Abstract
OBJECTIVE
The purposes of this article are to review the current management of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) based on the 2012 National Comprehensive Cancer Network guidelines and to describe the role of imaging in a multidisciplinary approach.
CONCLUSION
The management of GEP-NETs has become complex, requiring a multidisciplinary approach. The World Health Organization classification of GEP-NETs has been revised; the U.S. Food and Drug Administration has approved molecular targeted agents (sunitinib, everolimus) for the treatment of pancreatic NETs; and the National Comprehensive Cancer Network clinical practice guidelines have been updated.
Keywords: gastroenteropancreatic neuroendocrine tumor, guidelines, molecular targeted therapy
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are groups of heterogeneous tumors on a clinicopathologic spectrum from functioning well-differentiated tumors to nonfunctioning poorly differentiated carcinomas. GEP-NETs have garnered considerable interest because tumor-producing peptide hormones cause characteristic symptoms and overexpression of somatostatin receptors, which have been used in functional imaging and biotherapy. The diagnosis and management of GEP-NETs have evolved in both medicine and surgery.
Unlike other epithelial tumors, malignant GEP-NETs, even unresectable and metastatic tumors, often have a relatively indolent course with less sensitivity to conventional chemotherapy. Therefore, aggressive surgical treatment and various novel therapies, including radiofrequency ablation, chemoembolization, radioembolization, and molecular targeted therapy (MTT), have been applied in the management of unresectable and metastatic GEP-NETs [1–3]. Multidisciplinary collaboration between oncologists, radiologists, and surgeons is essential for provision of optimal personalized therapy. The World Health Organization (WHO) classification of GEP-NETs was revised in 2010, and MTTs were approved by the U.S. Food and Drug Administration (FDA) in 2011 (Appendix 1). Consensus guidelines on GEP-NETs have been updated [2, 3], most recently by the National Comprehensive Cancer Network (NCCN) in 2012 [4]. In this article, we review current management of GEP-NETs, primarily based on the 2012 NCCN guidelines, and the roles of imaging in a multidisciplinary and personalized therapeutic approach.
APPENDIX 1.
Summary of World Health Organization 2000 and 2010 Classifications
| World Health Organization 2000 Classification
| ||||
|---|---|---|---|---|
| Location | Well-Differentiated Endocrine Tumor (Benign Behavior) | Well-Differentiated Endocrine Tumor (Uncertain Behavior) | Well-Differentiated Endocrine Carcinoma (Low-Grade Malignancy) | Poorly Differentiated Endocrine Carcinoma (High-Grade Malignancy) |
|
| ||||
| Stomach | Well differentiated | Well differentiated | Well or moderately differentiated | Small cell carcinoma |
| Confined to mucosa and submucosa | Confined to mucosa and submucosa | Invasion to muscularis propria or beyond or metastases | ||
| ≤ 1 cm | > 1 cm | |||
| No vascular invasion | Vascular invasion | |||
| Duodenum, jejunum | Well differentiated | Well differentiated | Well or moderately differentiated | Small cell carcinoma |
| Confined to mucosa and submucosa | Confined to mucosa and submucosa | Invasion to muscularis propria or beyond or metastases | ||
| ≤ 1 cm | > 1 cm | |||
| No vascular invasion | Vascular invasion | |||
| Ileum, colon, rectum | Well differentiated | Well differentiated | Well or moderately differentiated | Small cell carcinoma |
| Confined to mucosa and submucosa | Confined to mucosa and submucosa | Invasion to muscularis propriaor | ||
| ≤ 1 cm (ileum) | > 1 cm (small intestine) | beyond or metastases | ||
| ≤ 2 cm (colon) | > 2 cm (large intestine), vascular invasion | |||
| No vascular invasion | ||||
| Appendix | Well differentiated | Well differentiated | Well or moderately differentiated | Small cell carcinoma |
| Nonfunctioning | Confined to subserosa | Invasion to mesoappendix or beyond or metastases | ||
| Confined to appendix wall, ≤ 2 cm, | > 2 cm | |||
| No vascular invasion | Vascular Invasion | |||
| Pancreas | Well differentiated | Well differentiated | Well or moderately differentiated | Small cell carcinoma |
| Confined to pancreas | Confined to pancreas | Gross local invasion or metastases | Necrosis common | |
| < 2 cm | ≥ 2 cm | 2–10 mitoses per 10 HPF | > 10 mitoses per 10 HPF | |
| < 2 mitoses per 10 HPF | > 2 mitoses per 10 HPF | Ki-67 index > 5% | > 15% Ki-67–positive cells | |
| < 2% Ki-67–positive cells | > 2% Ki-67–positive cells | Prominent vascular or perineural invasion | ||
| No vascular invasion | Vascular invasion | |||
| World Health Organization WHO 2010 Classification
| |
|---|---|
| Neuroendocrine Tumor | Neuroendocrine Carcinoma |
|
| |
| Well or moderately differentiated | Small cell or large cell |
| Grade 1, < 2 mitoses per 10 HPF; Ki-67 index, ≤ 2% | Grade 3, > 20 mitoses per 10 HPF; Ki-67 index, > 20% |
| Grade 2, 2–20 mitoses per 10 HPF, Ki-67 index, 3–20% | |
| Location, size, extent, vascular invasion: TNM staging | |
Note—HPF = high power fields.
Revised World Health Organization Classification and Terminology
In the 2000 WHO classification, GEP-NETs were divided into well-differentiated endocrine tumors (with benign or uncertain behavior), well-differentiated endocrine carcinomas (low-grade malignancy), and poorly differentiated endocrine carcinomas (high-grade malignancy) [5, 6]. The most poorly differentiated endocrine carcinomas were called small cell carcinomas (Table 1).
TABLE 1.
Change of Terminology in World Health Organization (WHO) Classifications of Gastroenteropancreatic Neuroendocrine Tumors
| WHO 2000 | WHO 2010
|
||
|---|---|---|---|
| Term and Grade | No. of Mitosesa | Ki-67 Indexb | |
|
| |||
| Well-differentiated endocrine tumor | Neuroendocrine tumor (carcinoid) | ||
| Well-differentiated endocrine carcinoma | Grade 1 | < 2 | ≤ 2 |
| Grade 2 | 2–20 | 3–20 | |
| Poorly differentiated endocrine carcinoma (small cell carcinoma) | Neuroendocrine carcinoma (small cell or large cell), grade 3 | > 20 | > 20 |
| Mixed exocrine-endocrine carcinoma | Mixed adenoneuroendocrine carcinoma | ||
Note—Criteria summarized in [6].
Per 10 high power fields (high power field = 2 mm2 at 400× magnification in hotspot areas).
Percentage of stained cells among 500–2000 tumor cells in hotspot areas.
The revised 2010 WHO classification primarily regards all GEP-NETs as potentially malignant tumors and no longer differentiates well-differentiated endocrine tumors and well-differentiated endocrine carcinomas. It classifies these tumors as NET grade 1 or 2 or neuroendocrine carcinoma grade 3 on the basis of the Ki-67 proliferation index or mitotic index, each of which is associated with a progressively poorer prognosis [6] (Table 1). Other parameters, such as tumor location, size, extent, and vascular invasion were shifted to the American Joint Committee on Cancer TNM staging system, seventh edition.
