Abstract
Facial expressions play a critical role in social interactions by eliciting rapid responses in the observer. Failure to perceive and experience a normal range and depth of emotion seriously impact interpersonal communication and relationships. As has been demonstrated across a number of domains, abnormal emotion processing in individuals with psychopathy plays a key role in their lack of empathy. However, the neuroimaging literature is unclear as to whether deficits are specific to particular emotions such as fear and perhaps sadness. Moreover, findings are inconsistent across studies. In the current experiment, eighty adult incarcerated males scoring high, medium, and low on the Hare Psychopathy Checklist-Revised (PCL-R) underwent fMRI scanning while viewing dynamic facial expressions of fear, sadness, happiness and pain. Participants who scored high on the PCL-R showed a reduction in neuro-hemodynamic response to all four categories of facial expressions in the face processing network (inferior occipital gyrus, fusiform gyrus, STS) as well as the extended network (inferior frontal gyrus and orbitofrontal cortex), which supports a pervasive deficit across emotion domains. Unexpectedly, the response in dorsal insula to fear, sadness and pain was greater in psychopaths than non-psychopaths. Importantly, the orbitofrontal cortex and ventromedial prefrontal cortex, regions critically implicated in affective and motivated behaviors, were significantly less active in individuals with psychopathy during the perception of all four emotional expressions.
Keywords: amygdala, emotion, facial expressions, fear, fMRI, insula, happiness, pain, sadness, psychopathy, ventromedial prefrontal cortex
Facial expressions play a central role in social interactions by conveying social and affective information. The expressions we see in the faces of others elicit rapid responses that serve important adaptive functions. However, faces are also a special class of stimuli which are associated with activity in a reliable collection of subcortical and cortical regions. Visual information from faces is processed via a distributed network with core and extended components (Haxby et al., 2000; 2002; Zhen et al., 2013). Portions of the inferior and middle occipital gyri, fusiform gyrus, and superior temporal sulcus form the ‘core’ system for face processing. Identity or unchangeable aspects of the face are processed by the fusiform gyrus, while expression, gaze, and other variable aspects of the face are processed by the superior temporal sulcus (Kanwisher et al., 1997). Additional processing occurs in the ‘extended’ face processing system, which incorporates affective and evaluative information, and includes the amygdala, inferior frontal gyrus (IFG), OFC, and ventral striatum (Breiter et al., 1996; Fusar-Poli et al., 2009).
Emotional Processing, Empathy and Psychopathy
Individuals classified as psychopaths are callous, shallow, and superficial. They often lack fear of punishment, have difficulty regulating their emotions, and do not experience insight into or empathy for those that their behavior affects (Blair, Mitchell and Blair, 2008; Hare, 2003; van Honk and Schutter, 2006). Empathy, the social-emotional response that is induced by the perception of another person's affective state, particularly if they are in need or are feeling vulnerable, is a fundamental component of the emotional experience, which plays a vital role in social interaction. Empathy is a complex construct encompassing affective, motivational and cognitive components (Davidov et al., 2013; Decety and Jackson, 2004; Singer and Decety, 2011). As such, the disturbance or malfunctioning of any one of these components can lead to the empathetic deficits seen in psychopaths.
Numerous studies have reported deficits in emotion perception and recognition in individuals with psychopathy, especially for fear and sadness (Marsh and Blair, 2008 for a review), but some other studies have not found any deficiency, or have only found deficits for facial expressions that were lower in intensity (Glass and Newman, 2006; Hasting et al., 2008; Pham and Philippot, 2010). A recent meta-analysis found evidence of significant impairments associated with psychopathic traits for positive as well as negative emotions across both facial and vocal modalities (Dawel et al., 2012).
Emotion processing deficits in psychopathy, especially related to fear, are a potential source of difficulty in instrumental conditioning which, during development, prevent the normal inhibition of aggression and violence and induces the acquisition of healthy prosocial behavior (Blair 2006). To most individuals, the presentation of distress cues such as fearful or sad facial expressions is aversive (Bandura and Rosenthal, 1966). Typically, viewing faces in distress or sadness may lead to the interruption of aggression (Perry and Perry, 1974) and the initiation of prosocial behavior (Hoffman, 1975) or concern for the other (Decety and Howard, 2013). If distress cues are not processed in a typical manner, they may not contribute to the development of functional empathy and the motivation to care for another. For instance, in one fMRI study, individuals with high callous traits showed significantly less amygdala and medial prefrontal cortex activity than those with low callous traits when the eyes of a faces were occluded, but not when they were isolated (Han et al., 2012). Another study using an approach/avoidance task with angry faces showed that psychopaths lack typical automatic avoidance behavior of social threat cues (von Borries et al., 2012).
Alternatively, the mechanisms for instrumental learning may be intact, but fail to produce typical effects due to abnormal intake of the affective information that characteristically drives learning behavior. For instance, evidence exists that psychopaths show executive deficits only in tests with affective components (LaPierre et al., 1995) and are sometimes found to perform poorly on tests of emotion expression identification, particularly for the expression of fear (Marsh and Blair 2008). A recent study demonstrated impaired cognitive empathy in psychopathy using a semi-naturalistic empathic accuracy paradigm (Brook and Kosson, 2013). This study found inverse associations between psychopathy and empathic accuracy scores, as well as robust group differences between psychopathic and non-psychopathic inmates. These results expand upon prior findings examining the performance of individuals with psychopathic traits on tasks related to cognitive empathy that demonstrate overall impaired recognition of emotion from morphed affective faces and speech cues. In addition, functional neuroimaging studies suggest that high callous-unemotional trait is associated with reduced amygdala activity to fearful facial expressions (Marsh et al., 2008; Jones et al., 2009). Taken together, these results indicate that atypical emotional processing clearly contributes to the dysfunction of empathy in psychopathy.
