW, et al. Glutamine transport in isolated human hepatocytes and transformed liver cells. Hepatology. 1995;21:511–20. [PubMed] [Google Scholar]
- 27.Drogat B, Bouchecareilh M, North S, et al. Acute L-glutamine deprivation compromises VEGF-a upregulation in A549/8 human carcinoma cells. J Cell Physiol. 2007;212:463–72. doi: 10.1002/jcp.21044. [DOI] [PubMed] [Google Scholar]
- 28.Huber KR, Mayer EP, Mitchell DF, et al. Cell cycle phase perturbations by 6-diazo-5-oxo-L-norleucine and acivicin in normal and neoplastic human cell lines. Br J Cancer. 1987;55:653–6. doi: 10.1038/bjc.1987.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brower M, Carney DN, Oie HK, et al. Growth of cell lines and clinical specimens of human non-small cell lung cancer in a serum-free defined medium. Cancer Res. 1986;46:798–806. [PubMed] [Google Scholar]
- 30.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 31.Souba WW, Pacitti AJ. How amino acids get into cells: mechanisms, models, menus, and mediators. JPEN J Parenter Enteral Nutr. 1992;16:569–78. doi: 10.1177/0148607192016006569. [DOI] [PubMed] [Google Scholar]
- 32.Boerner P, Saier MH., Jr. Adaptive regulatory control of System A transport activity in a kidney epithelial cell line (MDCK) and in a transformed variant (MDCK-T1). J Cell Physiol. 1985;122:308–15. doi: 10.1002/jcp.1041220221. [DOI] [PubMed] [Google Scholar]
- 33.Sastrasinh S, Sastrasinh M. Glutamine transport in submitochondrial particles. Am J Physiol. 1989;257:F1050–8. doi: 10.1152/ajprenal.1989.257.6.F1050. [DOI] [PubMed] [Google Scholar]
- 34.Rivera S, Azcon-Bieto J, Lopez-Soriano FJ, et al. Amino acid metabolism in tumour-bearing mice. Biochem J. 1988;249:443–9. doi: 10.1042/bj2490443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souba WW, Strebel FR, Bull JM, et al. Interorgan glutamine metabolism in the tumor-bearing rat. J Surg Res. 1988;44:720–6. doi: 10.1016/0022-4804(88)90106-0. [DOI] [PubMed] [Google Scholar]
- 36.Pacitti AJ, Chen MK, Bland KI, et al. Mechanisms of accelerated hepatic glutamine efflux in the tumour-bearing rat. Surg Oncol. 1992;1:173–82. doi: 10.1016/0960-7404(92)90031-f. [DOI] [PubMed] [Google Scholar]
- 37.Knox WE, Horowitz ML, Friedell GH. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969;29:669–80. [PubMed] [Google Scholar]
- 38.Chen MK, Salloum RM, Austgen TR, et al. Tumor regulation of hepatic glutamine metabolism. JPEN J Parenter Enteral Nutr. 1991;15:159–64. doi: 10.1177/0148607191015002159. [DOI] [PubMed] [Google Scholar]
- 39.Rennie MJ, MacLennan PA, Hundal HS, et al. Skeletal muscle glutamine transport, intramuscular glutamine concentration, and muscle-protein turnover. Metabolism. 1989;38:47–51. doi: 10.1016/0026-0495(89)90140-6. [DOI] [PubMed] [Google Scholar]
- 40.Salloum RM, Copeland EM, 3rd, Bland KI, et al. Selective stimulation of brush border glutamine transport in the tumor-bearing rat. J Surg Res. 1991;50:391–7. doi: 10.1016/0022-4804(91)90208-4. [DOI] [PubMed] [Google Scholar]
- 41.Pacitti AJ, Inoue Y, Souba WW. Characterization of Na(+)-independent glutamine transport in rat liver. Am J Physiol. 1993;265:G90–8. doi: 10.1152/ajpgi.1993.265.1.G90. [DOI] [PubMed] [Google Scholar]
- 42.Pacitti AJ, Inoue Y, Souba WW. Tumor necrosis factor stimulates amino acid transport in plasma membrane vesicles from rat liver. J Clin Invest. 1993;91:474–83. doi: 10.1172/JCI116225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langstein HN, Norton JA. Mechanisms of cancer cachexia. Hematol Oncol Clin North Am. 1991;5:103–23. [PubMed] [Google Scholar]
