Abstract
A growing number of reports indicate that anti-inflammatory actions of fish oil (FO) are beneficial against systemic lupus erythematosus (SLE). However, the majority of pre-clinical studies were performed using 5–20% FO, which is higher than the clinically relevant dose for lupus patients. The present study was performed in order to determine the effective low dose of FDA-approved concentrated FO (Lovaza®) compared to the commonly used FO-18/12 (18-Eicosapentaenoic acid [EPA]/12-Docosahexaenoic acid [DHA]). We examined the dose-dependent response of Lovaza® (1% and 4%) on an SLE mouse strain (NZB×NZW)F1 and compared the same with 1% and 4% placebo, as well as 4% FO-18/12, maintaining standard chow as the control. Results show for the first time that 1% Lovaza® extends maximal lifespan (517 d) and 4% Lovaza® significantly extends both the median (502 d) and maximal (600 d) life span of (NZB×NZW)F1 mice. In contrast, FO-18/12 extends only median lifespan (410 d) compared to standard chow diet (301 d). Additionally, 4% Lovaza® significantly decreased anti-dsDNA antibodies, reduced glomerulonephritis and attenuated lipopolysaccharide-induced pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) in splenocytes compared to placebo. 4% Lovaza® was also shown to reduce the expression of inflammatory cytokines, including IL-1β, IL-6 and TNF-α, while increasing renal anti-oxidant enzymes in comparison to placebo. Notably, NFκB activation and p65 nuclear translocation were lowered by 4% Lovaza® compared to placebo. These data indicate that 1% Lovaza® is beneficial, but 4% Lovaza® is more effective in suppressing glomerulonephritis and extending life span of SLE-prone short-lived mice, possibly via reducing inflammation signaling and modulating oxidative stress.
Keywords: Fish oil, inflammation, kidney disease, lifespan, lupus, survival
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune autoimmune disease characterized by diverse clinical manifestations including skin rashes, joint pain, glomerulonephritis, thrombocytopenia, hemolytic anemia, atherosclerosis, and increased risk of cardiovascular disease.1–3 Anti-dsDNA autoantibodies are the major pathogenic mediators in SLE.4 The diverse clinical manifestations in SLE appear to be associated with the production of different pathogenic autoantibodies, particularly to nuclear antigens and by an abnormal production of pro-inflammatory cytokines.5
Fat, sourced from either saturated or omega-6 polyunsaturated fatty acids (PUFA), are associated with more severe autoimmune disease and decreased life span in mice; whereas low-fat diets have been reported to retard the development of these diseases. 6,7 It is known that the type of dietary fat radically affects the onset of autoimmune disease in animal models. Alexander et al.,8 reported that 94% of lupus prone mice fed a diet containing fish oil reached 10 months of age, whereas only 35% of corn oil fed mice and no mice fed a diet with saturated fat survived that long. These results are in agreement with our own observations that omega-3 PUFA lowered the severity of autoimmune disease in mice, while both saturated and polyunsaturated (n-6) dietary lipids exacerbated the disease.7,9 Autoimmune-prone mice fed saturated fats experience more severe nephritis and glomerular pathology.10,11 Several researchers have reported that mice fed saturated fat diets produce higher levels of autoantibodies than those on low fat or high unsaturated fat diets.10,12,13 High fat diets in mice have also been linked with increases in proteinuria,6 prostaglandin (PGE2) production, cytokine levels (interleukin 6),14 and macrophage function.6 These results have led some researchers to suggest that dietary fat restriction, especially saturated fat, may be an effective therapeutic approach to alleviate murine lupus nephritis.11
Consumption of n-3 fatty acids containing fish oil suppresses inflammatory processes, suggesting its usefulness in prevention and amelioration of organ-specific and systemic autoimmune diseases.15 Fish oil contains two main bioactive components: EPA and DHA, which are responsible for its anti-inflammatory effects. A recent study showed reduced levels of EPA and omega-3 index (EPA + DHA) in RBCs of female SLE patients compared to age-matched healthy female controls. It also demonstrated a significant increase in the ratio of inflammatory arachidonic acid to the anti-inflammatory EPA,16 while consumption of EPA reversed this ratio17 in SLE patients. Furthermore, clinical studies have shown conflicting results for the efficacy of omega-3 PUFA in IgA nephropathy, which may relate to varying doses, proportions of eicosa-pentaenoic acid (EPA) and docosahexaenoic acid (DHA), duration of therapy and sample size of study population.18 Therefore, the primary objective of the current study is to establish the minimal dose of FDA-approved concentrated fish oil (Lovaza®) – containing 46.5% EPA and 37.5% DHA19 – required for suppressing renal disease and prolonging maximal lifespan in lupus-prone (NZB×NZW)F1 mice. Accordingly, we selected n-6 PUFA as the placebo control group and compared the survival of mice fed Lovaza® with the commonly used FO-18/12. Short-lived (NZB×NZW)F1 mice were used in the study due to their ability to spontaneously develop SLE, which is characterized by development of nephritis, owing to the formation of anti-dsDNA antibodies from a relatively early age.20
Materials and methods
Lovaza®, FO-18/12, placebo and control diet
Lovaza® and placebo were obtained from Glaxo SmithKline Waltham, MA. The FO-18/12 was generously provided by Ocean Nutrition, Canada. The lab chow (LC) rodent control diet was purchased from Harlan, USA (Catalog No. Harlan Teklad LM-485 Mouse/Rat Sterilizable Diet).
Animals and experimental diets
The complete study was conducted in two phases (Figure 1). In the first experimental phase, two-month-old female (NZB×NZW)F1 mice were purchased from Jackson Laboratories, Bar Harbor, ME. They were acclimatized for one week and then divided into six dietary groups. In this study, female mice were selected because the lupus progression is more pronounced in females than males. Female mice die at an average of 35 weeks of age and males at 58 weeks.21 Each group contained 20 mice and were fed semi-purified American Institute of Nutrition (AIN)-93 diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza® or 4% FO-18/12. One group was fed the standard LC diet ad libitum. In the 1% Lovaza®, 4% Lovaza® and 4% FO-18/12 diets, 1% n-6 fatty acids was added to prevent essential fatty acid deficiency. The composition of the semi-purified AIN-93 diets is provided in Table 1. Both the placebo and fish oil containing diets were supplemented with equal amounts of vitamin E to prevent peroxidative damage during storage.22 These mice were monitored for proteinuria from three months onwards and survival throughout the life span.
Figure 1.
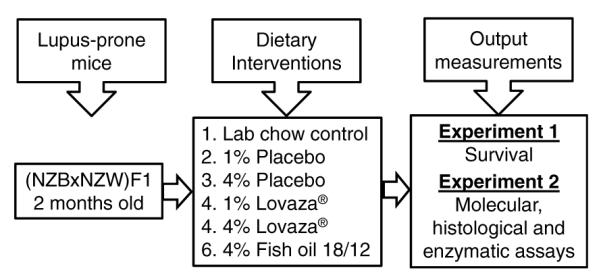
Schematic presentation of experimental design: Two-month old (NZB×NZW)F1 lupus-prone mice were fed diets containing 1%, 4% placebo, 1%, 4% Lovaza® and 4% fish oil-18/12 (FO-18/12) with standard diet (LC) as a control. In phase 1 experiment, median and maximal survival rate was monitored and measured from two months onwards in lupus-prone short-lived (NZB×NZW)F1 mice. In phase 2, a set of mice (n =6/group) were sacrificed after six months of dietary interventions for the evaluation of serum, molecular, histological and enzymatic assays as the dose-dependent and comparative output measurements of dietary interventions
Table 1.