In the current investigation, a large sample of incarcerated offenders scoring low, medium, and high on the PCL-R were scanned with functional MRI while passively viewing dynamic stimuli of expressive faces in order to probe well-characterized brain networks related to facial emotion processing and empathy in healthy adults. The large sample size and incarcerated control participants eschew methodological issues present in some previous neuroimaging research in psychopathy (Koenigs et al., 2010). The use of a passive viewing design reduces the risk of acquiring false negatives by potentially inhibiting affective processing through the use of a cognitive task. Dynamic video clips depicted facial expressions of fear, happiness, sadness, and pain. Judgments of facial affect are influenced by changing the velocity of an expressing face, suggesting that the dynamic display of facial expressions provides unique temporal information about the expressions, which is not available in static displays (Kamachi et al., 2001; Wehrle et al., 2000).
In a number of functional neuroimaging investigations of empathy for pain and distress in healthy individuals, expressive faces were used as stimuli. These studies found activity in key regions involved in the perception of physical pain including the anterior cingulate cortex (ACC) and anterior insula (aINS) (e.g., Botvinick et al., 2005; Decety and Michalska, 2010; Lamm et al., 2007; Saarela et al., 2006). In addition, when dynamic stimuli were used, or when participants were asked to focus on the negative affect in the face of the other, activation was detected in the dorsomedial prefrontal cortex (dmPFC) (Botvinick et al., 2005; Lamm et al., 2007).
Fearful and sad faces were chosen because of their use as communicative signals of negative emotional states and because of findings of selective deficits in psychopathy when processing these emotions (Dolan and Fullam, 2006). Pain expressions were included because of their relevance to empathy and caring. Analyses were conducted in a groupwise manner, by comparing participants with PCL-R total score greater than or equal to 30 to participants whose score was equal to or less than 20, and also in correlation analyses with Factor 1 and Factor 2 subscores as continuous variables. It was predicted that participants high in psychopathy would show reduced activation in affect-specific nodes of the face processing network, as well as the amygdala, OFC, and insula. Additionally, having a range of emotional expressions allowed us to examine whether the deficits in psychopaths perception of emotions are specific to fear or are more pervasive.
METHODS
Participants
80 adult males incarcerated in a medium-security North American correctional facility between the ages of 18 and 50 volunteered for the research study and provided informed consent to the procedures described here, which were approved by the Institutional Review Boards of the University of New Mexico and the University of Chicago. Before entering the scanner, the inmates completed a number of assessments, including demographic information, IQ, and assessments of psychopathy and other psychiatric disorders. Demographic information included age, race, handedness (Annett, 1970), and the Hollingshead socioeconomic status index (Hollingshead and Redlich, 1958). Intelligence quotient was estimated using the vocabulary and block design subtests from the Wechsler Adult Intelligence Scale (WAIS-III, Wechsler, 2000). All participants completed the Structured Clinical Interview (SCID) for Axis 1 and Axis II disorders (First et al., 1997), the State-Trait Anxiety scale (Spielberger, 2002), Beck Depression Inventory (Beck et al., 1996), and underwent a medical history including interview and file review to assess history of central nervous system abnormalities and drug and alcohol abuse. Participants underwent the PCL-R (Hare 2003), including file review and interviews, conducted by trained research assistants under the supervision of Dr. Kiehl. Participants scoring 30 or above on the PCL-R were assigned to the high-psychopathy group (n=27). Medium- and low-psychopathy groups were comprised of volunteers scoring between 21 and 29 on the PCL-R (n=25) and volunteers scoring at or below 20 (n=28), respectively. These groups were matched to high scorers on age, race and ethnicity, IQ, comorbidity for DSM-IV Axis II disorders (American Psychiatric Association 2000), and past drug abuse and dependence. Participants were paid one dollar per hour for their participation in the study, a typical rate for institutional labor compensation.
MRI Acquisition
Scanning was conducted on a Mind Research Network's 1.5 Tesla Siemens Magnetom Avanto mobile unit equipped with advanced SQ gradients and a twelve-element head coil. Functional images were collected using an EPI gradient-echo pulse sequence with TR/TE = 2000/39 ms, flip angle = 90°, field of view = 240×240 mm, matrix = 64×64 cm, in-plane resolution = 3.4×3.4 mm, slice thickness = 5mm, and 30 slices, full-brain coverage. Task presentation was implemented using the commercial software package E-Prime (Psychology Software Tools, Inc., Pittsburgh PA). The tasks described here were collected in a single session lasting one hour, including two functional tasks and structural imaging.
High-resolution T1-weighted structural MRI scans were acquired using a multiecho MPRAGE pulse sequence (repetition time = 2530 ms, echo times = 1.64 ms, 3.50 ms, 5.36 ms, 7.22 ms, inversion time = 1100 ms, flip angle = 7°, slice thickness = 1.3 mm, matrix size = 256 × 256) yielding 128 sagittal slices with an in-plane resolution of 1.0 mm × 1.0 mm.
Task Design
Participants were presented with sixty-four video stimuli of actors expressing happiness, sadness, fear, and pain. These clips were created in the Dr. Decety Lab and validated with a group of 40 healthy volunteers. Clips were 2.2-seconds in duration and were interspersed with 32 instances of a dynamically scrambled baseline stimulus designed to control for visual motion, color, brightness, and basic composition. Stimuli were presented in a pseudo-randomized order and timing parameters were generated using Optimize Design (Wager and Nichols, 2003). After eight of the clips, the participant was asked whether the previous clip had featured a male or a female subject. Data in this task were acquired in one eight-minute run. Participants’ gaze in the scanner was monitored via an infrared camera to ensure that they paid attention to the stimuli.
Image processing and analysis
The functional images were processed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) in Matlab (Mathworks Inc., Sherborn, MA, USA). For each participant, functional data were realigned to the first image acquisition of the series and re-sampled to a voxel size of 2×2×2 mm3. Structural T1 images were co-registered to the mean functional image and segmented using the ‘New Segment’ routine. A group-level structural template and individual flow fields were created using DARTEL, and the flow fields were in turn used to spatially normalize functional images to standard MNI space. Data were smoothed with an 8 mm full-width at half maximum (FWHM) isotropic Gaussian kernel. Ten participants were eliminated from further analysis due to image quality issues related to movement, leaving a total of 70 (n=22, 24, 24 for low, intermediate, and high psychopathy, respectively).
Statistics were calculated at the first level using the general linear model. The design matrix included three regressors for each stimulus category (detailed above), representing the event onsets and their time and dispersion derivatives. Movement parameters from the realignment output were included as regressors of no interest. All participants were entered into two second-level pooled analyses (one for the Pain Interactions task and one for the Pain expressions task), and full brain results were reported at a statistical cutoff of FWE-corrected p<0.05.