Regarding terminology, the term “neuroendocrine tumor” has been recommended instead of the historical terms “carcinoid” and “islet cell tumor” since the 2000 WHO classification [5]. The term “carcinoid” has been retained and used interchangeably with “neuroendocrine tumor” for lesions in the gastrointestinal tract, and the term “islet cell tumor of the pancreas” is also used for NETs of the pancreas. Neuroendocrine carcinoma (grade 3, poorly differentiated or extrapulmonary small cell carcinoma) should be managed in the same way as small cell lung cancer according to the 2012 NCCN guidelines. The rationale is that its clinicopathologic features, which are similar to those of small cell lung cancer, differ fundamentally from those of other NETs [4, 7]. We focus on the management of NETs (grades 1 and 2) and use the term GEP-NETs to stand for these grade 1 and 2 NETs.
Management of Gastroenteropancreatic Neuroendocrine Tumors: Overview
Initial Evaluation
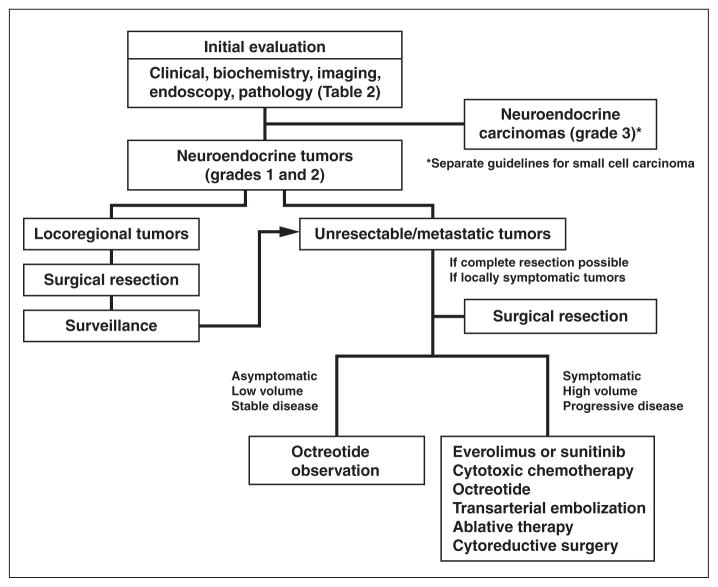
The overall management of GEP-NETs is summarized in Figure 1 [4]. The goals of initial evaluation are the diagnosis of GEP-NETs, localization of the tumor, and staging [1]. Approximately 15–30% of GEP-NETs are functioning tumors with hormone-related symptoms, and 70–85% of GEP-NETs are nonfunctioning and detected incidentally or because the patient has symptoms of local mass effect or metastasis [1]. Once GEP-NETs are suspected, laboratory and imaging examinations are performed systematically as summarized in Table 2. Multiphasic contrast-enhanced abdominal and pelvic CT or MRI is recommended for all patients with suspected GEP-NETs [4]. Endoscopy or endoscopic ultrasound evaluation is recommended as appropriate for detection of GEP-NETs of the stomach, duodenum, and rectum. An octreotide scan can be used as the first imaging tool, especially in cases in which CT or endoscopic ultrasound findings are inconclusive [3, 4]. Fluorine-18–labeled FDG PET/CT can be used for evaluation of high-grade tumors, but the applicability of FDG PET/CT to low-grade GEP-NETs is limited because of the lower metabolic activity of low-grade tumors. Thus the 2012 NCCN guidelines do not include PET/CT for this purpose [8]. Pathologic diagnosis and grade determination are essential in the management of GEP-NETs. Imaging-guided biopsy of the primary tumor or metastatic sites should be performed if possible [3].
Fig. 1.
Chart shows algorithm for management of gastroenteropancreatic neuroendocrine tumors.
TABLE 2.
Initial Evaluation of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs)
| Clinical Presentation | Expected Common GEP-NET | Biochemical Marker | Imaging or Endoscopya |
|---|---|---|---|
|
| |||
| Carcinoid syndrome: flushing, diarrhea | Liver metastases from neuroendocrine tumors in the ileum, cecum, and right colon | 5-HIAA (24-h urine), chromogranin A | Concomitant CT or MR enterography, small-bowel imaging, octreotide scan, colonoscopy |
| Recurrent peptic ulcer disease | Gastrinoma (in pancreas or stomach and duodenum) | Gastrin, vitamin B12 blood level | Esophagogastroduodenal endoscopic ultrasound, octreotide scan for patients with normal gastrin blood level |
| Hypoglycemia, weight gain | Insulinoma (whole pancreas) | Proinsulin, insulin-to-glucose ratio, C-peptide | Endoscopic ultrasound (for pancreatic evaluation) |
| Watery diarrhea, hypokalemia, achlorhydria | VIPoma (pancreas or duodenum) | VIP, electrolytes | Octreotide scan |
| Diabetes mellitus, migratory necrolytic erythema | Glucagonoma (pancreatic body, tail) | Glucagon, glucose, CBC | Octreotide scan |
| Symptoms related to mass effect, such as bowel obstruction and ischemia, biliary obstruction, and liver failure | Nonfunctioning GEP-NETs with metastases | Chromogranin A | Octreotide scan |
Note—5-HIAA = 5-hydroxyindoleacetic acid, VIP = vasoactive intestinal polypeptide.
Considered in addition to multiphasic abdominal and pelvic CT or MRI.
In the initial evaluation of GEP-NETs, it is crucial to divide resectable locoregional tumors from unresectable locoregional and metastatic tumors because the management and prognosis of the two categories differ markedly [9]. Imaging plays an important role in this determination. The seventh edition of the American Joint Committee on Cancer TNM staging system is used [10]. NETs of the stomach; duodenum, jejunum, and ileum; colon and rectum; and appendix have separate staging systems, and pancreatic NET shares the staging system with pancreatic adenocarcinoma [11].
Locoregional Gastroenteropancreatic Neuroendocrine Tumors
For locoregional GEP-NETs, the primary goal is complete surgical resection, which can have an excellent outcome [4, 12]. For peripherally located small pancreatic insulinomas and gastrinomas, enucleation may be considered, but for most other GEP-NETs and deep-seated tumors, conventional pancreatectomy is recommended. For most NETs of the bowel, conventional surgical bowel resection should be considered. At times, endoscopic resection may be considered for small localized tumors in the stomach and rectum. Regional lymph-adenectomy is recommended because GEP-NETs are associated with risk of nodal metastasis, even of small tumors [4, 13].
Unresectable and Metastatic Gastroenteropancreatic Neuroendocrine Tumors
The management of unresectable and metastatic GEP-NETs is complex and continues to evolve. The most common site of distant metastasis is the liver (85%), followed by peritoneum (18%), bones (8%), and lungs (4%) [14]. For patients with limited metastatic (mostly liver) disease and resectable primary tumor, surgical resection of both metastatic and primary tumors should be considered first if complete resection is possible. For asymptomatic low-volume disease with an indolent course, observation or octreotide treatment is recommended with surveillance until clinically significant disease progression occurs. In cases of significant tumor-related symptoms, tumor burden, or disease progression, several options can be considered, including MTT (everolimus or sunitinib), cytotoxic chemotherapy, octreotide, and liver-directed therapy (chemoembolization or radioembolization, ablative therapy, or cytoreductive surgery) [4, 9] (Table 3).