Composition of AIN-93 semi-purified diets containing placebo, Lovaza® and fish oil-18/12
| Diet Ingredients* | 1% Placebo | 4% Placebo | 1% Lovaza® | 4% Lovaza® | 4% FO-18/12 |
|---|---|---|---|---|---|
| Casein | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 |
| Corn starch | 50.43 | 47.43 | 50.43 | 47.43 | 42.73 |
| Dextronized corn starch | 14.50 | 14.50 | 14.50 | 14.50 | 14.50 |
| Sucrose | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 |
| Cellulose | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| AIN-93 mineral mix | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 |
| AIN-93 vitamin mix | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| l-cystine | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| TBHQ | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin E | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| †Lovaza® | 0 | 0 | 1 | 4 | 0 |
| Placebo/Corn oil | 2 | 5 | 1 | 1 | 1 |
| ‡Flsh oil -18/12 | 0 | 0 | 0 | 0 | 4 |
AII diet ingredients were purchased from MP Biomedicals (Irvine, CA).
Lovaza® and placebo were obtained from Glaxo SmIthKllne (Waltham,MA).
Fish oil -18/12 (18 - Elcosapentaenolc add /12 Docosahexaenolc add) was supplied by Ocean Nutrition, Nova Scotia, Canada).
In the second phase of the study, two-month-old (NZB×NZW)F1 mice were fed the same doses, as in phase I, of placebo, Lovaza®, FO-18/12 and chow diet after acclimatization. Each group contained 15 mice. Six mice from each group were sacrificed at eight months of age and examined for liver, renal histopathology and development of anti-dsDNA antibodies levels. In these mice, the spleen was removed aseptically and processed for splenocyte culture. Fresh diets were prepared weekly and stored in daily served packages. Remaining food was discarded routinely. The animals were maintained on a 12/12 hour light/dark cycle. All animal procedures were conducted according to the ‘Guide for the Care and Use of Laboratory Animals’ (NIH Publication No. 85–23, revised 1996) and were approved by the Institutional Animal Care and Use Committees at the University of Texas Health Science Center at San Antonio, Texas.
Study protocol
Mice were examined daily for morbidity and mortality. Body weights were monitored routinely on alternate weeks and proteinuria was measured biweekly starting from three months onward. Anesthetized mice were bled from the orbital plexus for assessment of triglycerides and anti-dsDNA antibodies. Moribund animals were killed and necropsied when proteinuria measurement was 3+ and the body weight loss greater than 50%.
Analysis of lifespan
Mice in the survival groups were supplemented with, semi-purified diet containing Lovaza®, FO-18/12, placebo and LC and were allowed to live out their life. The lifespan of individual mice was determined by recording the age of spontaneous death. The survival curves were compared statistically using the log-rank test23 and the median and maximum survivals were calculated for each group. Median and maximal survivals for each experimental group were compared to the respective placebo and LC control group by performing a Student’s t-test upon log-transformed survival times. The median and maximal survivals for each group were compared to the LC and placebo control groups using a score test adapted from Wang et al.23 All comparisons were made individually among each experimental group, placebo and LC group.
Blood collection and necropsy
At the age of eight months, blood was collected from second phase experimental mice and centrifuged at 10,000 g for 5 min at 4°C. Serum was stored at −80°C until analysis of triglycerides and anti-dsDNA antibodies. The mice were sacrificed by cervical dislocation. Livers were weighed and part of the livers and kidneys were collected in 4% formalin for histological analysis. The remaining part of kidneys were immediately frozen in liquid nitrogen then stored at −80°C for enzymatic and immunoblotting assays.
Anti-dsDNA antibodies and triglycerides measurement
Serum anti-dsDNA antibodies were analysed by ELISA kits (Alpha Diagnostics International, San Antonio, TX) as previously described.7 Serum triglycerides were measured using colorimetric kits (Cayman Chemical Company, MI) following manufacturer’s protocol.
Proteinuria analysis
Proteinuria was measured biweekly using Chemstrips (Roche Diagnostic, Indianapolis, IN). In this semi-quantitative protein analysis method, a designation of trace corresponds to<30 mg/mL, 1+= 30–100 mg/dL, 2+to 100–500 mg/dL, and 3+=>500 mg/dL. This is consistent with the criteria applied in previous studies of murine lupus. Proteinuria > 100 mg/dL (≥2+) was interpreted as an evidence of significant end-stage renal disease.
Renal and liver histology
Kidneys and livers were fixed in 4% buffered formalin. These were processed separately and cut into five-micro-meter-thick sections. The transverse renal sections were stained with hematoxylin (H) and eosin (E). Severity of kidney damage was evaluated in a blinded fashion using a semiquantitative scoring system on a scale of 0–4 (0 = no apparent changes, fine mesangial staining, 1+=mild mesangial expansion, 2+=moderate mesangial expansion, 3+=severe mesangial expansion and 4+=severe mesangial expansion as in 3+ but with glomerular distortion, loss of glomerular capillary structure and obsolescence). At least 25 glomeruli per section per mouse were analysed. Livers were stained with oil red O.24 Relative vacuole area was assessed in liver section using a light microscope with a digitalized camera and a MetaVue image analysis system (Olympus America, Center Valley, PA). The area (mm2) of lipid vacuoles was measured in six different fields in each slide section by two independent blinded operators.
Splenocyte preparation and culture
Spleens were aseptically removed and placed in 5 mL of RPMI 1640 media (Gibco, Grand Island, NY) supplemented with 25 mmol/L HEPES, 2 mmol/L glutamine, 100,000 U/L penicillin and 100 mg/L streptomycin (Gibco, Grand Island, NY). Single-cell suspensions were made by teasing the spleen between frosted ends of two sterile glass slides. After a 5-min centrifugation at 100 g to separate cells from debris, the cells were washed twice in RPMI medium.
Splenic lymphocytes were isolated by layering over Histopaque (Sigma, St. Louis, MO), centrifuging at 1000g for 15 min at 22°C and then washing twice in RPMI 1640 complete medium. Cell viability was determined by trypan blue exclusion method and cells were plated in six-well plates at a density of 10 × 106 cells/well. Bacterial lipopoly-saccharide (LPS) was added at a concentration of 5 mg/mL and incubated for 24 h at 37°C in a humidified atmosphere of air/CO2 95 : 5 (mol%).25 Multi-Analyte ELISArray™ was used analysed for cytokines (SA Biosciences Inc. San Diego, CA) in supernatant.
Cytokines measurement in conditioned medium of cultured splenocytes
The cytokines assay sensitivity was approximately 1 pg/mL. In brief, each well of a flat-bottom 96-well micro titer plate was coated separately with 100 ml of purified anti-IL-1β, IL-4, IL-6, IL-12, IL-17 A, TNF-α and TGF-β1 antibodies (4 mg/mL in binding solution) overnight at 4°C. The plates were rinsed four times with washing buffer; culture medium was added, and then allowed to incubate for 2 h at room temperature. After incubation, the plates were washed four times with washing buffer, followed by the addition of biotinylated anticytokine antibodies. The plates were incubated at room temperature for 1 h and then washed four times with washing buffer. Streptavidin-ALP conjugate was added, and the plates were incubated for 30 min at room temperature. The plates were again washed four times with washing buffer, and chromogen substrate was added. The plates were then incubated at room temperature to achieve the desired maximum absorbance and were read at 450 nm in an ELISA reader (Dynex Technologies, UK).