Second level analyses were conducted by comparing the extremes of the sample distribution of PCL-R scores, and then using PCL-R scores as a continuous regressor over the entire sample. Participants with PCL-R total score at or above 30 were selected for the psychopathy group, while participants scoring at 20 or below comprised the incarcerated control group. Regions of interest (ROIs) were created from the existing literature. Coordinates were taken from studies that reported functional neuroimaging results for the perception of facial expressions of pain (Botvinick et al., 2005; Budell et al., 2010; Decety et al., 2009; Lamm et al., 2007; Saarela et al., 2006; Simon et al., 2006). ROI data are reported for significant contrast image peaks within 10mm of these a priori coordinates. Beyond existing literature on the processing of empathy-inducing stimuli in healthy populations, there may be additional cortical or subcortical brain regions that contribute to abnormal processing of these regions in psychopathy. For this reason, additional regions of note that survive statistical cutoff of p<0.001 uncorrected and a spatial extent threshold of k=100 voxels are also reported in the groupwise analysis.
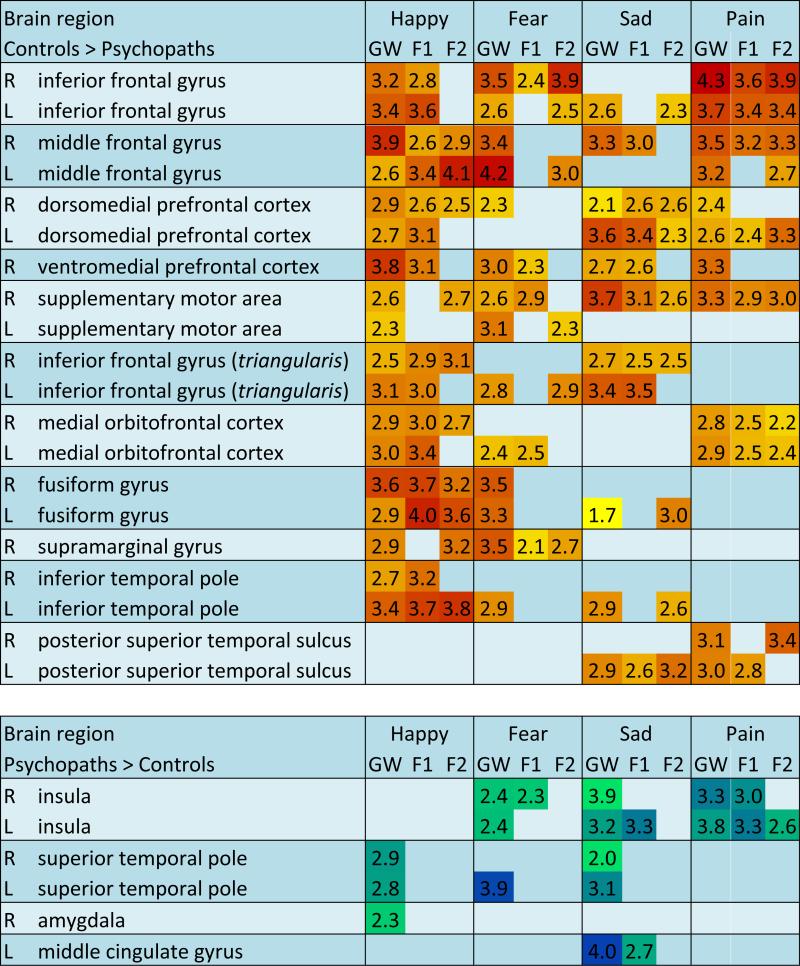
To investigate the effect of PCL-R scores, factor 1 and factor 2 scores were entered as covariates into the groupwise analysis. Peak activations predicted by PCL-R scores were then extracted from regions reported above using a sphere with radius 8mm (see Table 1).
Table 1. Emotional expressions and PCL-R scores.
Summary and comparison of all analyses in control participants and psychopaths scoring >30 on the PCL-R. Color scales are from yellow to red for increasingly negative T values and green to blue for increasingly positive T values for the appropriate contrast (GW, groupwise comparison; F1, Factor 1 score as a covariate; F2, Factor 2 score as a covariate) in each region of interest. ROI clusters must have reached significance of T>2.0 to be included in the table. L, left hemisphere; R, right hemisphere.
|
RESULTS
Happy Expressions
Pooled group analysis
In all participants (n=70), the contrast of happy facial expressions versus the dynamic baseline revealed clusters of activity surviving a statistical threshold of p<0.05, corrected for multiple comparisons using family wise error (FWE), bilaterally in the fusiform gyrus, middle and inferior occipital gyri, right pSTS, and right middle temporal gyrus. At a relaxed cutoff of p<0.0001 with a spatial extent threshold of k=50 voxels, additional activations were seen in IFG, SMA, right supramarginal gyrus, and bilateral amygdala (full results, Supplementary Table 1).
Groupwise contrasts
In direct comparison between groups, in response to happy facial expressions, control participants had greater activation than the high-scoring psychopaths bilaterally in the fusiform gyrus, IFG, vmPFC, dmPFC, inferior temporal pole, middle frontal gyrus, and SMA. The high-scoring psychopathy group had greater activation than controls in right amygdala and bilateral superior temporal pole.
Correlations with PCL-R Factor scores
Many of the clusters that displayed group differences in BOLD response to dynamic happy facial expressions also were significantly correlated with one or both PCL-R Factor scores, entered as a continuous variable for all seventy participants. Regions that were negatively correlated with both Factor scores included bilateral fusiform gyrus, right IFG, right OFC, right dmPFC, left inferior temporal pole, and bilateral middle frontal gyrus. Several other clusters were significantly correlated with only Factor 1 scores. These included right middle occipital gyrus, bilateral IFG, right vmPFC, left OFC, and right inferior temporal pole. Two regions were correlated only with the Factor 2 scores. These were the right supramarginal gyrus and the right SMA. None of the clusters found to be significantly more active in high-scoring psychopaths than in the low-scoring control group were significantly correlated with either Factor scores when the middle-scoring participants were added and the Factor scores were treated as a continuous variable.