- 44.van den Heuvel AP, Jing J, Wooster RF, et al. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther. 2012;13 doi: 10.4161/cbt.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hori S, Nishiumi S, Kobayashi K, et al. A metabolomic approach to lung cancer. Lung Cancer. 2011;74:284–92. doi: 10.1016/j.lungcan.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Long J, Wang H, Lang Z, et al. Expression level of glutamine synthetase is increased in hepatocellular carcinoma and liver tissue with cirrhosis and chronic hepatitis B. Hepatol Int. 2011;5:698–706. doi: 10.1007/s12072-010-9230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–59. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- 48.Carrascosa JM, Martinez P, Nunez de Castro I. Nitrogen movement between host and tumor in mice inoculated with Ehrlich ascitic tumor cells. Cancer Res. 1984;44:3831–5. [PubMed] [Google Scholar]
- 49.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaira K, Oriuchi N, Imai H, et al. Expression of L-type amino acid transporter 1 (LAT1) in neuroendocrine tumors of the lung. Pathol Res Pract. 2008;204:553–61. doi: 10.1016/j.prp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Kaira K, Takahashi T, Murakami H, et al. Relationship between LAT1 expression and response to platinum-based chemotherapy in non-small cell lung cancer patients with postoperative recurrence. Anticancer Res. 2011;31:3775–82. [PubMed] [Google Scholar]
- 52.Imai H, Kaira K, Oriuchi N, et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–28. [PubMed] [Google Scholar]
- 53.Kaira K, Oriuchi N, Takahashi T, et al. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am J Transl Res. 2011;3:468–78. [PMC free article] [PubMed] [Google Scholar]
- 54.Hassanein M, Hoeksema MD, Shiota M, et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19:560–70. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 56.Marin YE, Namkoong J, Cohen-Solal K, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–86. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Shin SS, Wall BA, Goydos JS, et al. AKT2 is a downstream target of metabotropic glutamate receptor 1 (Grm1). Pigment Cell Melanoma Res. 2010;23:103–11. doi: 10.1111/j.1755-148X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Namkoong J, Shin SS, Lee HJ, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 59.Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci U S A. 2001;98:6372–7. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010;70:859–62. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volm M, Drings P, Wodrich W, et al. Expression of oncoproteins in primary human non-small cell lung cancer and incidence of metastases. Clin Exp Metastasis. 1993;11:325–9. doi: 10.1007/BF00058052. [DOI] [PubMed] [Google Scholar]
- 63.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–4. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 64.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van den Heuvel AP, Jing J, Wooster RF, et al. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther. 2012;13:1185–94. doi: 10.4161/cbt.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver Z, Difilippantonio S, Carretero J, et al. Temporal molecular and biological assessment of an erlotinib-resistant lung adenocarcinoma model reveals markers of tumor progression and treatment response. Cancer Res. 2012;72:5921–33. doi: 10.1158/0008-5472.CAN-12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–9. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 69.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hara K, Yonezawa K, Weng QP, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–94. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 71.Ahluwalia GS, Grem JL, Hao Z, et al. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46:243–71. doi: 10.1016/0163-7258(90)90094-i. [DOI] [PubMed] [Google Scholar]
- 72.Baskerville A, Hambleton P, Benbough JE. Pathological features of glutaminase toxicity. Br J Exp Pathol. 1980;61:132–8. [PMC free article] [PubMed] [Google Scholar]
- 73.Unger C, Mueller C, Bausch MP, et al. A phase I schedule optimization study of pegylated glutaminase (PEG-PGA) plus 6-diazo-5-oxo-l-norleucine (DON) in patients (pts) with advanced solid tumors. Journal of Clininal Oncology. 2011;29:3049. [Google Scholar]