TABLE 3.
Therapeutic Methods for the Management of Unresectable and Metastatic Gastroenteropancreatic Neuroendocrine Tumors (NETs)
| Condition | Therapeutic Method | Category of Evidencea
|
Considerations | |
|---|---|---|---|---|
| Pancreatic NET | Gastrointestinal NET | |||
|
| ||||
| Complete resection possible | Complete resection of primary and metastatic lesions | 2A | 2A | Resection of small asymptomatic primary tumors in the presence of unresectable metastases is not indicated |
| Asymptomatic stable disease, and low tumor burden | Observation | 2A | 2A | According to PROMID trial results, octreotide treatment is recommended for gastrointestinal NETs, and observation strategy needs a critical reevaluation |
| Octreotide | § | 2A | Octreotide treatment of pancreatic neuroendocrine tumors is not included in NCCN 2012 guidelines | |
| Clinically significant tumor burden, progressive disease | Octreotide | 2B | 2A | Pancreatic NET: MTT or cytotoxic chemotherapy first or consider other methods |
| MTT | 2A | 3 | Gastrointestinal NET: octreotide and consider other methods | |
| Cytotoxic chemotherapy | 2A | 3 | Ablative therapy or cytoreductive surgery: only if nearly complete resection or removal can be achieved | |
| Liver-directed therapy (transarterial embolization, ablative therapy, cytoreductive surgery) | 2B | 2B | ||
Note—PROMID = Placebo-Controlled, Double-Blind, Prospective Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors, LAR = long-acting repeatable, MTT = molecular targeted therapy.
National Comprehensive Cancer Network (NCCN) categories of evidence and consensus [4]: 1, on the basis of high-level evidence, there is NCCN consensus that the intervention is appropriate; 2A, on the basis of lower-level evidence, there is NCCN consensus that the intervention is appropriate; 2B, on the basis of lower-level evidence, there is NCCN agreement but not consensus that the intervention is appropriate; 3, on the basis of any level of evidence, there is major disagreement that the intervention is appropriate.
In the 2012 NCCN guidelines, MTTs were added to the recommended treatment of unresectable and metastatic pancreatic NETs with category 2A evidence (Table 3). MTTs for unresectable and metastatic NETs in gastrointestinal tract, however, were recommended with category 3 evidence. Liver-directed therapies require category 2B evidence for treatment of clinically significant hepatic metastases from GEP-NETs [4]. Considering these evidence levels, the use of MTTs for pancreatic NETs, either alone or in combination with other therapies, will continue to increase. Radiologists, as part of multidisciplinary teams, should understand these trends in clinical management and be familiar with the various therapeutic modalities and the effects of these treatments on imaging findings.
Advances in Treatment of Gastroenteropancreatic Neuroendocrine Tumors
Somatostatin Analogues
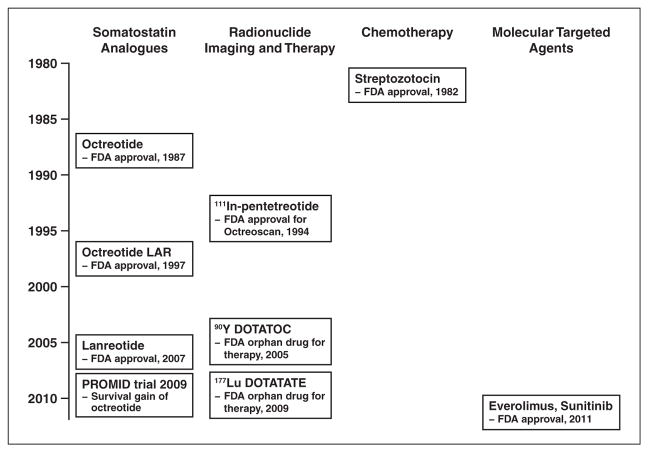
The expression of somatostatin receptors (SSTRs) is a unique characteristic of NETs that enables targeted therapy and functional imaging. Somatostatin is a peptide that binds SSTRs and stimulates hormonal activity [6]. The expression of SSTR is especially elevated in well-differentiated NETs in comparison with poorly differentiated neuroendocrine carcinomas [15, 16]. Octreotide, which targets SSTRs, has been used to control hormone-related symptoms since FDA approval in 1987 (Fig. 2). The once-monthly long-acting depot (also called long-acting repeatable [LAR]) form of octreotide approved by the FDA in 1997 is widely used [11]. Lanreotide, another somatostatin analogue targeting SSTR, is similar to octreotide in clinical efficacy. Several studies [17–19] have shown that in addition to controlling the effects of hormonal symptoms, octreotide has tumor-stabilizing effects. According to results of the Placebo-Controlled, Double-Blind, Prospective Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors (PROMID), the use of octreotide can have a survival advantage for patients with metastatic midgut carcinoid tumors [19].
Fig. 2.
Chart shows evolution of medical treatment of gastroenteropancreatic neuroendocrine tumors. Octreoscan is Covidien product. FDA = U.S. Food and Drug Administration, LAR = long-acting repeatable, PROMID = Placebo-Controlled, Double-Blind, Prospective Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors.
Peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogues adds therapeutic radiation effects to somatostatin analogues and is an effective option in therapy for unresectable and metastatic GEP-NETs. Since 1992, when the first PRRT was administered with 111In-labeled octreotide, PRRT agents such as 90Y-labeled octreotide and 177Lu-labeled octreotide have been developed. As of this writing, PRRT is not available in the United States [20].
Molecular Targeted Therapy
The goal of MTT is inactivation of overexpressed or overactivated signaling cascades to inhibit tumor growth and various functions of tumors by targeting growth factors, growth factor receptors, receptor tyrosine kinases, and intracellular signaling cascades [8]. Several growth-promoting targets are overexpressed in GEP-NETs, including vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and insulinlike growth factor 1 receptor (IGF-1R). The phosphatidylinositol-3′-kinase (PI3K)–Akt–mammalian target of rapamycin (mTOR) and the Ras–Raf–mitogen-activated protein extracellular signal-regulated kinase (MEK)-Erk1/2 intracellular signaling pathways are also important regulators of survival and proliferation of GEP-NETs activated by various growth factors [18] (Fig. 3).
Fig. 3.