Fear Expressions
Pooled group analysis
In the pooled sample of all participants, the contrast of expressions of fear versus the dynamic baseline revealed clusters of activity surviving a statistical threshold of FWE- p<0.05 bilaterally in the fusiform gyrus, middle and inferior occipital gyri, and pSTS. At a relaxed cutoff of p<0.0001 with a spatial extent threshold of k=50 voxels, additional activations were detected in bilateral IFG, amygdala, left SMA, right supramarginal gyrus, and ventral striatum (Full results, Supplementary Table 2).
Groupwise contrasts
In direct comparison between groups, control participants had greater activation in bilateral fusiform gyrus, middle occipital gyrus, insula, IFG, vmPFC, SMA, right amygdala, and middle frontal gyrus. Psychopaths exhibited greater activation than low-scoring controls in aINS and left superior temporal pole.
Correlations with PCL-R Factor scores
Clusters of interest from the contrast of fear expressions versus dynamic baseline were tested for correlations with Factor 1 and Factor 2 subscores of the PCL-R across the entire sample of seventy participants. Regions that were significantly negatively correlated with both Factor 1 and Factor 2 scores were bilateral middle occipital gyrus, right IFG, and right supramarginal gyrus.
Regions with significant negative correlation with Factor 1 scores were the left insula, right vmPFC and OFC, and right SMA. Other regions had significant negative correlation only with Factor 2, including right insula, left inferior frontal gyrus, left middle frontal gyrus, and left SMA. Finally, several clusters were significantly less active in the high-scoring psychopaths (PCL-R≥30) than the low-scoring (PCL-R≤20) participants, but were not negatively correlated with either factor score in the entire continuous sample of seventy participants. These were bilateral fusiform gyrus, dmPFC, inferior temporal pole, and right middle frontal gyrus. Clusters from the reverse contrast, in which activation was greater in the high-scoring psychopaths than in the low-scoring incarcerated controls, were not significantly correlated with either Factor score, with the exception of the right insular cluster which had significant positive correlation with Factor 1 scores only.
Sad Expressions
Pooled group analysis
In the pooled sample of all participants, viewing facial expressions of sadness versus the dynamic baseline revealed clusters of activity surviving a statistical threshold of FWE- p<0.05 bilaterally in the fusiform gyrus, middle occipital gyrus, and right pSTS. At a relaxed cutoff of p<0.0001 with a spatial extent threshold of k=50 voxels, additional activations were detected bilaterally in the IFG, amygdala, and temporal poles (Supplementary Table 3).
Groupwise contrasts
In a direct comparison between groups, control participants showed greater activation than the high-scoring psychopaths in left fusiform gyrus, pSTS, bilateral IFG and amygdala, vmPFC, dmPFC, SMA, middle fontal gyrus, and SMA. The psychopath group had greater activation than controls bilaterally in the aINS, middle cingulate gyrus, and bilateral superior temporal pole.
Correlations with PCL-R Factor scores
Clusters of significantly different BOLD activation between the high- and low-scoring groups when perceiving sad expressions versus dynamic baseline were tested for significant correlation with Factor 1 and Factor 2 scores across the entire seventy-participant sample. Several of the clusters that were found to be significantly more active in the low-scoring control group were also negatively correlated with both Factor 1 and Factor 2 scores. These clusters were located in the left pSTS, right IFG, bilateral dmPFC, and right SMA. Clusters that were negatively correlated only with Factor 1 were in the left IFG and right middle frontal gyrus. The left fusiform gyrus, another left IFG cluster, and the left inferior temporal pole were negatively correlated with Factor 2 scores only. Clusters from the reverse contrast, which were more active in the high-psychopath group than in low-scoring controls, were not significantly correlated with PCL-R Factor scores with the exception of two clusters in the left aINS and left middle cingulate gyrus, which were positively correlated with Factor 1 scores only.
Pain Expressions
Pooled group analysis
In response to dynamic expressions of pain, participants showed robust hemodynamic activation in the face network of expected cortical and brain regions during the perception of facial expression of pain (Lamm, Decety & Singer, 2011 for a meta-analysis). At the whole-group level, clusters (FWE-corrected p<0.05) were detected bilaterally in the fusiform gyrus, occipital gyri, temporal gyri, IFG, and pSTS. At a relaxed cutoff of p<0.0001 with a spatial extent threshold of k=50 voxels, additional activations were seen bilaterally in the aINS, SMA, and temporal poles (Supplementary Table 4).
Groupwise contrasts
In direct comparison between groups, control participants had greater activation bilaterally in the IFG, midcingulate cortex, angular gyrus, putamen, pSTS, supramarginal gyrus, dmPFC, and dorsal ACC. At a relaxed whole-brain cutoff of p<0.001, uncorrected, additional clusters of greater activation in the control group were observed in vmPFC and medial OFC. Psychopaths exhibited greater activation in the aINS, postcentral gyrus, inferior parietal lobule, precentral gyrus and right amygdala.
Correlations with PCL-R Factor scores
A number of clusters found to be significantly more active in the control group, including the middle cingulate cortex, IFG, dmPFC, and left angular gyrus, were negatively correlated with both Factor 1 and 2 scores. The right angular gyrus and left pSTS were correlated with Factor 1 only, and right STS, dorsal ACC, and striatum were correlated with scores on Factor 2. In the reverse direction, the response in the aINS was positively correlated with both scores on Factor 1 and 2. Two clusters from left postcentral gyrus and right precentral gyrus were correlated with Factor 1 scores only.
DISCUSSION
Emotion expression and recognition play critical roles in social interactions and interpersonal relationships. Understanding the neural underpinnings of emotion recognition in individuals with psychopathy is critical because of the centrality of abnormal affective responses to the clinical disorder. In addition, individuals affected by psychopathy provide a natural experiment in which emotional processes are altered, enabling identification of downstream effects (Marsh, 2013). One theory proposes that specific deficits in experiencing fear and sadness contribute to the development of psychopathy (Blair, 2006). Another account conjectures that psychopathy is associated with abnormal attention to socially relevant cues, and that dysfunction in attentional mechanisms causes emotion recognition deficits (Dadds et al., 2006). The response modulation hypothesis holds that individuals with psychopathy are capable of normal emotional responses but have difficulty processing affective information when it is peripheral to their primary attentional focus (Newman & Lorenz, 2003). Neuroimaging studies have produced mixed findings in support of either theory, often due to limited sample size. Therefore whether emotion perception deficits in psychopathy are restricted to fear or whether they are more pervasive remains an important empirical and theoretical question.