- 74.Mueller C, Al-Batran S, Jaeger E, et al. A phase I trial of PEGylated glutaminase (PEG-PGA) in combination with 6-diazo-5-oxo-L-norleucine (DON) in advanced, refractory, solid tumors. Journal of Clininal Oncology. 2007;25:14040. [Google Scholar]
- 75.Mueller C, Al-Batran S, Jaeger E, et al. A phase IIa study of PEGylated glutaminase (PEG-PGA) plus 6-diazo-5-oxo-L-norleucine (DON) in patients with advanced refractory solid tumors. Journal of Clininal Oncology. 2008;26:2533. [Google Scholar]
- 76.Harzmann R, Esser N, Loewe R, et al. Glutaminase (PEG-PGA) increases antitumoral efficacy of cytotoxic agents. Journal of Clininal Oncology. 2004;22:3183. [Google Scholar]
- 77.Suwa H, Ohshio G, Imamura T, et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77:147–52. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mira JP, Benard V, Groffen J, et al. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci U S A. 2000;97:185–9. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang JB, Erickson JW, Fuji R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–19. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erickson JW, Cerione RA. Glutaminase: a hot spot for regulation of cancer cell metabolism? Oncotarget. 2010;1:734–40. doi: 10.18632/oncotarget.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaurav K, Goel RK, Shukla M, et al. Glutamine: A novel approach to chemotherapy-induced toxicity. Indian J Med Paediatr Oncol. 2012;33:13–20. doi: 10.4103/0971-5851.96962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kisner DL, Catane R, Muggia FM. The rediscovery of DON (6-diazo-5-oxo-L-norleucine). Recent Results Cancer Res. 1980;74:258–63. doi: 10.1007/978-3-642-81488-4_30. [DOI] [PubMed] [Google Scholar]
- 83.Weber G, Prajda N. Targeted and non-targeted actions of anti-cancer drugs. Adv Enzyme Regul. 1994;34:71–89. doi: 10.1016/0065-2571(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 84.Shelton LM, Huysentruyt LC, Seyfried TN. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int J Cancer. 2010;127:2478–85. doi: 10.1002/ijc.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ovejera AA, Houchens DP, Catane R, et al. Efficacy of 6-diazo-5-oxo-L-norleucine and N-[N-gamma-glutamyl-6-diazo-5-oxo-norleucinyl]-6-diazo-5-oxonorleucine against experimental tumors in conventional and nude mice. Cancer Res. 1979;39:3220–4. [PubMed] [Google Scholar]
- 86.Nichols KE, Chitneni SR, Moore JO, et al. Monocytoid differentiation of freshly isolated human myeloid leukemia cells and HL-60 cells induced by the glutamine antagonist acivicin. Blood. 1989;74:1728–37. [PubMed] [Google Scholar]
- 87.Maroun JA, Fields AL, Pater JL, et al. Phase II study of acivicin in colorectal carcinoma: a National Cancer Institute of Canada study. Cancer Treat Rep. 1984;68:1121–3. [PubMed] [Google Scholar]
- 88.Patel PH, Chaganti RS, Motzer RJ. Targeted therapy for metastatic renal cell carcinoma. Br J Cancer. 2006;94:614–9. doi: 10.1038/sj.bjc.6602978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakatsugawa M, Hirohashi Y, Torigoe T, et al. Novel spliced form of a lens protein as a novel lung cancer antigen, Lengsin splicing variant 4. Cancer Sci. 2009;100:1485–93. doi: 10.1111/j.1349-7006.2009.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shukla K, Ferraris DV, Thomas AG, et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55:10551–63. doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer research. 2010;70:8981–7. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehnert JM, Semlani N, Wen Y, et al. A phase I trial of riluzole and sorafenib in patients with advanced solid tumors and melanoma. Journal of Clininal Oncology. 2012;30:TPS3112. [Google Scholar]
- 93.Mehnert JM, Lee JH, Shirk J, et al. A phase II trial of riluzole, an antagonist of metabotropic glutamate receptor 1 (GRM1) signaling, in metastatic melanoma. Journal of Clininal Oncology. 2010;28:TPS309. [Google Scholar]
