Abstract
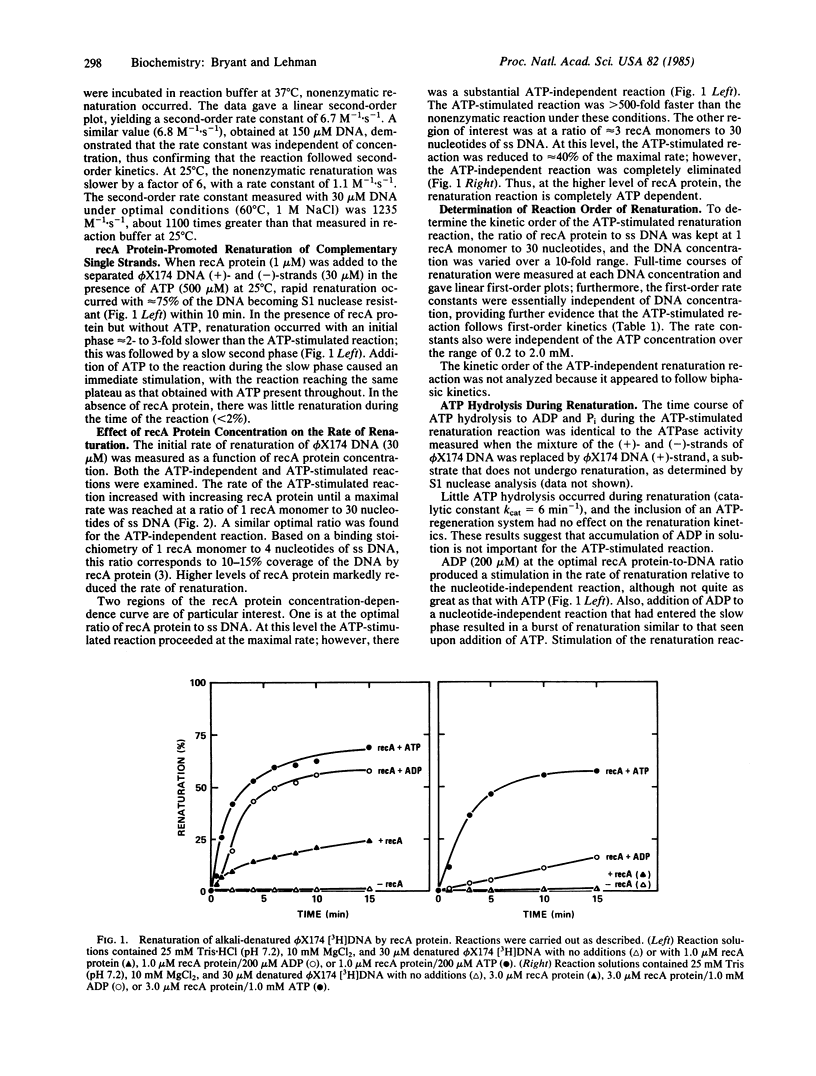
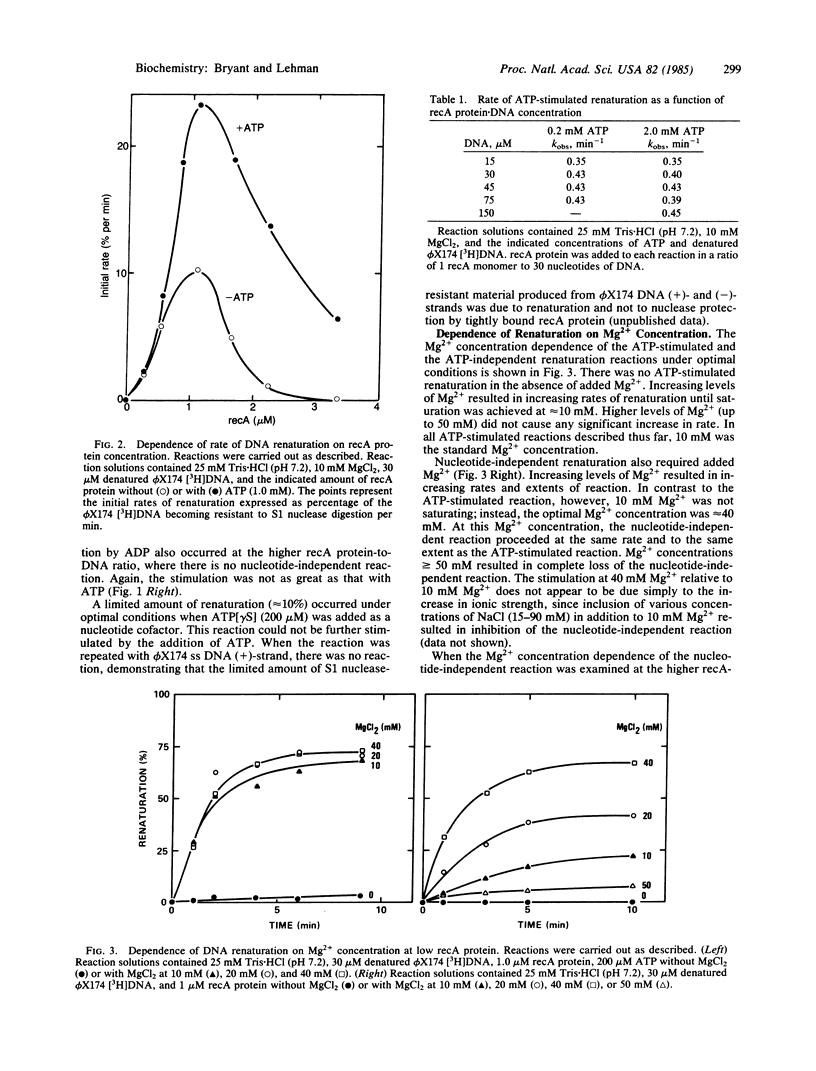
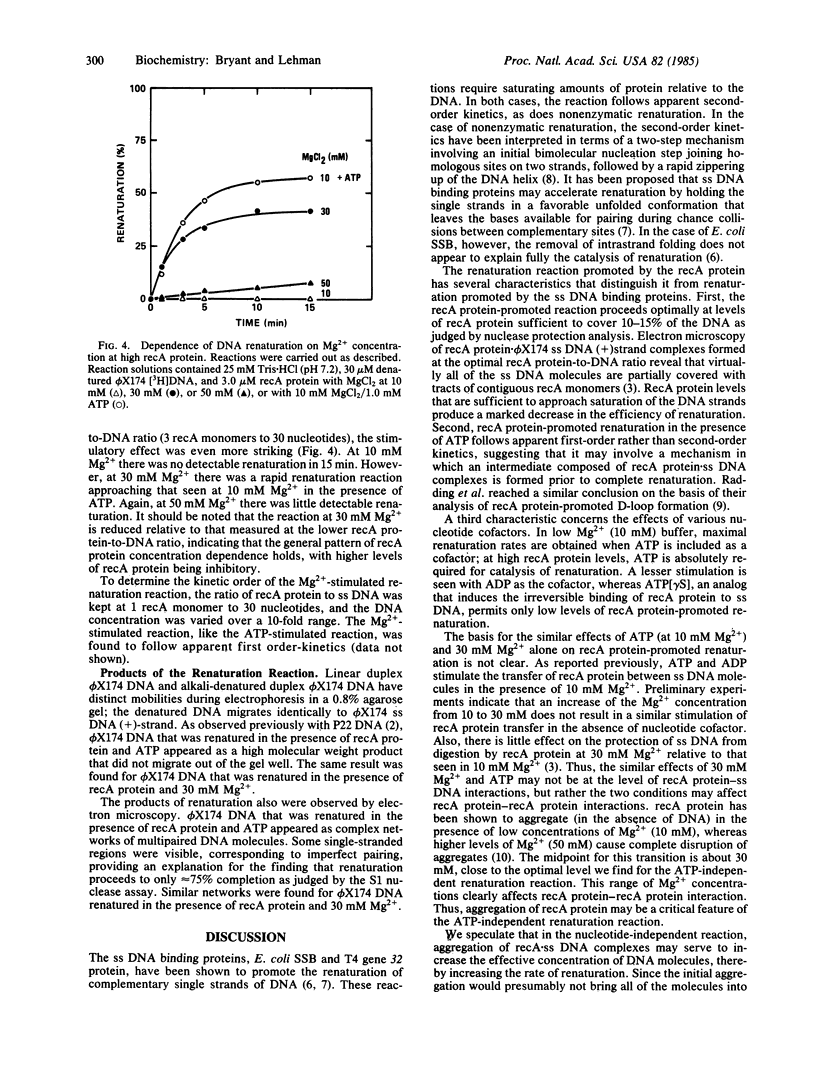
The renaturation of complementary DNA strands by the recA protein of Escherichia coli has been found to exhibit the following features. (i) Optimal renaturation occurs at recA protein levels below that required to saturate the DNA strands; saturating amounts of recA protein significantly reduce the rate of reaction. (ii) The reaction proceeds in the absence of a nucleotide cofactor but is markedly stimulated by ATP in the presence of 10 mM Mg2+. A similar stimulation occurs in the absence of ATP when the Mg2+ concentration is increased from 10 mM to 30-40 mM. (iii) Both the ATP-stimulated and the Mg2+-stimulated reactions follow apparent first-order kinetics. These results, taken together with the known effects of ATP and Mg2+ on the state of aggregation of recA protein, suggest that the association of recA monomers may play an important role in recA protein-promoted DNA renaturation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Baldwin R. L. Catalysis of DNA reassociation by the Escherichia coli DNA binding protein: A polyamine-dependent reaction. J Mol Biol. 1977 Sep 25;115(3):441–454. doi: 10.1016/0022-2836(77)90164-4. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3433–3437. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M., McEntee K., Lehman I. R. A simple and rapid procedure for the large scale purification of the recA protein of Escherichia coli. J Biol Chem. 1981 May 10;256(9):4676–4678. [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Radding C. M., Shibata T., DasGupta C., Cunningham R. P., Osber L. Kinetics and topology of homologous pairing promoted by Escherichia coli recA-gene protein. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):385–390. doi: 10.1101/sqb.1981.045.01.052. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