In the current study, individuals scoring high on psychopathy had consistently less activation than controls in relevant brain regions during the viewing of dynamic video clips of happy, sad, fearful, and pain expressions. All four facial expressions evoked greater activation in controls than in psychopaths in the face processing network including the fusiform gyrus as well as the extended network, particularly the inferior frontal gyrus, orbitofrontal cortex and ventromedial prefrontal cortex. As such, the data do not support a fear-specific deficit, as has been hypothesized elsewhere (Marsh and Blair, 2008); rather they are consistent with a pervasive deficit across emotion (Dawel et al., 2012).
In healthy participants, face viewing is known to elicit robust activity in the fusiform gyrus, occipital cortex and pSTS, known as the core face processing network, as well as several supporting regions including the IFG, amygdala, OFC, and aINS (Haxby et al., 2000; Skelly and Decety, 2012; Trautmann et al., 2009). Nodes of the core network were selectively less active in the psychopath group: the fusiform gyrus was less responsive to happy and fearful faces, and the pSTS, thought to process the expressive or changeable components of the face, was less active in the left hemisphere to sad faces and less active bilaterally to expressions of pain. Middle and inferior occipital participation in face processing was robust and did not differ between groups.
Regions that belong to the extended face processing network had variable differences between groups and among expression categories. Greater neuro-hemodynamic activity in the OFC was elicited in controls compared to psychopaths in response to all expressions, and the vmPFC was more active in controls to all four emotions. These sub-regions of the prefrontal cortex, reciprocally connected to the amygdala and insula, are involved in sensory integration and represent the affective value of reinforcers. These regions play an important role in facial emotion recognition (Heberlein et al., 2008; Rempel-Clower, 2007), empathic concern and moral sensitivity (Decety, Michalska, & Kinzler, 2012). Extensive work in psychopathy has documented abnormal processing (and atypical effective connectivity) of affective information in both the OFC and vmPFC (Blair, 2007; Decety et al., 2013a,b; Kiehl, 2006; Sobhani & Bechara, 2011).
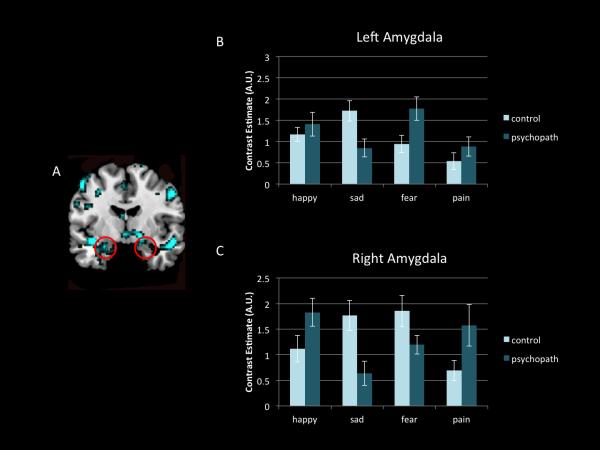
Of note, the response in the amygdala was statistically equivalent between groups for all categories of emotions except for happy faces (Figure 1). No significant differences were detected in the left amygdala to any expression set, or in the right amygdala in response to fear, sadness, or pain (although response in the right amygdala was reduced for sad and fearful faces in psychopaths, without reaching significance). Both high-scoring psychopaths and low-scoring controls exhibited significant amygdala activation to most expression categories, and (with the exception of the right amygdala in response to happy and sad faces) that activity did not differ between groups. This somewhat surprising result may be due to the use of dynamic displays of emotive expressions. Though it is well-established that the human amygdala is engaged in the perception of facial expressions of emotion (Zald, 2003), the great majority of these studies utilized static images of faces (Trautmann et al., 2009). To wit, one neuroimaging study demonstrated no significant differences between emotion categories when facial stimuli were dynamic rather than static (Van der Gaag et al., 2007). The fact that the amygdala was strongly recruited in all participants including psychopaths may at least be seen as evidence of focused attention on the dynamic stimuli during scanning (Williams et al., 2005). These findings also provide some support for the response modulation hypothesis which suggests that individuals with psychopathy may up-regulate emotional processing when attention to salient stimuli is particularly engaged (Dadds et al., 2006; Newman and Lorenz, 2003). Another possibility is that rather than representing the level of emotion expressed, the amygdala may act to direct processing resources toward the most salient elements of a stimulus in order to resolve ambiguity (Adolphs, 2010). Recently, Cunningham and Brosch (2012) proposed that amygdala processing reflects an early stimulus evaluation check that determines the relevance of a stimulus with respect to the observer's ongoing motivational state. Activation of the amygdala may be a contextualized aspect of affective responding, however the response outcome varies greatly across individuals. It is worth mentioning that psychopathic traits are not exclusively associated with amygdala hyporeactivity. A study that included a large number of participants (N = 200) with a self-report psychopathy scale found that amygdala reactivity to fearful facial expressions was negatively associated with the interpersonal facet of psychopathy, whereas reactivity to angry expressions was positively associated with the lifestyle facet (Carré et al., 2013). Thus the role of the amygdala in emotion perception research with psychopaths seems more complex than just a broad dysfunction, and subdividing this region into sub-nuclei would facilitate the clarification of apparently inconsistent findings. A more anatomically specific segmentation of the amygdala into basolateral and central nuclei is warranted in future studies with psychopaths. Both empirical research and theory point to different, even antagonistic, functions of these subregions (Bos et al., 2013; Moul, Killcross, and Dadds, 2012).
Figure 1.