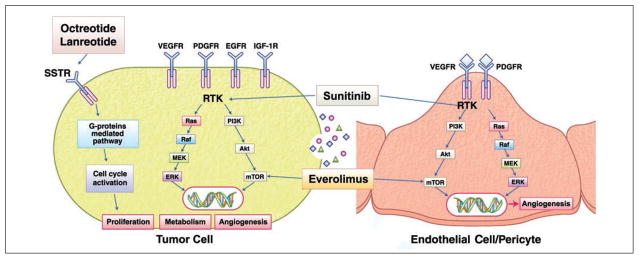
Diagram shows targeting intracellular signaling pathways in gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Several targets are overexpressed in GEP-NETs, including vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR), and insulinlike growth factor 1 receptor (IGF-1R), which play role in angiogenesis and tumor growth. Sunitinib inhibits receptor tyrosine kinases of VEGFR and PDGFR, and everolimus inhibits mammalian target of rapamycin (mTOR). Somatostatin receptor (SSTR) is also overexpressed in GEP-NETs and plays role in biologic functions, including hormone secretion and cell cycle activation. Somatostatin analogues such as octreotide and lanreotide inhibit release of hormones and have tumor stabilization effect. RTK = receptor tyrosin kinase, PIT3K = phosphatidylinositol-3′-kinase, MEK = mitogen-activated protein–ERK, ERK = extracellular signal-regulated kinase.
Sunitinib is a multitargeted tyrosine kinase inhibitor with activity against VEGFR, PDGFR, c-Kit, and Flt-3. Everolimus is an mTOR inhibitor blocking the PI3K-Akt-mTOR signaling cascade (Fig. 3). Both everolimus and sunitinib have been confirmed to have anti-tumor activity and to improve progression-free survival among patients with advanced pancreatic NETs [3, 4]. In addition, many targeted agents, such as bevacizumab, temsirolimus, imatinib, and sorafenib, are being investigated in clinical trials.
Cytotoxic Chemotherapy
Streptozocin-based chemotherapy for the treatment of pancreatic NETs was approved by the FDA in 1982. Streptozocin with 5-fluorouracil is still an effective option for unresectable and metastatic pancreatic NETs (category 2A), especially grade 2 tumors [11]. Commonly used regimens are streptozocin with 5-fluorouracil or doxorubicin, temozolomide with capecitabine, and cisplatin with etoposide [18]. However, cytotoxic chemotherapy has minimal benefit in the management of unresectable and metastatic gastrointestinal NETs (carcinoids) and is recommended if there is category 3 evidence (i.e., no other option is feasible) [4, 11].
Liver-Directed Therapies
Liver-directed therapies are cytoreductive surgery, ablative therapy (radiofrequency ablation, cryotherapy, microwave ablation), and transarterial embolization (TAE) therapy (chemoembolization, radioembolization). These therapies are recommended to patients with liver-predominant metastatic disease [4]. The rationale of these therapies is that aggressive debulking of the hepatic metastatic tumor volume has survival benefits despite the risks of invasive procedures and recurrence after treatment [21].
When surgery seems unfeasible or a patient has inoperable disease, ablative therapy may be considered if there are fewer than five liver metastatic lesions and they are smaller than 5 cm [22]. Repeated sessions of TAE therapy may be considered for patients with widespread liver metastases [18]. Although many protocols of TAE therapy have been reported in uncontrolled series, no single protocol shows confirmed superiority. Radioembolization with 90Y microspheres has been developed to combine a profound embolization effect with an internal radiation therapeutic effect. Currently, 90Y microspheres are approved as a medical device for the treatment of patients with liver metastases from colon cancer or unresectable hepatocellular carcinoma (HCC). The superiority of radioembolization over TAE has not been proved [18].
Role of Imaging
The traditional roles of CT and MRI, such as detection, differential diagnosis, staging, presurgical and preprocedural planning, and postsurgical and postprocedural assessment of tumors of pancreas and bowel have been extensively reviewed [23, 24] and can be applied to management of locoregional GEP-NETs [25].
For management of unresectable and metastatic GEP-NETs, the use of imaging to assess treatment response and identify complications and toxicities related to treatment is important but challenging. Conventional size criteria such as the Response Evaluation Criteria in Solid Tumors (RECIST) are widely used for assessment of the efficacy of cytotoxic chemotherapy [26]. However, accurate radiologic criteria have not been established for assessment of the treatment of patients with metastatic GEP-NETs treated with MTT. The Choi criteria used in gastrointestinal stromal tumors or modified RECIST used in HCC (mRECIST) may be alternative methods of response assessment (Appendix 2), but the utility of these methods in response assessment of GEP-NETs has not been proven [27].
APPENDIX 2.
Alternative Tumor Response Criteria in Management of Gastroenteropancreatic Neuroendocrine Tumors
| Status | Choi Criteria [35] | Modified RECIST Assessment for HCC [36] |
|---|---|---|
|
| ||
| Complete response | Disappearance of all lesions | Disappearance of intratumoral arterial enhancement in all target lesions and all nontarget lesions |
| No new lesions | ||
| Partial response | Decrease in size of 10% or more or a decrease in tumor attenuation (HU) of 15% or more at CT | At least a 30% decrease in the SLD of viable (arterially enhancing) target lesions, taking as reference the baseline SLD of target lesions |
| No new lesions | Persistence of one or more nontarget lesions | |
| No obvious progression of nonmeasurable disease | ||
| Stable disease | Does not meet criteria for complete response, partial response, or progression | Any cases that do not qualify for either partial response or progressive disease |
| Persistence of one or more nontarget lesions | ||
| Progressive disease | Increase in tumor size 10% or more and does not meet criteria for partial response by tumor attenuation at CT | An increase of at least 20% in the SLD of viable (enhancing) target lesions, taking as reference the SLD of viable (enhancing) target lesions |
| New lesions | Lesions recorded since treatment started | |
| New intratumoral nodules or increase in the size of existing intratumoral tumor nodules | New lesion > 1 cm meeting criteria for HCC Unequivocal progression of existing nontarget lesions | |
Note—RECIST = Response Evaluation Criteria in Solid Tumors, HCC = hepatocellular carcinoma; SLD = sum of longest diameters.
In clinical trials, it is imperative to adhere to predefined quantitative criteria. However, in daily practice at our cancer institute, a comprehensive approach that includes quantitative and qualitative image analysis and clinical assessments including tumor markers is frequently beneficial for more accurate assessment of treatment response. The complexity of treatment response assessment in the various therapeutic modalities and the unique toxicity profiles of each therapeutic modality highlight the importance of imaging in the era of MTT, as summarized in Table 4.
TABLE 4.