Gene expression using quantitative real-time RT-PCR
In order to measure the gene expression, kidneys were homogenized under liquid nitrogen conditions using a Kinematica tissue pulverizer and RNA was isolated using RNeasy Mini Kit following manufacturer’s instructions (Qiagen, Valencia, CA). Total RNA concentration was assessed in a NanoDropTM 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). mRNA expression of genes encoding IL-1β, IL-6 and TNF-α was measured using real time RT-PCR. Real time RT-PCR (50 ng RNA starting material) was carried out using TaqMan® RNA-to-CT 1-step kit (Applied Biosystems, Foster City, CA) in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) using fluorescent TaqMan methodology. Real time quantitative RT-PCR was performed for each of the following genes, using ready-to-use primer and probe sets pre-developed by Applied Biosystems (TaqMan Gene Expression Assays): IL-1β (Mm00434228_mL), IL-6 (Mm00466190_mL) and TNF-α (Mm00443258_mL), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh, Mm99999915_gL) as an endogenous control. The gene levels were normalized to GAPDH gene as the housekeeping gene, and the results are reported as 2–ΔCt values.
Measurement of glutathione peroxidase (GPx), catalase, and malondialdehyde in kidneys
The kidneys were rinsed in ice-cold physiological saline and minced with scissors. Ten per cent homogenates were prepared in 0.01 M Tris-HCl buffer (pH 7.4), centrifuged at 10,000 g and the supernatant was used for measurement of glutathione peroxidase (Northwest Life Science Specialties, LLC, Vancouver, WA) and catalase (Northwest Life Science Specialties, LLC, Vancouver, WA). For the determination of malondialdehyde (Northwest Life Science Specialties, LLC, Vancouver, WA) part of kidneys was homogenized in 1.15% KCl solution. The protein content of kidneys homogenates was determined using the bicinchoninic acid (BCA) protein assay reagent (Pierce Chemical Company, Rockford, IL).
Detection of NF-κB activity and nuclear p65 in kidneys
Kidney pieces were sonicated on ice in cell lysis buffer (Active Motif, Carlsbad, CA). Soluble proteins of the super-natant were analysed by sandwich ELISA detecting phosphorylization of the NF-κB subunit p65 and p50, (TransAM® Kits NFκB p50, p52, p65 & Family Kits Active Motif, Carlsbad, CA), following manufacturer’s recommendations. A family kit was used to screen the members of the NF-κB family (p50, p52, p65, c-Rel and RelB). This kit measures the antibodies specific for the activated form of p50, p52 and p65. Further, nuclear p65 levels were quantified by immunoblotting (Cell Signaling Technology, Danvers, MA). Actin served as loading control.
Statistical analysis
Results are expressed as mean ± SEM. Data were analysed using one-way ANOVA followed by Newman–Keuls/ Kruskal–Wallis non-parametric multiple comparison post hoc test for specific differences between pairs of groups. P < 0.05 were considered significant. The survival curves were compared statistically using the log-rank test.
Results
Lovaza® extends lifespan of lupus-prone mice
Our previous results demonstrated that Menhaden FO attenuates kidney disease and moderately extends life span of (NZB×NZW)F1 mice.26 Furthermore, DHA-enriched FO significantly extends the lifespan in lupus-prone mice.7 However, all these interventions were carried out using 10% FO and are relatively difficult to translate to a clinical dose for lupus patients. This study investigates the dose-dependent effect of Lovaza® compared to placebo and FO-18/12 on lifespan and progression of kidney disease in lupus prone mice. LC, containing neither EPA nor DHA, served as control. Lovaza®, FO-18/12 and placebo diets were isocaloric. Results show that median life span of LC control animals was 301 d, and 4% FO-18/12 moderately extended median life span to 410 d. In contrast, 1% and 4% Lovaza® significantly increased median life span to 419 d and 502 d respectively compared to LC and 4% placebo (320 d) controls. 4% FO-18/12 had minimal effect on maximal life span (450 d) compared to Lovaza®, but significantly extended median life span than LC control. Likewise, the maximal life span of 1% and 4% Lovaza® fed mice was significantly increased to 517 d and 600 d respectively compared to LC (446 d) and 4% placebo (435 d) diet fed mice (Figure 2). Furthermore, the body weights data is also in agreement with the survival data. While the LC control mice did not survive till 16 months of age, a moderate increase in body weight was observed in mice fed 4% Lovaza® compared to all the other groups (Table 2). These results indicate that Lovaza® (both 1% and 4%) significantly extends both median and maximal life span compared to LC control in lupus-prone short-lived mice.
Figure 2.
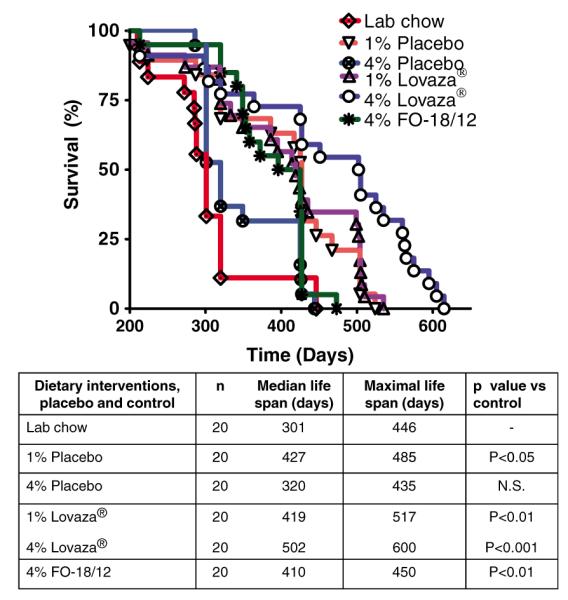
4% Lovaza® improves median and maximal lifespan of short-lived SLE-prone (NZB×NZW) F1 female mice. Two-month-old mice were fed semi-purified diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza®, 4% FO-18/12 and standard LC diet as a control and monitored for survival rate. Both doses of Lovaza® and 4% FO-18/12 significantly increases lifespan of (NZB×NZW)F1 mice compared to 1% placebo, 4% placebo and LC control. Both maximum and median survival rates were increased in 4% Lovaza® fed mice compared to 4% FO-18/12 fed mice. Values are expressed as percentage of mice in each dietary group alive. Results were analysed by Log-rank followed by Chi square test. (A color version of this figure is available in the online journal)
Table 2.
Body weights in Lovaza® and FO-18/12 fed (NZBxNZW)F1 mice
| Body weight (g) at different months of age* |
||||
|---|---|---|---|---|
| Groups (diet) | 4 months | 12 months | 16 months | 20 months |
| Lab chow | 36.2±1.0 (15) | 48.5±1.6 (2) | - | - |
| 1 % Placebo | 35.0±0.7 (15) | 43.8±1.0 (14) | 49.5±2.0 (6) | 43.7±0 (1) |
| 4% Placebo | 35.7±0.5 (15) | 45.8±0.6 (15) | 48.5±1.0 (11) | 49.0±1.6 (4) |
| 1 % Lovaza® | 33.0±0.5 (15) | 44.0±0.7 (15) | 48.0±1.3 (15) | 45.6±1.2 (6) |
| 4% Lovaza® | 35.1±0.6 (15) | 46.0±1 (15) | 50.2±1 (14) | 47.2±2.3 (11) |
| 4% FO-18/12 | 36.0±0.6 (15) | 46.1±1 (15) | 47.0±1.5 (13) | 34.5 ±0 (1) |
Numbers in parentheses indicate the number of mice in the group.