Amygdala response for each facial expression does not show significant difference between psychopaths and controls. (A) All emotional expressions for all participants greater than baseline thresholded for viewing purposes at p<0.001, with spatial threshold of k>50 voxels (red circles indicate amygdala). (B) Mean contrast estimates in the left amygdala for each emotional expression type for controls participants (individuals who scored <20 on the PCL-R) and for psychopaths (individuals who scored >30 on the PCL-R) (C) Mean contrast estimates in the right amygdala for each emotional expression type for each group.
Of all regions reported here, the inferior frontal gyrus had the most consistent and robust group differences during the perception of emotion, showing significantly less activity in the psychopath group in all four expressions. Although the IFG was not emphasized in the early foundational conceptualization of the face processing network (Haxby et al., 2000; 2002), it was later associated with the semantic aspects of facial processing (Ishai et al., 2000). Recent investigations using natural dynamic emotional expressions, as used here, have noted robust and persistent activation of this region during emotion perception and extensive interconnectivity between the IFG and other nodes of the face processing network (Foley et al. 2012). During perception of facial expressions of pain, this region is found to code for both the presence and the amount of pain in facial expressions (Budell et al., 2010; Saarela et al., 2006). Interestingly, the participation of the IFG in pain perception processing as well as gray matter volume in that region, have been linked with individual differences in trait empathy, although it is not clear whether they are associated with cognitive or emotional empathy (Banissy et al., 2012; Hooker et al., 2010), or perhaps with a more general attentional function of that region that relates to top-down control of cognitive and emotional processing (Tops and Boksem, 2011). Activity in the IFG during the perception of expressions of basic emotions was found to be correlated with participant scores on the empathy quotient (EQ) scale (Chakrabarti et al., 2006) and connectivity between the IFG and aINS during observation of facial expressions of pain was correlated with subjects’ self-reported ratings of trait empathy (Saarela et al., 2006). The consistent observation of reduced IFG activity in our sample of emotionally-impaired individuals supports the existing literature and highlights the IFG as a region of interest for future investigations of psychopathy and more generally the role of that region in emotion processing.
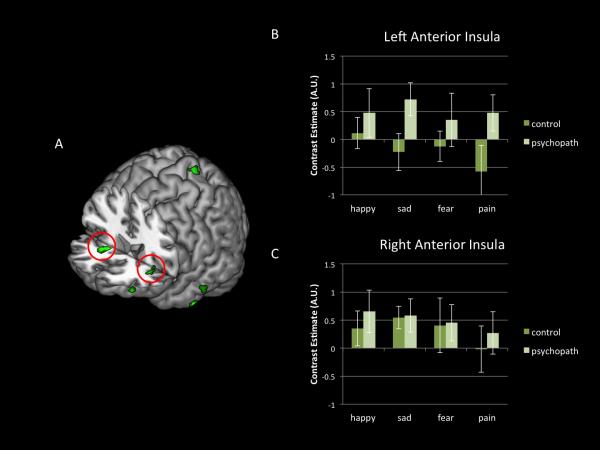
Another surprising and unexpected result was the response of the aINS in participants scoring high on psychopathy when perceiving facial expressions of pain, fear, and sadness (Figure 2). This response was positively correlated with scores on Factors 1 and 2 of the PCL-R. The aINS is the most consistently activated region across all studies of empathy for pain (Bird et al., 2010; Lamm, Decety and Singer, 2011), even when there is no explicit cognitive demand to empathize with another individual (Valentini, 2010). Moreover, gray matter reduction has been observed in the insula in individuals scoring high on psychopathy tests (de Oliveira-Souza et al., 2008). Thus, finding a heightened response in individuals who are characterized as lacking empathy may sound surprising. However, this result replicates two recent fMRI studies in psychopaths, which have also reported greater activation in the aINS in individuals scoring high on the PCL-R when they were presented with stimuli depicting facial expressions of pain and bodily injuries (Decety et al., 2013a) and when they imagined themselves to be in pain (Decety et al., 2013b). The anterior insula is a polysensory region with extensive reciprocal connections to limbic forebrain area, which is considered as the integral hub of the salience network, which assists target brain regions in the generation of appropriate behavioral responses to salient stimuli (Harsay et al., 2012; Menon and Uddin, 2010). As with the amygdala findings reported above, the observed increased activity in the aINS in participants scoring high on psychopathy supports the response modulation hypothesis (Newman and Lorenz, 2003), and further suggests that in some circumstances the engagement of emotional processing in psychopathy may extend beyond fear to all negative emotions. Whether this response in the insula reflects a non-specific increase in arousal or a specific negative affective reaction that does not reach emotional awareness (perhaps due to the lack of co-activation of the orbitofrontal cortex) remains open to interpretation or speculation. A careful inspection of the clusters in the anterior insula indicates that the dorsal region was activated. The dorsal anterior insula is functionally connected to a set of regions previously described as a cognitive control network (Dosenbach et al., 2007). Subdividing the anterior insula into dorsal and ventral regions, which have distinct patterns of functional connectivity (Deen et al., 2011) is necessary to better understand the role of this region in emotional processing and psychopathy. The ventral subdivision appears more important for reactive processing (monitoring for the need to undertake remedial action), homeostatic regulation, and hedonic valence, whereas the dorsal aspect of the aINS seems to be involved in attentional and executive mechanisms for prospective control (Ullsperger et al., 2010). Of particular note, further investigations on the possible dissociation between dorsal and ventral functions in individuals who vary in psychopathic traits, particularly on Factor 1 of the PCL-R, are necessary.
Figure 2.
The response in the dorsal insula is greater in psychopaths. (A) Greater activity in psychopaths as compared to controls for pain expressions (red circles indicate anterior dorsal insular cortex) (B) Mean contrast estimates in the left anterior insula for each emotional expression for control participants (individuals who scored <20 on the PCL-R) and for psychopaths (individuals who scored >30 on the PCL-R) (C) Mean contrast estimates in the right anterior insula for each emotional expression for each group.