Imaging Findings of Treatment Response, Recurrence, and Toxicities of Various Therapies for Gastroenteropancreatic Neuroendocrine Tumors
| Therapy | Treatment Response Pattern | Recurrence | Radiologically Evident Toxicities and Complications |
|---|---|---|---|
|
| |||
| Molecular targeted therapy (sunitinib, everolimus) | Typical: decrease in density and stable to mild decrease in size | Typical: increase in size and density, new separate enhancing masses | Bowel complications (colitis, pneumatosis intestinalis, perforation) |
| Atypical: increase in size with decrease in density; increase in size with increase in density; appearance of previously inconspicuous liver lesions (pseudoprogression) | Atypical: new intratumoral nodule or nodules or increased tumor density without necessarily increased size of lesions | Cholecystitis, pancreatitis | |
| Thromboembolic events | |||
| Noninfectious pneumonitis | |||
| Somatostatin analogues (octreotide, lanreotide, pasireotide) | No change in tumor size or morphologic or enhancement pattern | Increase in size | Gallstone and calculous cholecystitis or cholangitis |
| Less commonly, mild decrease in size | New separate enhancing masses | Hematoma or abscess in injection sites | |
| Cytotoxic chemotherapy (streptozotocin-based regimen) | Decrease in size of tumors or necrosis | Increase in size | Immunosuppression: opportunistic infection or neutropenic colitis |
| New separate enhancing masses | Enterocolitis related to oral administration | ||
| Liver-directed therapy (transarterial embolization, ablative therapy, cytoreductive surgery) | Treated portions: complete or partial devascularization | Locally recurrent or residual tumor: solid enhancing intralesional or perilesional nodules or masses | Abscess, cholangitis, cholecystitis, hemorrhage, injury to adjacent organs |
Treatment Response Patterns
Conventional Medical Treatment
The common and expected imaging response pattern of metastatic GEP-NETs to somatostatin analogues is stable disease with no unequivocal changes in tumor size or morphologic and enhancement patterns because of the indolent course of GEP-NETs and tumor-stabilizing effect of octreotide [25]. Octreotide occasionally causes mild reduction of tumor burden (Fig. 4). The common response pattern of metastatic GEP-NETs to cytotoxic chemotherapeutic regimens is a decrease in the size of the tumor or necrosis, which is identical to that of conventional chemotherapy (Fig. 5). Progression of disease during octreotide or cytotoxic chemotherapy is seen as an increase in the size or number of metastatic lesions.
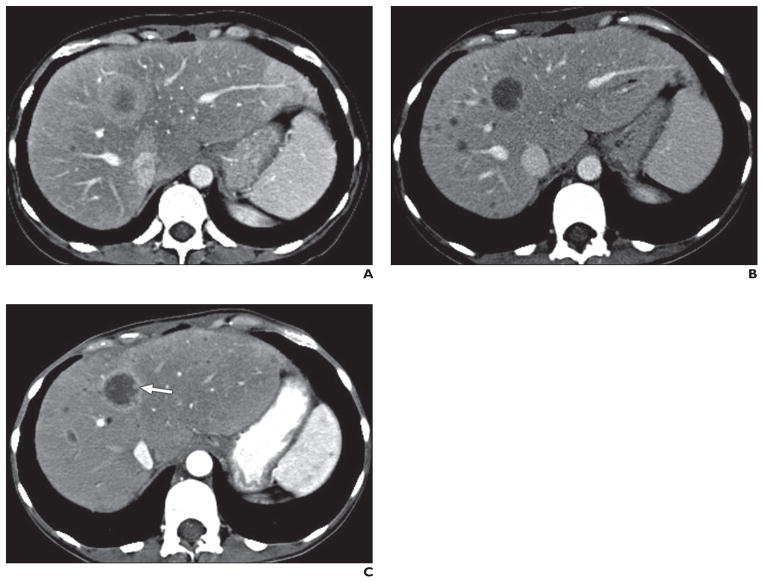
Fig. 4. 48-year-old man with asymptomatic mesenteric mass confirmed as metastatic neuroendocrine tumor (grade 1) and liver metastasis treated with octreotide.
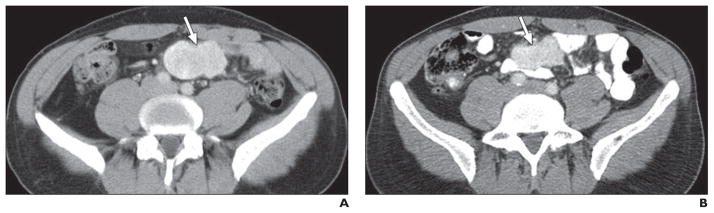
A, Baseline axial contrast-enhanced CT image shows well-defined solid hypervascular tumor (arrow) in mesentery. Liver metastatic lesions are not visible.
B, Axial contrast-enhanced CT image obtained 11 months after start of octreotide treatment shows mild decrease in size of tumor (arrow) without marked change in its enhancing and solid nature, likely representing tumor-stabilizing effect of octreotide, which can result in mild reduction of tumor size.
Fig. 5. 55-year-old man with pancreatic neuroendocrine tumor (grade 2) and multiple liver metastatic lesions treated with cytotoxic chemotherapy.
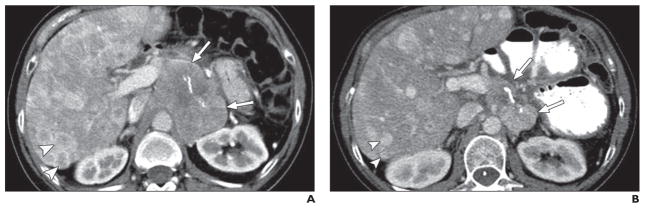
A, Axial contrast-enhanced CT image before treatment shows large, solid mass (arrows) in pancreatic tail and multiple hypervascular liver metastatic lesions (arrowheads). Internal linear calcification is present in pancreatic mass.
B, Axial contrast-enhanced CT image obtained 3 months after treatment shows marked decrease in size of pancreatic mass (arrows) and liver metastatic lesions (arrowheads) without change in solid enhancing nature of tumors, likely representing partial response to cytotoxic chemotherapy.
Molecular Targeted Therapy
GEP-NETs are primarily hypervascular tumors related to overexpression of VEGFR or PDGFR pathways. Sunitinib directly targets receptor tyrosine kinases of VEGFR and PDGFR; everolimus targets the common signaling cascades of these pathways; and both act as cytostatic agents [8]. Therefore, the typical CT response pattern to these targeted agents is a decrease in tumor attenuation and enhancement with treatment and a stable to mild decrease in tumor size. However, various treatment response patterns are frequently encountered, especially during assessment of the treatment response of liver metastatic lesions at contrast-enhanced CT. In our experience, there are three representative atypical response patterns and a feature of atypical progressive disease [28] (Table 4).
Increase in size with decrease in density
Sometimes tumors have decreased density suggestive of response to treatment but have increased size, which may lead to misinterpretation of tumor progression according to size criteria [27] (Fig. 6). These changes may be attributed to cystic change, intratumoral edema, or visualization of previously unidentified portions of isodense tumor [28].
Fig. 6. 50-year-old woman with multiple liver metastatic lesions from pancreatic neuroendocrine tumor treated with sunitinib.
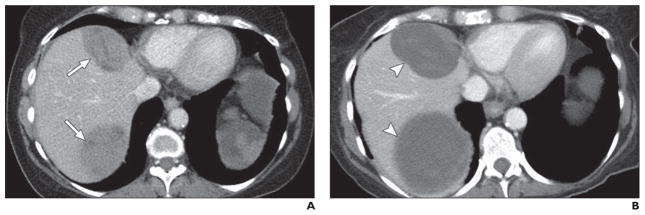
A, Axial contrast-enhanced CT image obtained before sunitinib treatment shows two solid masses (arrows) in liver.
B, Axial contrast-enhanced CT image obtained 6 months after sunitinib treatment shows masses (arrowheads) are larger. However, overall tumor attenuation and enhancing solid component have decreased, suggesting cystic change responding to treatment.