- 94.Yip D, Le MN, Chan JL, et al. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Topkan E, Yavuz MN, Onal C, et al. Prevention of acute radiation-induced esophagitis with glutamine in non-small cell lung cancer patients treated with radiotherapy: evaluation of clinical and dosimetric parameters. Lung Cancer. 2009;63:393–9. doi: 10.1016/j.lungcan.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 96.Topkan E, Parlak C, Topuk S, et al. Influence of oral glutamine supplementation on survival outcomes of patients treated with concurrent chemoradiotherapy for locally advanced non-small cell lung cancer. BMC Cancer. 2012;12:502. doi: 10.1186/1471-2407-12-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rajagopalan KN, DeBerardinis RJ. Role of glutamine in cancer: therapeutic and imaging implications. J Nucl Med. 2011;52:1005–8. doi: 10.2967/jnumed.110.084244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gambhir SS. Molecualr imaging of cancer: from molecules to humans. Introduction. J Nucl Med. 2008;49(Suppl 2):1S–4S. doi: 10.2967/jnumed.108.053751. [DOI] [PubMed] [Google Scholar]
- 99.Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol. 2012;23:362–9. doi: 10.1016/j.semcdb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Lieberman BP, Ploessl K, Wang L, et al. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J Nucl Med. 2011;52:1947–55. doi: 10.2967/jnumed.111.093815. [DOI] [PubMed] [Google Scholar]
- 101.Qu W, Zha Z, Ploessl K, et al. Synthesis of optically pure 4-fluoroglutamines as potential metabolic imaging agents for tumors. Journal of the American Chemical Society. 2011;133:1122–33. doi: 10.1021/ja109203d. [DOI] [PubMed] [Google Scholar]
- 102.Qu W, Zha Z, Ploessl K, et al. Synthesis of optically pure 4-fluoroglutamines as potential metabolic imaging agents for tumors. J Am Chem Soc. 2011;133:1122–33. doi: 10.1021/ja109203d. [DOI] [PubMed] [Google Scholar]
- 103.Lieberman BP, Ploessl K, Wang L, et al. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:1947–55. doi: 10.2967/jnumed.111.093815. [DOI] [PubMed] [Google Scholar]
- 104.Ploessl K, Wang L, Lieberman BP, et al. Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:1616–24. doi: 10.2967/jnumed.111.101279. [DOI] [PubMed] [Google Scholar]
- 105.Qu W, Oya S, Lieberman BP, et al. Preparation and characterization of L-[5-11C]-glutamine for metabolic imaging of tumors. J Nucl Med. 2012;53:98–105. doi: 10.2967/jnumed.111.093831. [DOI] [PubMed] [Google Scholar]
- 106.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakatsugawa M, Horie K, Yoshikawa T, et al. Identification of an HLAA*0201-restricted cytotoxic T lymphocyte epitope from the lung carcinoma antigen, Lengsin. Int J Oncol. 2011;39:1041–9. doi: 10.3892/ijo.2011.1089. [DOI] [PubMed] [Google Scholar]
- 108.Kramer BS, Birch R, Greco A, et al. Phase II evaluation of acivicin in lung cancer: a Southeastern Cancer Study Group Trial. Cancer Treat Rep. 1986;70:1031–2. [PubMed] [Google Scholar]
- 109.Bonomi P, Finkelstein D, Chang A. Phase II trial of acivicin versus etoposide-cisplatin in non-small cell lung cancer. An Eastern Cooperative Oncology Group study. Am J Clin Oncol. 1994;17:215–7. doi: 10.1097/00000421-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 110.Iwamoto FM, Kreisl TN, Kim L, et al. Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer. 2010;116:1776–82. doi: 10.1002/cncr.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katt WP, Ramachandran S, Erickson JW, et al. Dibenzophenanthridines as inhibitors of glutaminase C and cancer cell proliferation. Mol Cancer Ther. 2012;11:1269–78. doi: 10.1158/1535-7163.MCT-11-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ploessl K, Wang L, Lieberman BP, et al. Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J Nucl Med. 2012;53:1616–24. doi: 10.2967/jnumed.111.101279. [DOI] [PubMed] [Google Scholar]