CONCLUSIONS
Overall these functional MRI data do not support a specific dysfunction in psychopathy in the perception of facial expression of fear. Rather, they are consistent with the view that emotion perception deficits in psychopathy are pervasive across emotions (Dadds et al., 2006; Dawel et al., 2012). Importantly, the reduction in activation in the orbitofrontal cortex and ventromedial prefrontal cortex during emotion processing is in agreement with the affective neuroscience literature on psychopathy. These regions, critical for monitoring ongoing behavior, estimating consequences, and incorporating emotional learning into decision making, have consistently been featured in theories of psychopathy and remain the most common prefrontal regions implicated in neuroimaging and effective connectivity investigations of this clinical disorder (Decety et al., 2013b; Motzkin, Newman, Kiehl and Koenigs, 2011). Increasing attention to the role of different regions of the IFG and the aINS in cognition and emotion could have important implications for research on psychopathy. Similarly, segmenting the amygdala into sub-nuclei (which was not done here) will illuminate the psychobiological mechanisms underlying psychopathy. It is important to note that our study, given the task (passive viewing), cannot contribute to disentangling the extent to which the neural processing associated with emotion experience overlaps with the processing related to experiencing emotion. Current neuroimaging evidence is inconclusive as to whether they rely on similar or distinct computational and physiological processes (Blair, 2011; Decety, 2011; Cheng et al., 2012). Meta-analyses of studies involving the generation of an emotional experience and studies involving the perception of emotion have shown a striking dissociation between the two modes especially in the IFG (Wager et al., 2008). This aspect—what neural substrates are shared and what are not shared between perceiving and experiencing emotion—is critical for a better understanding of affective processing in psychopathy and for advancing theories of emotion.
LIMITATIONS
There are several limitations to this research that need to be acknowledged. Because the design of our study relied on passive viewing of dynamic visual stimuli, it is difficult to know how motivated the participants were while doing the task in the scanner. Manipulation of motivation to attend to emotionally expressive faces elicits selective changes both in the magnitude of responses in certain nodes of the face-processing network and in the task-related functional connectivity among them (Skelly and Decety, 2012; Lelieveld et al., 2013). Finally the exclusive use of incarcerated male offenders as participants may limit the generalizability of our findings. Future research is needed to replicate these findings in female participants.
Supplementary Material
Acknowledgements
This study was supported by NIMH R01 grant 1R01MH087525-01A2 (J. Decety, PI) and by NIMH R01 grant MH070539-01 (K. Kiehl, PI).
References
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Bandura A, Rosenthal T. Vicarious classical conditioning as a function of arousal level. Journal of Personality and Social Psychology. 1966;3:54–62. doi: 10.1037/h0022639. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Kanai R, Walsh V, Rees G. Inter-individual differences in empathy are reflected in human brain structure. Neuroimage. 2012;62:2034–2039. doi: 10.1016/j.neuroimage.2012.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory—II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–1525. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The emergence of psychopathy for the neuropsychological approach to developmental disorders. Cognition. 2006;101:414–442. doi: 10.1016/j.cognition.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Science. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Should affective arousal be grounded in perception-action coupling? Emotion Review. 2011;3:109–110. [Google Scholar]
- Blair RJR, Mitchell D, Blair K. The Psychopath: Emotion and the Brain. Blackwell Publishing Ltd; London: 2005. [Google Scholar]
- Bos PA, van Honk J, Ramsey NF, Stein DJ, Hermans EJ. Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology. 2013;38:808–817. doi: 10.1016/j.psyneuen.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brook M, Kosson DS. Impaired cognitive empathy in criminal psychopathy: Evidence from a laboratory measure of empathic accuracy. Journal of Abnormal Psychology. 2013;1:156–166. doi: 10.1037/a0030261. [DOI] [PubMed] [Google Scholar]
- Budell L, Jackson PL, Rainville P. Brain responses to facial expression of pain: emotional or motor mirroring? Neuroimage. 2010;53:355–363. doi: 10.1016/j.neuroimage.2010.05.037. [DOI] [PubMed] [Google Scholar]
- Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Social Neuroscience. 2013;8:122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Social Neuroscience. 2006;1(3-4):364–84. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hung A, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Development and Psychopathology. 2012;24:623–636. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience: amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21:54–59. [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, Solak E, Abeygunawardane AI. Attention to the eyes and fear-recognition deficits in child psychopathy. British Journal of Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Davidov M, Zahn-Waxler C, Roth-Hanania R, Knafo A. Concerns for others in the first year of life: Theory, evidence, and avenues for research. Child Development Perspectives. 2013;7:126–131. [Google Scholar]
- Dawel A, O'Kearney R, McKone E, Palermo R. Not just fear and sadness: Meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neuroscience and Biobehavioral Reviews. 2012;36:2288–2304. doi: 10.1016/j.neubiorev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio A, Tovar-Moll F, Moll J. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic gray matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Decety Dissecting the neural mechanisms mediating empathy. Emotion Review. 2011;3:92–108. [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Howard LH. The role of affect in the neurodevelopment of morality. Child Development Perspectives. 2013;7:49–54. [Google Scholar]
- Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010;13:886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey B. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cerebral Cortex. 2012;22:209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- Decety J, Skelly LR, Kiehl KA. Brain response to empathy-eliciting scenarios in incarcerated individuals with psychopathy. JAMA Psychiatry. 2013a;70:638–645. doi: 10.1001/jamapsychiatry.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski CL, Kiehl KA. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Frontiers in Human Neuroscience. 2013b;7:489. doi: 10.3389/fnhum.2013.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2011;21:1498–506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychological Medicine. 2006;36:1563–1569. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME. Distinct brain networks for adaptive and stable task control in humans. Proceedings National Academy of Science USA. 2007;(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Foley E, Rippon G, Thai NJ, Longe O, Senior C. Dynamic facial expressions evoke distinct activation in the face perception network: a connectivity analysis study. Journal of Cognitive Neuroscience. 2012;24:507–520. doi: 10.1162/jocn_a_00120. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Glass SJ, Newman JP. Recognition of facial affect in psychopathic offenders. Journal of Abnormal Psychology. 2006;115:815–820. doi: 10.1037/0021-843X.115.4.815. [DOI] [PubMed] [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RW, Mitchell DG. Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Cognition and Affective Science. 2012;7:958–68. doi: 10.1093/scan/nsr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist: Revised. Multi-Health Systems; New York: 2003. [Google Scholar]