Increase in size with increase in density
MTTs with antiangiogenic action sometimes cause intratumoral hemorrhage, which can result in an increase in density with a variable size change. If tumor size and density are increased by hemorrhage (usually early-stage hemorrhage), accurate interpretation of treatment response is difficult and may be confused with progression, even when new criteria such as the Choi criteria and mRECIST are used. Useful hints suggestive of intratumoral hemorrhage are intratumoral fluid-fluid levels and internal changes in tumor architecture (e.g., a homogeneous lesion becomes heterogeneous) (Fig. 7). Intratumoral hemorrhage can be confirmed with additional imaging with unenhanced CT or MRI. Early-stage intratumoral hemorrhage manifests itself as hyperattenuation on unenhanced CT images and hyperintensity on T1-weighted MR images. These lesions usually have decreased size and density at follow-up imaging but sometimes exhibit fluctuation in size and density due to recurrent or intermittent hemorrhage [28].
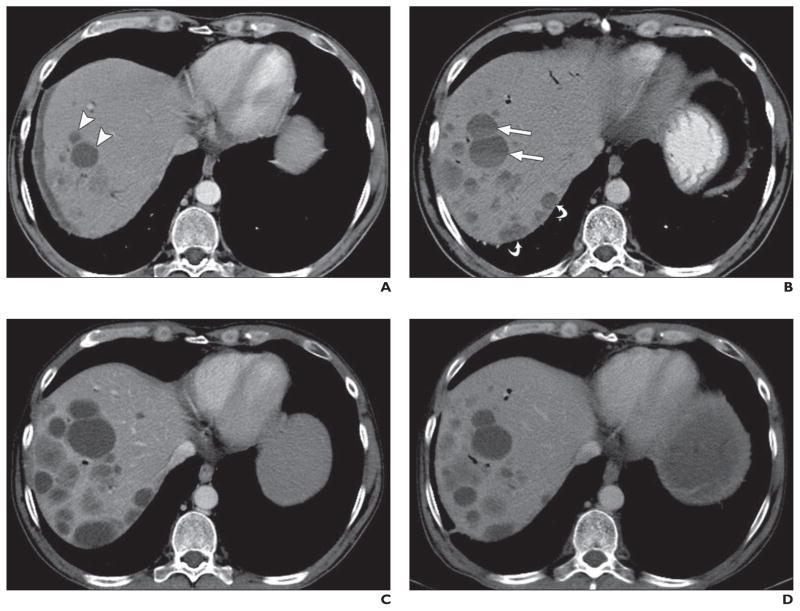
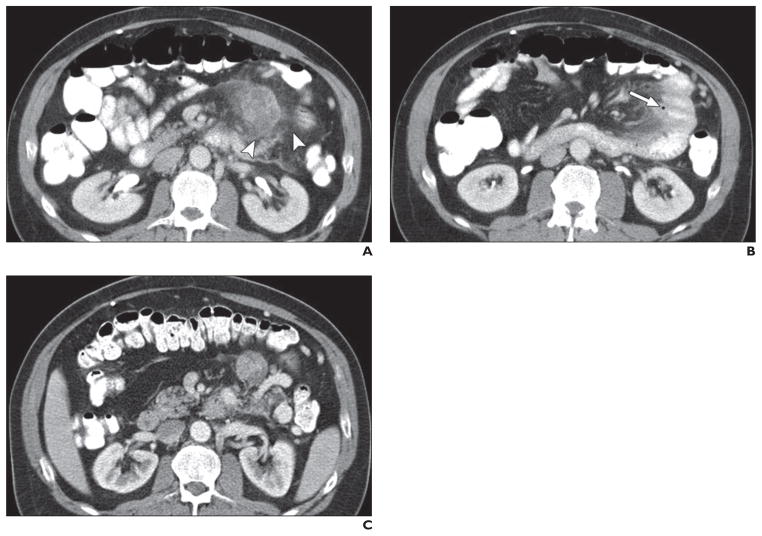
Fig. 7. 63-year-old man with liver metastatic lesions from pancreatic neuroendocrine tumor treated with sunitinib.
A, Axial contrast-enhanced CT image obtained before start of sunitinib treatment shows two low-attenuation lesions (arrowheads) in segment VIII of liver and multiple subtle hypodense lesions in segment VII, representing liver metastases.
B, Axial contrast-enhanced CT image obtained 3 months after start of sunitinib therapy shows two low-attenuation lesions in hepatic segment VIII have increased in size and attenuation and have fluid-fluid levels (straight arrows) indicative of intratumoral hemorrhage. Newly apparent low-attenuation peripheral lesions in hepatic segment VII (curved arrows) likely represent pseudoprogression. Blood chromogranin A level has decreased from 800 to 400 U/L, and sunitinib treatment was continued. Pneumobilia is secondary to interim biliary stent insertion.
C, Axial contrast-enhanced CT image obtained 6 months after start of sunitinib therapy shows hypodense lesions have grown, but attenuation has decreased as in cystic change. These findings were regarded as atypical response pattern, and sunitinib treatment was continued.
D, Axial contrast-enhanced CT image obtained 9 months after start of sunitinib therapy shows decrease in size of low-attenuation lesions.
Appearance of previously inconspicuous liver lesions
Sometimes isodense hepatic metastasis unidentified because of lack of contrast with normal parenchyma at baseline can be revealed as new low-attenuation lesions after treatment with MTTs (Fig. 8). These seemingly new lesions tend to further decrease in size and density on follow-up images. This is a potential pitfall that frequently leads to misinterpretation of progressive disease (pseudoprogression) and premature cessation of the current regimen, which is actually effective [28].
Fig. 8. 49-year-old woman with multiple liver metastatic lesions from pancreatic neuroendocrine tumor treated with sunitinib.
A, Baseline axial contrast-enhanced CT image shows large enhancing mass in segment IV of liver.
B, Follow-up contrast-enhanced CT image obtained 3 months after start of sunitinib treatment shows index mass in segment IV has decreased in size and attenuation, representing good response to treatment. However, multiple hypoattenuating nodules are newly apparent. Serum chromogranin A concentration decreased from 1090 to 210 U/L. Hence new liver nodules were considered pseudoprogression, and sunitinib treatment was continued.
C, Follow-up contrast-enhanced CT image obtained 14 months after start of sunitinib treatment shows reappearance of rim enhancement in index mass and development of several intratumoral nodules (arrow), representing true progression of tumor.
Atypical but true tumor progression
Atypical but true tumor progression can manifest itself as a new intratumoral nodule or nodules, increased tumor attenuation, or reappearance of rim enhancement without an increase in the size of the lesions [27] (Fig. 7).
If there is uncertainty in characterizing the treatment response pattern or if an atypical treatment response is suspected, radiologists in concert with referring oncologists should use all available imaging and clinical information for the most comprehensive treatment assessment. We should also consider the overall trend of response pattern [28]. If the pattern is mixed, in which most of the masses have decreased size and density and a few masses have grown or increased in density, then the findings of increased size or density may well represent an atypical response to MTT. In the case of pseudoprogression of liver metastasis, it is helpful to correlate the response pattern with tumors outside the liver. For example, if an extrahepatic lesion such as a primary tumor or lymph node metastatic lesion has decreased in density or size, we should consider the possibility of pseudoprogression when evaluating newly apparent liver lesions. Clinical and biochemical correlation is also helpful in cases of atypical response patterns. Tumor markers in blood (chromogranin A) and urine (5-hydroxyindoleacetic acid) are frequently used in monitoring treatment response. If there are no immediate management-related implications, reassessment at follow-up imaging in 6–12 weeks can be an alternative means of assessing response, depending on the clinical circumstances, because NETs are generally indolent or slowly progressing tumors [28].