Reduced anti-ds DNA antibodies, proteinuria and nephritis in Lovaza®-fed mice
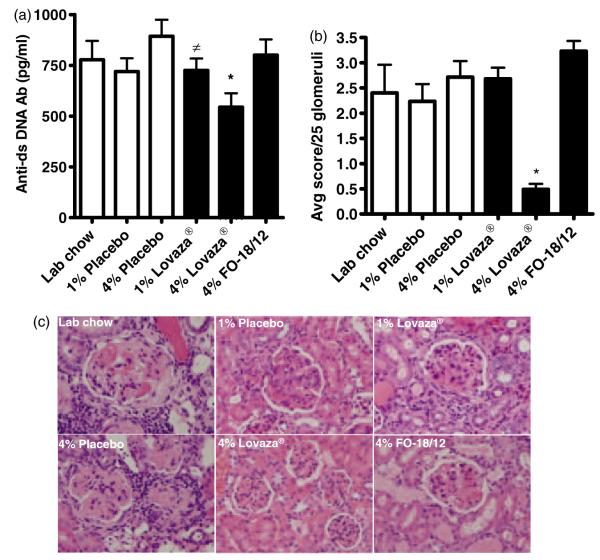
Lupus glomerulonephritis, proteinuria and production of anti-dsDNA antibodies are associated with the pathogenesis of SLE.7 Among all the dietary treatments, only 4% Lovaza®-fed mice exhibited lower serum anti-dsDNA antibodies (Figure 3A) and proteinuria (Table 3) compared to LC, placebo and 4% FO-18/12-fed mice. The histological evaluation of kidney was scored on a scale of 0–4, where 0=no apparent changes, fine mesangial staining, 1+=mild mesangial expansion, 2+=moderate mesangial expansion, 3+=severe mesangial expansion and 4+=severe mesangial expansion as in 3+ but with glomerular distortion, loss of glomerular capillary structure and obsolescence. Interestingly, only 4% Lovaza® significantly decreased kidney damage compared to FO-18/12, placebo and LC (Figure 3B and 3C). These results indicate that concentrated FO, Lovaza®, at a lower dose, failed to reduce anti-dsDNA antibodies and lupus glomerulonephritis and 4% Lovaza® may be considered a desired dose for testing translational studies.
Figure 3.
4% Lovaza® decreases serum anti-dsDNA antibodies and renal injury in (NZB×NZW)F1 female mice. Two-month-old mice were fed semi-purified diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza®, 4% FO-18/12 and standard LC diet as a control. At eight months of age, six mice/group were sacrificed for histological evaluation of kidney and serum anti-dsDNA by ELISA. Histological evaluation of kidney was scored on a scale of 0–4 (0=no apparent changes, fine mesangial staining, 1+=mild mesangial expansion, 2+=moderate mesangial expansion, 3+=severe mesangial expansion and 4+=severe mesangial expansion as in 3+ but with glomerular distortion, loss of glomerular capillary structure and obsolescence). (A) 4% Lovaza® fed mice exhibit significantly lower anti-dsDNA antibodies in serum (*P < 0.01 versus LC and placebo, ≠P < 0.05 versus placebo by ANOVA). (B) Histological evaluation of kidney demonstrates significantly decreased kidney damage in 4% Lovaza® fed mice compared to 1% Lovaza® , 4% FO-18/12, 4% placebo, 1% placebo and LC diet fed mice (25 glomeruli/mouse and 6 mice/group; *P < 0.01 versus LC, placebo and FO-18/12 by ANOVA). (C) Representative photomicrographs (40 × magnification) of H and E stained kidneys indicate reduced renal distortion in 4% Lovaza® fed mice. (A color version of this figure is available in the online journal)
Table 3.
Proteinuria levels in Lovaza® and FO-18/12 fed (NZBxNZW)FI mice
| Protein levels* |
||||||
|---|---|---|---|---|---|---|
| Groups (diet) | Age (Months) | Number of mice | Trace (<30 mg/dL) | +(30-100 mg/dL) | ++(100-500 mg/dL) | +++(>500 mg/dL) |
| Lab chow | 5a | 15 | 7 | 0 | 1 | 7 |
| 7 | 6 | 2 | 0 | 0 | 4 | |
| 11 | 1 | 0 | 0 | 0 | 1 | |
| 1 % Placebo | 5 | 15 | 10 | 0 | 1 | 4 |
| 7 | 13 | 6 | 1 | 0 | 6 | |
| 11 | 3 | 1 | 0 | 1 | 1 | |
| 13 | 1 | 0 | 0 | 0 | 1 | |
| 14 | 1 | 0 | 0 | 0 | 1 | |
| 4% Placebo | 5b | 15 | 13 | 0 | 0 | 2 |
| 7 | 14 | 9 | 1 | 2 | 2 | |
| 11 | 8 | 2 | 0 | 0 | 6 | |
| 13 | 4 | 1 | 1 | 0 | 2 | |
| 14 | 4 | 1 | 0 | 0 | 3 | |
| 1 % Lovaza® | 5 | 15 | 13 | 1 | 0 | 1 |
| 7 | 14 | 10 | 0 | 0 | 4 | |
| 11 | 9 | 1 | 1 | 3 | 4 | |
| 13 | 6 | 1 | 0 | 1 | 4 | |
| 14 | 6 | 1 | 0 | 0 | 5 | |
| 4% Lovaza® | 5c | 15 | 14 | 1 | 0 | 0 |
| 7 | 15 | 13 | 1 | 0 | 1 | |
| 11 | 13 | 3 | 2 | 2 | 6 | |
| 13 | 11 | 7 | 0 | 2 | 2 | |
| 14 | 10 | 3 | 1 | 1 | 5 | |
| 4% FO-18/12 | 5 | 15 | 14 | 1 | 0 | 0 |
| 7 | 15 | 5 | 1 | 5 | 4 | |
| 11 | 8 | 0 | 0 | 0 | 8 | |
| 13 | 1 | 0 | 0 | 0 | 1 | |
| 14 | 1 | 0 | 0 | 0 | 1 | |
Proteinuria was determined using Chemstrips (Roche Diagnostic Corporation, Indianapolis, Inc.) (n= 15).
Significantly different from 4% Lovaza® at five month onwards by one way ANOVA followed by Kruskal–Wallis non-parametric test. P <0.05 was considered statistically significant.
Significantly different from 4% Lovaza® at five month onwards by one way ANOVA followed by Kruskal–Wallis non-parametric test. P <0.05 was considered statistically significant.
Significantly different from 4% Lovaza® at five month onwards by one way ANOVA followed by Kruskal–Wallis non-parametric test. P <0.05 was considered statistically significant.
Lovaza® attenuates production of inflammatory cytokines
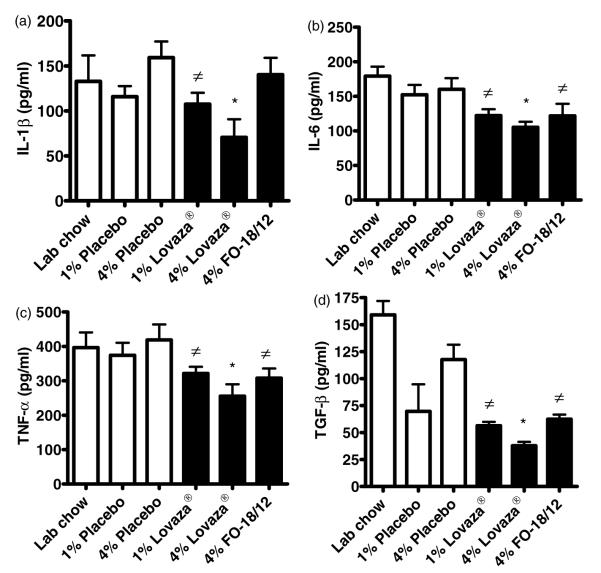
Pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and TGF-β contribute to inflammatory processes that lead to irreversible splenomegaly and kidney damage.27 Twenty-four hour cultures of splenocytes isolated from (NZB×NZW)F1 female mice fed 1% and 4% Lovaza® showed a significant reduction in LPS-induced cytokines (TNF-α, IL-1β, IL-6, and TGF-b) compared to that from mice fed LC and placebo diet (Figure 4A–D). Similarly, 1% Lovaza® also reduced IL-1β, IL-6, TNF-α and TGF-β compared to 4% placebo in cultured splenocytes. Splenocytes from 4% FO-18/12 fed mice exhibited reduced IL-6, TNF-α and TGF-β compared to that from 4% placebo fed mice (Figure 4A–D). These results suggest that while 1% Lovaza® and 4% FO-18/12 partially attenuate inflammatory response, a significant reduction in inflammatory cytokines was brought about by 4% Lovaza® in immune response initiating organ.