- Harsay HA, Spaan M, Wijnen JG, Ridderinkhof KR. Error awareness and salience processing in the oddball task: shared neural mechanisms. Frontiers in Human Neuroscience. 2012;6:246. doi: 10.3389/fnhum.2012.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings ME, Tangney JP, Stuewig J. Psychopathy and identification of facial expressions of emotion. Personality and Individual Differences. 2008;44:1474–1483. doi: 10.1016/j.paid.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Science. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. Journal of Cognitive Neuroscience. 2008;20:721–733. doi: 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. Developmental synthesis of affect and cognition and its implications for altruistic motivation. Developmental Psychology. 1975;11:607–22. [Google Scholar]
- Hollingshead AB, Redlich FC. Social class in mental illness. Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Research. 2010;1308:110–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kamachi M, Bruce V, Mukaida S, Gyoba J, Yoshikawa S, Akamatsu S. Dynamic properties influence the perception of facial expressions. Perception. 2001;30:875–887. doi: 10.1068/p3131. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4301–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigation the neural correlates of psychopathy: a critical review. Molecular Psychiatry. 2010;7:1–8. doi: 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- LaPierre D, Braun CMJ, Hodgins S. Ventral frontal deficits in psychopathy: neuropsychological test findings. Neuropsychologia. 1995;33:139–151. doi: 10.1016/0028-3932(94)00110-b. [DOI] [PubMed] [Google Scholar]
- Lelieveld G-J, van Dijk E, Guroglu B, van Beest I, van Kleef GA, Rombouts SARB, Crone EA. Behavioral and neural reactions to emotions of others in the distribution of resources. Social Neuroscience. 2013;1-2:52–62. doi: 10.1080/17470919.2012.735621. [DOI] [PubMed] [Google Scholar]
- Marsh AA. What can we learn about emotion by studying psychopathy? Frontiers in Human Neuroscience. 2013;7:181. doi: 10.3389/fnhum.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair RJR. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2008;32(3):454–65. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul C, Killcross S, Dadds MR. A model of differential amygdala activation in psychopathy. Psychological Review. 2012;119:789–806. doi: 10.1037/a0029342. [DOI] [PubMed] [Google Scholar]
- Newman JP, Lorenz AR. Response modulation and emotion processing: implications for psychopathy and other dysregulatory psychopathology. In: Davidson RJ, editor. Handbook of Affective Sciences. Oxford University Press; New York: 2003. pp. 904–929. [Google Scholar]
- Perry DG, Perry LC. Denial of suffering in the victim as a stimulus to violence in aggressive boys. Child Development. 1974;45:55–62. [PubMed] [Google Scholar]
- Pham TH, Philippot P. Decoding of facial expression of emotion in criminal psychopaths. Journal of Personality Disorders. 2010;24:445–459. doi: 10.1521/pedi.2010.24.4.445. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL. Role of orbitofrontal cortex connections in emotion. Annals of the New York Academy of Sciences. 2007;1121:72–86. doi: 10.1196/annals.1401.026. [DOI] [PubMed] [Google Scholar]
- Saarela MV, Hluschuk Y, Williams AC, Schurmann M, Lalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cerebral Cortex. 2006;17:230–237. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Simon D, Craig KD, Miltner WH, Rainville P. Brain responses to dynamic facial expressions of pain. Pain. 2006;126:309–318. doi: 10.1016/j.pain.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Singer T, Decety J. The Social neuroscience of empathy. In: Decety J, Cacioppo JT, editors. The Oxford Handbook of Social Neuroscience. Oxford University Press; New York: 2011. pp. 551–564. [Google Scholar]
- Skelly LR, Decety J. Passive and motivated perception of emotional faces: Qualitative and quantitative changes in the amygdala and the face processing network. PLoS One. 2012;7(6):e40371. doi: 10.1371/journal.pone.0040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani M, Bechara A. A somatic marker perspective of immoral and corrupt behavior. Social Neuroscience. 2011;6:640–652. doi: 10.1080/17470919.2011.605592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-trait anger expression inventory-2: Professional manual. Psychological Assessments Resources, Inc.; Lutz, Florida: 2002. [Google Scholar]
- Tops M, Boksem MAS. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Frontiers in Psychology. 2011;2:330. doi: 10.3389/fpsyg.2011.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann SA, Fehr T, Hermann M. Emotions in motion: dynamic compared to static facial expressions of disgust and happiness revel more widespread emotion-specific activations. Brain Research. 2009;1284:100–115. doi: 10.1016/j.brainres.2009.05.075. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel J, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Structure and Function. 2010;204:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Gaag C, Minderaa RB, Keysers C. Facial expressions: What the mirror neuron system can and cannot tell us. Social Neuroscience. 2007;2:179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Schutter DJLG. Unmasking feigned sanity: a neurobiological model of emotion processing in primary psychopathy. Cognitive Neuropsychiatry. 2006;11:285–306. doi: 10.1080/13546800500233728. [DOI] [PubMed] [Google Scholar]
- von Borries AK, Volman I, de Bruijn ER, Bulten BH, Verkes RJ, Roelofs K. Psychopaths lack the automatic avoidance of social threat: relation to instrumental aggression. Psychiatry Research. 2012;200:761–6. doi: 10.1016/j.psychres.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Valentini E. The role of anterior insula and anterior cingulate in empathy for pain. Journal of Neurophysiology. 2010;104:584–585. doi: 10.1152/jn.00487.2010. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, Lindquist KA, Duncan S, Kober H, Joseph J, Davidson M, Mize J. Handbook of Emotions. 3rd edition Guilford Press; New York: 2008. The neuroimaging of emotion. pp. 249–267. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition-UK: Administration and scoring manual. Psychological Corp.; London: 2000. [Google Scholar]
- Wehrle T, Kaiser S, Schmidt S, Scherer KR. Studying the dynamics of emotional expression using synthesized facial muscle movements. Journal of Personality and Social Psychology. 2000;78:105–119. doi: 10.1037//0022-3514.78.1.105. [DOI] [PubMed] [Google Scholar]
- Williams MA, McGlone F, Abbott DF, Mattingley JB. Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 2005;24:417–425. doi: 10.1016/j.neuroimage.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Review. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhen Z, Fang H, Liu J. The hierarchical brain network for face recognition. PLoS One. 2013;8(3):e59886. doi: 10.1371/journal.pone.0059886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.