Liver-Directed Therapies
The response patterns of GEP-NETs to ablative or TAE therapy are identical to those of HCC and liver metastasis from colon cancer in that the treated portions of tumors exhibit complete or partial devascularization (lack of contrast enhancement), and residual or recurrent tumors are solid enhancing nodules or masses [29]. For quantitative response criteria, the European Association for the Study of the Liver (EASL) guidelines for assessment of locoregional HCC treatment can be used. In these guidelines foci of tumor enhancement on CT or MR images are considered viable, whereas nonenhancing regions reflect tissue necrosis. The EASL guidelines define complete response as the absence of enhancing areas; partial response as a greater than 50% decrease in the enhanced areas; progressive disease as a greater than 25% increase in the size of a single, measurable lesion or the appearance of new lesions; and stable disease as a tumor response between partial response and progressive disease [30].
Monitoring Toxicities and Complications
Conventional Medical Treatment
An important radiologically evident adverse event in octreotide therapy is biliary calcium secretion and gallstone formation, which can result in calculous cholecystitis and cholangitis [31]. Radiologists should monitor the presence of gallstones and associated biliary dilatation or inflammation in patients treated with octreotide.
Somatostatin analogues are administered by subcutaneous injection (short-acting octreotide) or intramuscular injection (long-acting octreotide depot form). Octreotide injection can cause subcutaneous or intramuscular nodular opacities and accumulation of intralesional air in the anterior abdominal wall or gluteal area depending on the injection site. Local hemorrhage and, rarely, abscess formation can occur at the injection site, especially intramuscular injection sites of the long-acting octreotide depot form (Fig. 9).
Fig. 9.
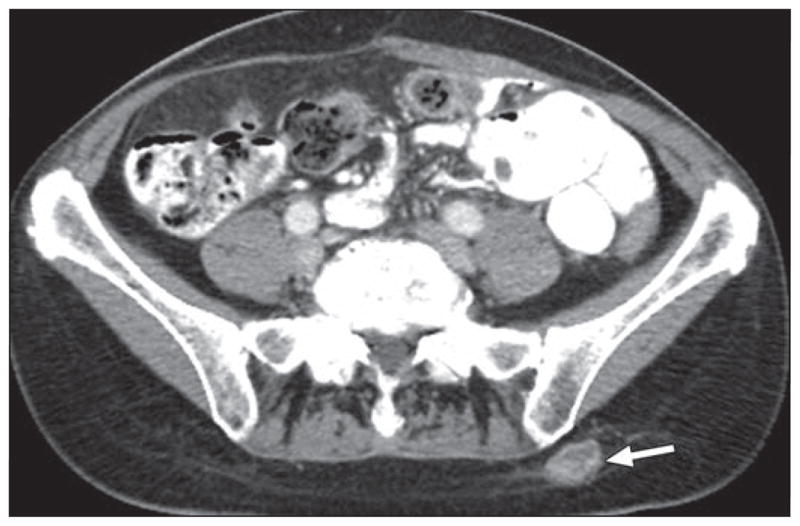
58-year-old woman with multiple liver metastatic lesions from neuroendocrine tumor in small bowel (not shown) treated with long-acting depot form of octreotide. Axial contrast-enhanced CT image shows ovoid high-attenuation subcutaneous soft-tissue-density lesion (arrow) in left gluteal area. Lesion corresponds to point of local tenderness at injection site, consistent with hematoma.
In patients treated with cytotoxic chemotherapy, the radiologically evident drug toxicities are related to immunosuppression and include opportunistic infections and neutropenic colitis. Oral agents such as temozolomide can cause enterocolitis related to the route of administration [32].
Molecular Targeted Therapy
In patients with GEP-NETs treated with sunitinib or everolimus, some overlapping and some separate radiologic patterns of drug toxicities are encountered (Table 4). Both sunitinib and everolimus have antiangiogenic activity and share toxicities related to ischemia and endothelial injury [32, 33]. Bowel complications such as ischemic colitis, pneumatosis intestinalis, and bowel perforation can occur as consequences of bowel-wall ischemia (Fig. 10). Less commonly, cholecystitis occurs as a consequence of gallbladder ischemia. Arterial and venous thromboembolic events also occur as a result of endothelial injury in patients treated with sunitinib. Noninfectious pneumonitis occurs in 2–36% of patients treated with everolimus (Fig. 11). This complication is dose dependent and is graded 1–4 [34]. These drug toxicities can be fatal when they are unrecognized and MTTs are continued. Early recognition by radiologists is extremely important for prompt management and to minimize complications [32].
Fig. 10. 59-year-old woman with acute abdominal pain and pancreatic neuroendocrine tumor and mesenteric nodal metastatic lesions treated with sunitinib for 7 months.
A, Axial contrast-enhanced CT image obtained at acute presentation shows haziness (arrowheads) around mesenteric mass and adjacent jejunal loop.
B, Axial contrast-enhanced CT image obtained at same examination as A at more inferior level shows small sealed perforation along mesenteric border of proximal jejunal loop (arrow). These findings were regarded as complication of sunitinib treatment, which include small-bowel ischemia and perforation. Patient was treated conservatively with antibiotics, and sunitinib treatment was stopped.
C, Axial contrast-enhanced CT image obtained 1 month after A and B shows resolution of bowel perforation and mesenteric haziness.
Fig. 11. 60-year-old man with metastatic pancreatic neuroendocrine tumor treated with everolimus.
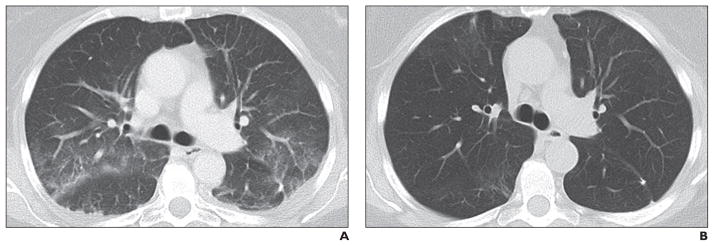
A, Axial CT image (lung window) obtained 6 months after start of everolimus therapy when patient reported nonproductive cough shows newly apparent multifocal sub-pleural ground-glass opacities along bronchovascular bundles, likely representing mild drug-associated noninfectious pneumonitis.
B, Follow-up CT image obtained 3 months after everolimus cessation shows ground-glass opacities have resolved.
Liver-Directed Therapies
The complications of TAE and ablative therapies, including abscess, cholangitis, cholecystitis, hemorrhage, and injury to adjacent organs, are well established and have been extensively reviewed [29, 30]. In case of transarterial radioembolization for liver metastasis, local radiation effects lead to peripheral ring enhancement around the radioembolized tumor. This enhancement may be incorrectly ascribed to disease progression of hypervascular metastasis. Additional MRI or reassessment with follow-up CT can be helpful [30].