Figure 4.
4% Lovaza® decreases LPS induced cytokines in cultured splenocytes from (NZB×NZW)F1 female mice. Two-month-old mice were fed semi-purified diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza®, 4% FO-18/12 and standard LC diet as a control. At eight months of age, splenocytes were isolated, cultured aseptically and stimulated with LPS for 24 h. 4% Lovaza® fed mice showed significantly decreased (A)IL-1β, (B)IL-6, (C) TNF-α and (D) TGF-β in conditioned medium of splenocyte culture by ELISA (5 mice/group). Results are expressed as mean±SEM. *P < 0.05 versus LC and placebo controls, ≠P < 0.05 versus placebo by ANOVA
Lovaza® attenuates triglyceridemia and liver adiposity
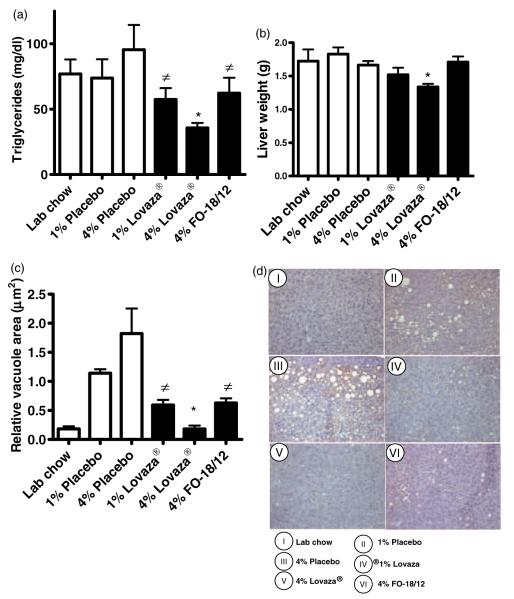
Triglyceride levels are enhanced in lupus patients and are an important predictor of cardiovascular disease.28 Our results showed that 4% Lovaza® (36±3 mg/dL) markedly reduced serum triglycerides in (NZB×NZW)F1 mice compared to LC (76±11 mg/dL) and 4% placebo (95±18 mg/dL) (Figure 5A); whereas 1% Lovaza® and 4% FO-18/12 (62±11 mg/dL) fed mice showed reduced triglycerides levels compared to 4% placebo (95 ± 18 mg/dL) fed mice. Only 4% Lovaza® was shown to reduce the liver weight (1.3±0.04g) when compared to 4% placebo (1.7±0.06 g) (Figure 5B). Interestingly, 4% FO-18/12 and both doses of Lovaza® lowered liver adiposity compared to 4% placebo measured in oil red O stained livers by determining the vacuole area (μm2) (Figure 5C–D). These results suggest that while a lower dose of Lovaza® and 4% FO-18/12 attenuates liver adiposity, only 4% Lovaza® was more effective for triglycerides management and reduced liver adiposity in lupus prone mice.
Figure 5.
4% Lovaza® decreases triglycerides, liver weight and lipid accumulation in liver of (NZB×NZW)F1 female mice. Two-month-old mice were fed semi-purified diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza®, 4% FO-18/12 and LC diet as a control. At eight months of age, six mice/group were scarified and serum triglycerides, liver weight and histology were performed. 4% Lovaza® fed mice exhibit significantly decreased (A) serum triglycerides (*P < 0.05 versus LC and placebo controls; #P < 0.05 versus placebo by ANOVA), (B) liver weight (*P < 0.05 versus LC, placebo controls and FO-18/12) and reduced (C) lipid vacuoles area compared to 4% placebo fed mice (*P < 0.05 versus placebo controls and FO-18/12, ≠P < 0.05 versus placebo by ANOVA). Results are expressed as mean±SEM. (D) Representative photomicrographs (40 × magnification) of oil red O stained livers indicate reduced lipid accumulation in 1% Lovaza®, 4% Lovaza® and 4% FO-18/12 fed mice, compared to 4% placebo fed mice. (A color version of this figure is available in the online journal)
Lovaza® enhances antioxidant enzymes and malondialdehyde in kidneys
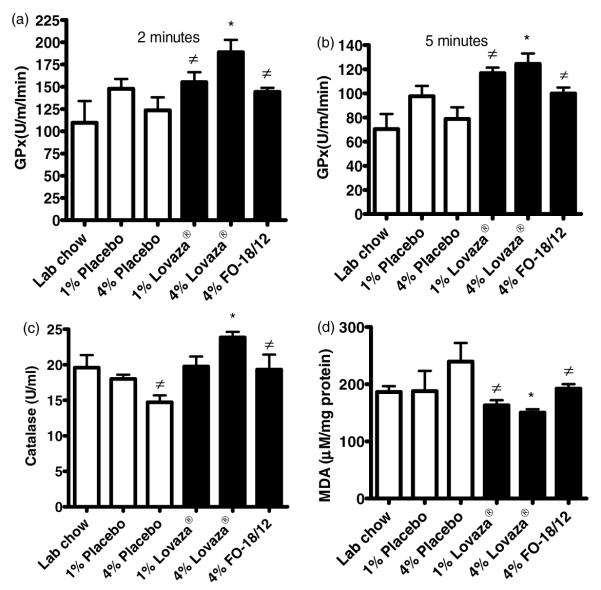
Altered redox balance is commonly observed in lupus patients.29 Our results show that 4% Lovaza® fed mice exhibited increased glutathione peroxidase (GPx) and catalase activity compared to 4% placebo fed mice (Figure 6A–C). In contrast, the malondialdehyde levels were significantly decreased in kidney of 4% Lovaza® fed mice compared to placebo fed mice (Figure 6D). These results indicate that relatively higher doses of Lovaza® are required to preserve the redox balance in kidneys of short-lived (NZB×NZW)F1 mice.
Figure 6.
4% Lovaza® increases glutathione peroxidase (GPx), catalase and increases malondialdehyde (MDA) in kidney homogenates of (NZB×NZW)F1 female mice. Two-month-old were fed semi-purified diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza®, 4% FO-18/12 and LC diet as a control. At eight months of age, GPx, catalase and malondialdehyde were measured in the kidney homogenates of mice from different groups (n = 6). 4% Lovaza® fed mice exhibit significantly increased GPx at 2 (A) and 5 (B) minutes and (C) catalase compared to 4% placebo and LC fed mice. (P < 0.05, ANOVA). (D) 4% Lovaza® fed mice exhibit significantly decreased MDA compared to 4% Placebo and LC fed mice. Results are expressed as mean ± SEM. (*P < 0.05 versus LC and placebo controls, #P < 0.05 versus placebo by ANOVA)
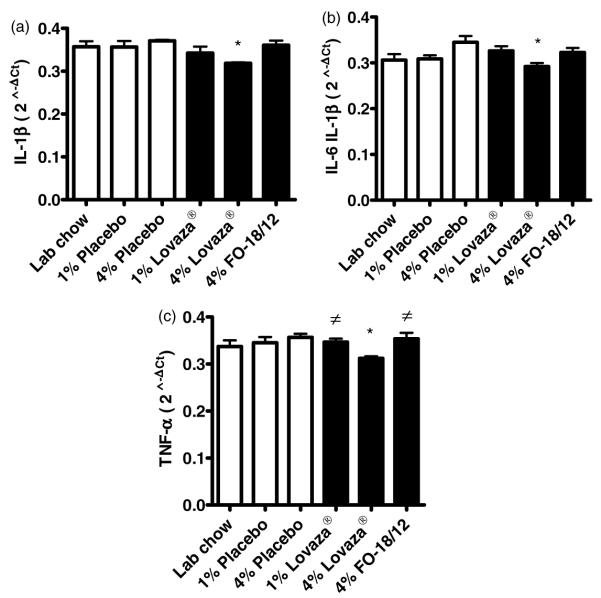
Pro-inflammatory gene expression is lowered in Lovaza® fed mice
Pro-inflammatory gene expression profile is significantly enhanced in kidneys of lupus prone mice. Our data indicate that only 4% Lovaza® down-regulates the expression of IL-1β, IL-6 and TNF-α in kidney compared to placebo and LC control (Figure 7A–C). Though 1% Lovaza® and 4% FO-18/ 12 fed mice also showed reduction in expression of inflammatory genes, the results were not significant.
Figure 7.
4% Lovaza® down-regulates the expression of (A) IL-1β, (B) IL-6 and (C) TNF-α in the kidney of lupus prone (NZB×NZW)F1 female mice. Two-month-old mice were fed semi-purified diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza®, 4% FO-18/12 and LC diet as a control. At eight months of age, RNA was isolated from mice kidney and gene expression was determined by quantitative real-time RT-PCR. Results are expressed as mean ± SEM. (*P < 0.05 versus LC and placebo controls, #P < 0.05 versus placebo by ANOVA)
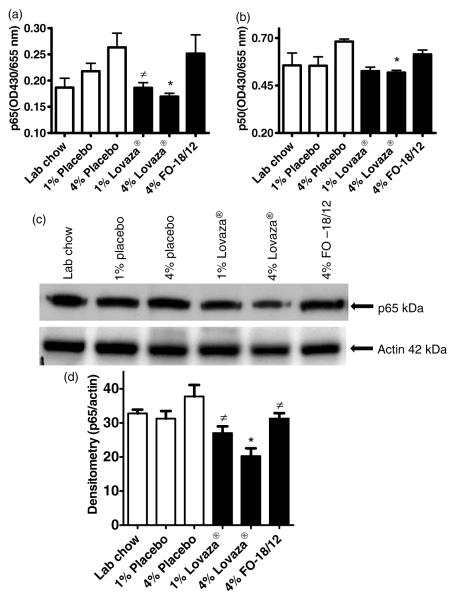
Lovaza® downregulates NF-κB activation and p65 nuclear translocation in kidneys
NF-κB is significantly involved in cytokines induction and is up-regulated in lupus patients and lupus prone mice kidney.7,30 We examined the effects of 4% Lovaza® on NF-κB activation in kidney. Amount of p65 and p50 were significantly lowered in the kidney nuclear extract of 1% and 4% Lovaza® fed mice compared to 4% placebo fed mice (Figure 8A and 8B). Furthermore, p65 nuclear trans-location was confirmed by immunoblotting in kidney nuclear extracts. Only 4% Lovaza® reduced p65 nuclear translocation more prominently than placebo and LC controls, as presented in representative immunoblot of p65 and actin (Figure 8C) and the densitometry results normalized with actin (Figure 8D). In summary, 4% Lovaza® attenuates NF-κB activation and p65 translocation in kidneys thus reducing the severity of glomerulonephritis in lupus-prone mice.
Figure 8.
4% Lovaza® down-regulates NF-κB (A/B), and p65 nuclear translocation in kidney of lupus prone (NZB×NZW)F1 female mice (C/D). Two-month-old mice were fed semi-purified diets containing 1% placebo, 4% placebo, 1% Lovaza®, 4% Lovaza®, 4% FO-18/12 and LC as a control. At eight months of age, NF-κB and p65 were determined in kidney nuclear protein. Results are expressed as mean ± SEM. (*P < 0.05 versus LC and placebo controls, ≠P < 0.05 versus placebo by ANOVA)
Discussion
SLE is a chronic, recurrent, potentially fatal multisystem inflammatory disorder that can be difficult to diagnose and later adversely affects the lifespan. Additionally, the organ damage in SLE progresses over time with both renal and cardiovascular organ systems most commonly affected.31 Accordingly, the development of novel therapies or dietary regimen/recommendations is critical for survival in SLE patients. Our study, for the first time, elucidates the dose dependent effect of the concentrated fish oil formulation Lovaza® on the life span, liver adiposity and glomerulonephritis in lupus-prone (NZB×NZW)F1 mice.
Our recent studies reported that n-3 fatty acids, particularly enriched in DHA (60%), extend median and maximal life span of short-lived lupus-prone BXW mice compared with a diet supplemented with n-6 fatty acids enriched corn oil.7 Menhaden FO, containing 20–25% EPA and DHA, extends median and maximal life span of short-lived lupus-prone (NZB×NZW)F1 mice compared with a diet supplemented with n-6 fatty acids-rich corn oil.32–34 Additional studies published from our laboratory reports that a combination of n-3 fatty acids and caloric restriction further extends the life span of (NZB×NZW)F1 mice compared to n-6 fatty acids fed ad libitum or with calorie restriction,34 suggesting that source of dietary fat (n-3 fatty acids versus n-6 fatty acids) was an important determinant of disease progression and severity in (NZB×NZW)F1 mice.
Although these findings are encouraging, there are obvious concerns that dietary regimens of fish oil with 10% or 30– 40% calorie restriction may be impractical for SLE patients, and that FO with low EPA/DHA content would have only moderate beneficial effects in the lupus patients. Lovaza® is an FDA approved fish oil formulation and is composed of 46.5% EPA and 37.5% DHA.19 In the current study, we investigated the dose dependent (1% and 4%) efficacy of Lovaza® on disease severity and longevity in (NZB×NZW)F1 mice. Our results demonstrate for the first time that 1% Lovaza® moderately extends the life span of (NZB×NZW)F1 mice, while 4% Lovaza® is a potent inhibitor of inflammatory cytokines and autoantibody production, reduces kidney disease, and significantly extends life span of lupus-prone short-lived (NZB×NZW)F1 mice.
Anti-dsDNA antibodies are characteristic biomarkers of diagnosis and prognosis of patients with SLE. Additionally, these antibodies have been found to be good markers of disease activity and predictors of outcome in lupus nephritis.35 It is important to note that this study demonstrates a downregulation of LPS-induced cytokines such as IL-1β, IL-6, TNF-α and TGF-β that control differentiation, maturation and activation of cells. Dietary interventions with Lovaza® brought about significant reduction in anti-dsDNA antibody and attenuated the development of glomerulonephritis. Furthermore, the pro-inflammatory gene expression (IL-1β, IL-6 and TNF-α) in kidney is a significant contributor towards initiating inflammatory response of lupus nephritis.27,36,37 Dietary Lovaza® suppresses inflammatory cytokines, particularly IL-1β, IL-6, TNF-α and TGF-β secretion by immune initiating organ and their expression (IL-1β, IL-6 and TNF-α) in kidney modulates both the initiation and propagation of auto-immune response resulting in delayed development of glomerulonephritis.