Summary
The treatment of patients with GEP-NETs has evolved and will continue to change with new therapeutic options. Various treatment options, including the use of MTTs, have been incorporated into clinical practice. The dramatic changes in management of GEP-NETs and sophisticated guidelines can be challenging. Radiologists in collaboration with oncologists, surgeons, and pathologists should adopt a multidisciplinary therapeutic approach that includes use of imaging and clinicopathologic data for optimized, focused care of patients with GEP-NETs.
Acknowledgments
M. Nishino supported by 1K23CA157631 (NCI) from the National Institutes of Health.
References
- 1.Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012;24:46–55. doi: 10.1097/CCO.0b013e32834c554d. [DOI] [PubMed] [Google Scholar]
- 2.Oberg K, Akerstrom G, Rindi G, Jelic S. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v223–v227. doi: 10.1093/annonc/mdq192. [DOI] [PubMed] [Google Scholar]
- 3.Ramage JK, Ahmed A, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2012;61:6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulke MH, Benson AB, 3rd, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012;10:724–764. doi: 10.6004/jnccn.2012.0075. [DOI] [PubMed] [Google Scholar]
- 5.Rindi G, Kloppel G. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology. 2004;80(suppl 1):12–15. doi: 10.1159/000080733. [DOI] [PubMed] [Google Scholar]
- 6.Anlauf M. Neuroendocrine neoplasms of the gastroenteropancreatic system: pathology and classification. Horm Metab Res. 2011;43:825–831. doi: 10.1055/s-0031-1291307. [DOI] [PubMed] [Google Scholar]
- 7.Howard S, O’Regan K, Jagannathan J, Krajewski K, Giardino A, Ramaiya N. Extrapulmonary small cell carcinoma: a pictorial review. AJR. 2011;197:W392–W398. doi: 10.2214/AJR.10.5757. web. [DOI] [PubMed] [Google Scholar]
- 8.Dong M, Phan AT, Yao JC. New strategies for advanced neuroendocrine tumors in the era of targeted therapy. Clin Cancer Res. 2012;18:1830–1836. doi: 10.1158/1078-0432.CCR-11-2105. [DOI] [PubMed] [Google Scholar]
- 9.Burns WR, Edil BH. Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34. doi: 10.1007/s11864-011-0172-2. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 11.Oberg K. Neuroendocrine tumors of the digestive tract: impact of new classifications and new agents on therapeutic approaches. Curr Opin Oncol. 2012;24:433–440. doi: 10.1097/CCO.0b013e328353d7ba. [DOI] [PubMed] [Google Scholar]
- 12.Klöppel G, Couvelard A, Perren A, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–166. doi: 10.1159/000182196. [DOI] [PubMed] [Google Scholar]
- 13.Parekh JR, Wang SC, Bergsland EK, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas. 2012;41:840–844. doi: 10.1097/MPA.0b013e31823cdaa0. [DOI] [PubMed] [Google Scholar]
- 14.Balachandran A, Tamm EP, Bhosale PR, et al. Pancreatic neuroendocrine neoplasms: diagnosis and management. Abdom Imaging. 2013;38:342–357. doi: 10.1007/s00261-012-9923-1. [DOI] [PubMed] [Google Scholar]
- 15.Gatto F, Hofland LJ. The role of somatostatin and dopamine D2 receptors in endocrine tumors. Endocr Relat Cancer. 2011;18:R233–R251. doi: 10.1530/ERC-10-0334. [DOI] [PubMed] [Google Scholar]
- 16.Rust E, Hubele F, Marzano E, et al. Nuclear medicine imaging of gastro-entero-pancreatic neuroendocrine tumors: the key role of cellular differentiation and tumor grade—from theory to clinical practice. Cancer Imaging. 2012;12:173–184. doi: 10.1102/1470-7330.2012.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:2963–2970. doi: 10.3748/wjg.v16.i24.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auernhammer CJ, Goke B. Therapeutic strategies for advanced neuroendocrine carcinomas of jejunum/ileum and pancreatic origin. Gut. 2011;60:1009–1021. doi: 10.1136/gut.2009.204453. [DOI] [PubMed] [Google Scholar]
- 19.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 20.Kvols LK, Revisiting CG. Moertel’s land of small tumors. J Clin Oncol. 2008;26:5005–5007. doi: 10.1200/JCO.2008.19.2161. [DOI] [PubMed] [Google Scholar]
- 21.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 22.Vogl TJ, Naguib NN, Zangos S, Eichler K, Hedayati A, Nour-Eldin NE. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol. 2009;72:517–528. doi: 10.1016/j.ejrad.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am. 2010;90:235–249. doi: 10.1016/j.suc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Tamm EP, Balachandran A, Bhosale PR, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50:407–428. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Horton KM, Kamel I, Hofmann L, Fishman EK. Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR. 2004;182:559–567. doi: 10.2214/ajr.182.3.1820559. [DOI] [PubMed] [Google Scholar]
- 26.Sundin A, Rockall A. Therapeutic monitoring of gastroenteropancreatic neuroendocrine tumors: the challenges ahead. Neuroendocrinology. 2012;96:261–271. doi: 10.1159/000342270. [DOI] [PubMed] [Google Scholar]
- 27.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR. 2012;198:737–745. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinagare AB, Jagannathan JP, Krajewski KM, Ramaiya NH. Liver metastases in the era of molecular targeted therapy: new faces of treatment response. AJR. 2013;200:W15–W28. doi: 10.2214/AJR.12.9498. web. [DOI] [PubMed] [Google Scholar]
- 29.Steward MJ, Warbey VS, Malhotra A, Caplin ME, Buscombe JR, Yu D. Neuroendocrine tumors: role of interventional radiology in therapy. RadioGraphics. 2008;28:1131–1145. doi: 10.1148/rg.284075170. [DOI] [PubMed] [Google Scholar]
- 30.Kim KW, Lee JM, Choi BI. Assessment of the treatment response of HCC. Abdom Imaging. 2011;36:300–314. doi: 10.1007/s00261-011-9683-3. [DOI] [PubMed] [Google Scholar]
- 31.Moser AJ, Giurgiu DI, Morgenstern KE, Abedin ZR, Roslyn JJ, Abedin MZ. Octreotide stimulates Ca++ secretion by the gallbladder: a risk factor for gallstones. Surgery. 1999;125:509–513. [PubMed] [Google Scholar]
- 32.Chikarmane SA, Khurana B, Krajewski KM, et al. What the emergency radiologist needs to know about treatment-related complications from conventional chemotherapy and newer molecular targeted agents. Emerg Radiol. 2012;19:535–546. doi: 10.1007/s10140-012-1052-1. [DOI] [PubMed] [Google Scholar]
- 33.Howard SA, Krajewski KM, Thornton E, et al. Decade of molecular targeted therapy: abdominal manifestations of drug toxicities–what radiologists should know. AJR. 2012;199:58–64. doi: 10.2214/AJR.11.7432. [DOI] [PubMed] [Google Scholar]
- 34.Albiges L, Chamming’s F, Duclos B, et al. Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:1943–1953. doi: 10.1093/annonc/mds115. [DOI] [PubMed] [Google Scholar]
- 35.Choi H, Charnsangavej C, Faria SC, et al. Correlation of CT and PET in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new CT response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 36.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]