NF-κB is a prevalent stress-responsive transcription factor and plays a pivotal role in inflammation and glomerulonephritis.38 NF-κB can be activated by several stimuli, such as pro-inflammatory cytokines (IL-1β, IL-6) and chemokines that lead to NF-κB translocation to the nucleus and promote the transcription of several genes. Our previous reports indicate that NF-κB is a downstream mediator of PI3K and Akt pathways, and plays a role in IL-18 induction and signaling.7 In the present investigation, we analysed NF-κB activation by ELISA and nuclear translocation of p65 by immunoblotting in kidneys after treatment with Lovaza®. Our results show a significant inhibition in NF-kB activity in 4% Lovaza® fed mice, followed by 1% Lovaza® and 4% FO-18/12 fed mice. These results corroborate previous studies demonstrating inhibition of NF-κB activation by n-3 fatty acids both in-vivo and in-vitro.7,39,40 Our results also show that reduced levels of nuclear NF-κBp65 and NF-κBp50 in kidneys of 1% and 4% Lovaza®-fed mice, suggesting that concentrated FO inhibits NF-κB activation by inhibiting nuclear translocation of p65. This finding is in agreement with a previous study which showed that FO attenuates LPS-induced nuclear p65 levels in cultured human THP-1 macrophages.40 Our study provides the first in vivo evidence that Lovaza® is a potent inhibitor of NF-κB activation, and suggests that reduced NF-κB activation might be a contributing factor in the inhibition of renal disease in Lovaza®-fed mice.
Lupus progression compromises survival in patients and requires multi-drug therapies to limit the severity of symptoms and to extend the lifespan. In multi-organ damage inflammatory rheumatic and SLE patients, over 50% of premature deaths are attributable to cardiovascular disease.3 In the present study, Lovaza® and FO-18/12 reduced triglycerides, indicating the ability of FO to attenuate hyperlipidemia and provides indirect contributory evidence on reduction of adverse cardiovascular events. Furthermore, the liver adiposity is also reduced in 1%, 4% Lovaza® and 4% FO-18/12 fed mice. Interestingly, we have earlier demonstrated that 10% FO-18/12 extends median and maximal life span to 414 d and 539 d respectively,7 whereas in the current study 4% FO-18/12 extends median and maximal life span of lupus-prone mice to 410 d and 450 d respectively. Furthermore, the higher efficacy of 1% Lovaza® over 10% FO-18/12 may be ascribed to the higher percentage of DHA and EPA in the former. This provides a rationale for consumption of concentrated FO Lovaza® in consultation with the physician according to individual need.
Studies on the use of non-concentrated regular fish oil, with or without non-steroidal anti-inflammatory drugs against rheumatoid arthritis patients, have been undertaken in the past based on anti-inflammatory action of fish oil.41–43 It now appears, based on our current results, that undertaking new clinical studies on RA and lupus patients using both Lovaza® capsules along with much lower dosages of anti-rheumatic drugs and other biologic agents may yield less toxicity and also much favorable results against the disease. It is known that the use of fish oil lowers drug toxicity44 and also reduces the severity of cardiovascular disease, which is more common in RA patients.45 Furthermore, it is apparent that fish oil lowers inflammatory cytokines which cause pain and disability,46 and results in our laboratory shows that Lovaza®, the concentrated fish oil formulation, reduces pain sensitivity47 and renders protection against bone loss as well.48,49 Thus, the use of FDA approved Lovaza® may have multiple benefits besides lowering RA disease severity.
There are certain limitations to the study that need to be addressed with diligence. Recent epidemiological studies indicate that 50% of deaths in rheumatic and SLE patients are attributed to cardiovascular disease.3 Moreover, (NZB×NZW)F1 mice develop hypertension, showing increased mean arterial blood pressure at nine months of age, which is a prominent feature of SLE and glomerulonephritis.50 However, the current study lacks data on blood pressure, left ventricle function or incidence of myocardial infarction. Recent studies also indicate that increased lipotoxicity, particularly developed by increased intake of n-6 fatty acids, primarily aggravates inflammatory response,51 which is not revealed in detail in this study. Consequently, future studies with specific emphasis on n-6 fatty acids vs fat, and combinations of n-3+n-6 fatty acids in pre-clinical and clinical settings will provide relevant guidance on lipid intake for lupus patients.
In summary, this study provides mechanistic basis and minimum effective dose of concentrated FO to extend the life span and attenuate lupus nephritis in lupus-prone mice.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants NCCAM K99AT006704 to GVH and NCCAM R01AT004259 to GF. All the authors declared no competing interests. The authors acknowledge the support from GlaxoSmithKline for providing Lovaza® and placebo samples. They also thank Ocean Nutrition for providing the fish oil 18/ 12 for this study.
REFERENCES
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 3.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 4.Helyer BJ, Howie JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963:197–197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- 5.Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:228. doi: 10.1186/ar3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin BF, Huang CH, Chiang BL, Jeng SJ. Dietary fat influences Ia antigen expression, cytokines and prostaglandin E2 production of immune cells in autoimmune-prone NZB × NZW F1 mice. Br J Nutr. 1996;75:711–22. doi: 10.1079/bjn19960175. [DOI] [PubMed] [Google Scholar]
- 7.Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J Immunol. 2010;184:5280–6. doi: 10.4049/jimmunol.0903282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander NJ, Smythe NL, Jokinen MP. The type of dietary fat affects the severity of autoimmune disease in NZB/NZW mice. Am J Pathol. 1987;127:106–21. [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes G, Venkatraman J, Khare A, Horbach GJ, Friedrichs W. Modulation of gene expression in autoimmune disease and aging by food restriction and dietary lipids. Proc Soc Exp Biol Med. 1990;193:16–22. doi: 10.3181/00379727-193-42983. [DOI] [PubMed] [Google Scholar]
- 10.Levy JA, Ibrahim AB, Shirai T, Ohta K, Nagasawa R, Yoshida H, Estes J, Gardner M. Dietary fat affects immune response, production of anti-viral factors, and immune complex disease in NZB/NZW mice. Proc Natl Acad Sci USA. 1982;79:1974–8. doi: 10.1073/pnas.79.6.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yumura W, Hattori S, Morrow WJ, Mayes DC, Levy JA, Shirai T. Dietary fat and immune function. II. Effects on immune complex nephritis in (NZB × NZW)F1 mice. J Immunol. 1985;135:3864–8. [PubMed] [Google Scholar]
- 12.Erickson KL, Adams DA, Scibienski RJ. Dietary fatty acid modulation of murine B-cell responsiveness. J Nutr. 1986;116:1830–40. doi: 10.1093/jn/116.9.1830. [DOI] [PubMed] [Google Scholar]
- 13.Jyonouchi H, Sun S, Goodman D, Yokoyama H, Sato S. Dietary fatty acid modulates actions of nucleotides on humoral immune responses. Nutrition. 1995;11:437–43. [PubMed] [Google Scholar]
- 14.Yaqoob P, Calder PC. The effects of dietary lipid manipulation on the production of murine T cell-derived cytokines. Cytokine. 1995;7:548–53. doi: 10.1006/cyto.1995.0074. [DOI] [PubMed] [Google Scholar]
- 15.Pestka JJ. n-3 polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82:251–8. doi: 10.1016/j.plefa.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghdassi E, Ma DW, Morrison S, Hillyer LM, Clarke S, Gladman DD, Urowitz MB, Fortin PR. Alterations in circulating fatty acid composition in patients with systemic lupus erythematosus: a pilot study. JPEN J Parenter Enteral Nutr. 2011;35:198–208. doi: 10.1177/0148607110386378. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura N, Kumasaka R, Osawa H, Yamabe H, Shirato K, Fujita T, Murakami R, Shimada M, Nakamura M, Okumura K, Hamazaki K, Hamazaki T. Effects of eicosapentaenoic acids on oxidative stress and plasma fatty acid composition in patients with lupus nephritis. In Vivo. 2005;19:879–82. [PubMed] [Google Scholar]
- 18.Fassett RG, Gobe GC, Peake JM, Coombes JS. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am J Kidney Dis. 2010;56:728–42. doi: 10.1053/j.ajkd.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Pratt CM, Reiffel JA, Ellenbogen KA, Naccarelli GV, Kowey PR. Efficacy and safety of prescription omega-3-acid ethyl esters for the prevention of recurrent symptomatic atrial fibrillation: a prospective study. Am Heart J. 2009;158:163–9. doi: 10.1016/j.ahj.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Lambert PH, Dixon FJ. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968;127:507–22. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolly CA, Muthukumar A, Avula CP, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB × NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr. 2001;131:2753–60. doi: 10.1093/jn/131.10.2753. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on ‘maximum lifespan’. Mech Ageing Dev. 2004;125:629–32. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Halade GV, Rahman MM, Williams PJ, Fernandes G. Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6J mice. J Nutr Biochem. 2011;22:459–69. doi: 10.1016/j.jnutbio.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21:1162–9. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrasekar B, Troyer DA, Venkatraman JT, Fernandes G. Dietary omega-3 lipids delay the onset and progression of autoimmune lupus nephritis by inhibiting transforming growth factor beta mRNA and protein expression. J Autoimmun. 1995;8:381–93. doi: 10.1006/jaut.1995.0030. [DOI] [PubMed] [Google Scholar]
- 27.Aringer M, Smolen JS. Tumour necrosis factor and other proinflammatory cytokines in systemic lupus erythematosus: a rationale for therapeutic intervention. Lupus. 2004;13:344–7. doi: 10.1191/0961203303lu1024oa. [DOI] [PubMed] [Google Scholar]
- 28.Touma Z, Gladman DD, Ibanez D, Urowitz MB. Ability of non-fasting and fasting triglycerides to predict coronary artery disease in lupus patients. Rheumatology (Oxford) 2012;51:528–34. doi: 10.1093/rheumatology/ker339. [DOI] [PubMed] [Google Scholar]
- 29.Taysi S, Gul M, Sari RA, Akcay F, Bakan N. Serum oxidant/antioxidant status of patients with systemic lupus erythematosus. Clin Chem Lab Med. 2002;40:684–8. doi: 10.1515/CCLM.2002.117. [DOI] [PubMed] [Google Scholar]
- 30.Enzler T, Bonizzi G, Silverman GJ, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–15. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Gill JM, Quisel AM, Rocca PV, Walters DT. Diagnosis of systemic lupus erythematosus. Am Fam Physician. 2003;68:2179–86. [PubMed] [Google Scholar]
- 32.Chandrasekar B, Fernandes G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994;200:893–8. doi: 10.1006/bbrc.1994.1534. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes G. Dietary lipids and risk of autoimmune disease. Clin Immunol Immunopathol. 1994;72:193–7. doi: 10.1006/clin.1994.1129. [DOI] [PubMed] [Google Scholar]
- 34.Venkatraman JT, Chandrasekar B, Kim JD, Fernandes G. Effects of n-3 and n-6 fatty acids on the activities and expression of hepatic antioxidant enzymes in autoimmune-prone NZBxNZW F1 mice. Lipids. 1994;29:561–8. doi: 10.1007/BF02536628. [DOI] [PubMed] [Google Scholar]
- 35.Illei GG, Tackey E, Lapteva L, Lipsky PE. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum. 2004;50:2048–65. doi: 10.1002/art.20345. [DOI] [PubMed] [Google Scholar]
- 36.Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, Avalos-Diaz E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus. 1998;7:154–8. doi: 10.1191/096120398678919949. [DOI] [PubMed] [Google Scholar]
- 37.Maczynska I, Millo B, Ratajczak-Stefanska V, Maleszka R, Szych Z, Kurpisz M, Giedrys-Kalemba S. Proinflammatory cytokine (IL-1beta, IL-6, IL-12, IL-18 and TNF-alpha) levels in sera of patients with sub-acute cutaneous lupus erythematosus (SCLE) Immunol Lett. 2006;102:79–82. doi: 10.1016/j.imlet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Kalergis AM, Iruretagoyena MI, Barrientos MJ, Gonzalez PA, Herrada AA, Leiva ED, Gutieérrez MA, Riedal CA, Bueno SM, Jacobelli SH. Modulation of nuclear factor-kappaB activity can influence the susceptibility to systemic lupus erythematosus. Immunology. 2009;128:e306–14. doi: 10.1111/j.1365-2567.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18:1206–16. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- 40.Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM. Docosahexaenoic acid induces an anti-inflammatory profile in lipopo-lysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem. 2007;18:250–8. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Caughey GE, James MJ, Proudman SM, Cleland LG. Fish oil supplementation increases the cyclooxygenase inhibitory activity of paracetamol in rheumatoid arthritis patients. Complement Ther Med. 2010;18:171–4. doi: 10.1016/j.ctim.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 42.Dawczynski C, Schubert R, Hein G, Muller A, Eidner T, Vogelsang H, Basu S, Jahreis G. Long-term moderate intervention with n-3 long-chain PUFA-supplemented dairy products: effects on pathophysiological biomarkers in patients with rheumatoid arthritis. Br J Nutr. 2009;101:1517–26. doi: 10.1017/S0007114508076216. [DOI] [PubMed] [Google Scholar]
- 43.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107:S171–84. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- 44.Zaitone SA, Moustafa YM, Mosaad SM, El-Orabi NF. Effect of evening primrose oil and omega-3 polyunsaturated fatty acids on the cardiovascular risk of celecoxib in rats. J Cardiovasc Pharmacol. 2011;58:72–9. doi: 10.1097/FJC.0b013e31821c8353. [DOI] [PubMed] [Google Scholar]
- 45.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 46.Campbell RC, Batley M, Hammond A, Ibrahim F, Kingsley G, Scott DL. The impact of disease activity, pain, disability and treatments on fatigue in established rheumatoid arthritis. Clin Rheumatol. 2012;31:717–22. doi: 10.1007/s10067-011-1887-y. [DOI] [PubMed] [Google Scholar]
- 47.Veigas JM, Williams PJ, Halade G, Rahman MM, Yoneda T, Fernandes G. Fish oil concentrate delays sensitivity to thermal nociception in mice. Pharmacol Res. 2011;63:377–82. doi: 10.1016/j.phrs.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharya A, Rahman M, Sun D, Fernandes G. Effect of fish oil on bone mineral density in aging C57BL/6 female mice. J Nutr Biochem. 2007;18:372–9. doi: 10.1016/j.jnutbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharya A, Rahman M, Banu J, Lawrence RA, McGuff HS, Garrett IR, Fischbach M, Fernandes G. Inhibition of osteoporosis in autoimmune disease prone MRL/Mpj-Fas(lpr) mice by N-3 fatty acids. J Am Coll Nutr. 2005;24:200–9. doi: 10.1080/07315724.2005.10719466. [DOI] [PubMed] [Google Scholar]
- 50.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension. 2006;48:988–93. doi: 10.1161/01.HYP.0000243612.02929.df. [DOI] [PubMed] [Google Scholar]
- 51.Halade GV, Jin YF, Lindsey ML. Roles of saturated vs. polyunsaturated fat in heart failure survival: not all fats are created equal. Cardiovasc Res. 2012;93:4–5. doi: 10.1093/cvr/cvr298. [DOI] [PMC free article] [PubMed] [Google Scholar]