Abstract
Hemochromatosis is an inherited genetic disorder of iron metabolism which can also occur as a secondary result of iron-overload. It leads to organ damage such as cardiomyopathy, liver cirrhosis, hypogonadism, and diabetes. This paper discusses a case of secondary hemochromatosis associated with repeated transfusions, presenting as asymptomatic hypoparathyroidism and subclinical hypothyroidism with multiple organ involvement. The 29-year-old female, who had severe aplastic anemia, received multiple transfusions totaling approximately 1,400 units of red blood cells over 15 years. During her routine laboratory examination, hypocalcemia was detected with decreased intact parathyroid hormone and increased thyroid stimulating hormone. Serum ferritin, iron, and total iron binding capacity had increased to 27,583.03 ng/mL, 291 µg/dL, and 389 µg/dL, respectively. She had unusually bronze skin and computed tomography revealed iron deposition in the thyroid, liver, and heart. Multiorgan involvement as seen in this case is rare in hemochromatosis associated with secondary transfusions. To the best of the author's knowledge, this is the first case report in Korea of hypoparathyroidism and subclinical hypothyroidism due to iron deposition in the parathyroid and thyroid gland.
Keywords: Hypoparathyroidism, Hypothyroidism, Hemochromatosis
INTRODUCTION
Hemochromatosis is a hereditary or secondary disorder resulting from iron-overload and iron-impregnation in the organs, leading to organ damage [1]. Hereditary hemochromatosis is an autosomal recessive disease common in Caucasians and caused by a gene mutation related to iron absorption in the bowels. Some case reports from Korea suggest that end-stage renal disease patients with hemodialysis or patients with aplastic anemia had secondary hemochromatosis caused by repeated transfusions [2]. Unlike hereditary hemochromatosis where iron is impregnated in parenchymal cells, repeated transfusion induced-iron-overload mainly impregnates iron into reticuloendothelial cells which has been considered to be less harmful organ failure than parenchymal iron accumulation [3]. The authors report a case of a patient with aplastic anemia who received a total of approximately 1,400 units of red blood cells (RBCs) over 15 years. She was admitted for asymptomatic hypocalcaemia and diagnosed with secondary hemochromatosis accompanied by hypoparathyroidism, hypothyroidism, hepatic dysfunction, and cardiac dysfunction. To the best of the author's knowledge, this is the first case report of secondary hemochromatosis presenting as hypoparathyroidism and hypothyroidism.
CASE REPORT
A 29-year-old female admitted to our hospital for evaluation of asymptomatic hypocalcemia. Fifteen years ago she was diagnosed with aplastic anemia, treated with antilymphocyte globulin and antithymocyte globulin, and had routine transfusions of RBCs of 2 units per week (about 1,400 units over 15 years). In addition, starting 10 years ago, selective iron-chelating agent (deferasirox 1,500 mg/day) was administered due to increased serum ferritin levels. Continued hypocalcaemia was detected in blood tests at regular follow-up visits and the patient visited the endocrinologic department as an outpatient for additional examinations and treatment. She has no histories of cervical irradiation, thyroid disease or related family history. She had a blood pressure of 120/80 mm Hg, pulse rate of 78 beats per minute, temperature of 36.8℃, and a respiratory rate of 20 breaths per minute. Her skin was unusually dark in color with conjunctiva pallor. Chest examination showed that breathing was clear, heartbeat was regular and no unusual sounds were detected. Her abdomen was flat and soft with no unusual sounds, and the liver and spleen were not palpated. Neither leg had pitting edema. Neurological tests showed no local neurological symptoms, Chvostek sign or Trousseau sign.
In the laboratory findings, peripheral blood test indicated a white blood cell count of 700/mm3 (neutrophil 150), hemoglobin level of 8.0 g/dL, and platelet count of 37,000/mm3. A serum biochemical test reported blood urea nitrogen levels of 10.1 mg/dL, serum creatinine of 0.7 mg/dL, albumin levels of 3.9 g/dL, aspartate aminotransferase levels of 53 IU/L, alanine aminotransferase levels of 60 IU/L, alkaline phosphatase of 94 IU/L, and total bilirubin of 0.5 mg/dL. In a serum electrolyte test, sodium level was 140 mEq/L and potassium level was 4.3 mEq/L, both of which are in the normal range. However, total calcium was 6.7 mEq/dL (albumin corrected calcium 6.78 mEq/dL; normal range, 8.4 to 10.2), and ionized calcium was 1.78 mEq/L (normal range, 2.2 to 2.6). Serum ferritin levels were 27,583.03 ng/mL (normal range, 4.63 to 274.6), iron levels were 291 µg/dL (normal range, 65 to 157), and total iron binding capacity was 389 µg/dL (normal range, 256 to 426) which showed an increase. The transferrin saturation level was high to 74.8% (normal range, 22% to 46%).
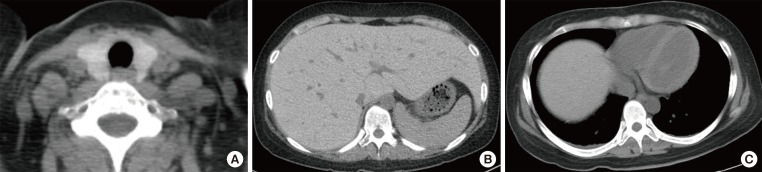
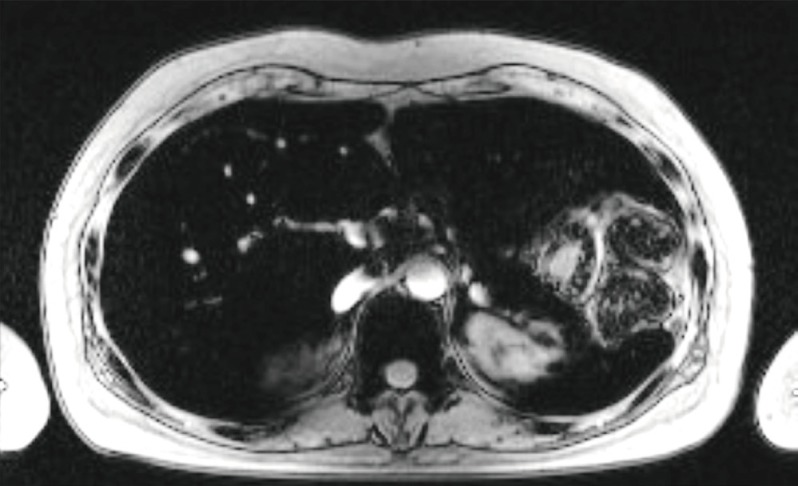
She was diagnosed with hypocalcaemia induced by hypoparathyroidism based on test results showing intact parathyroid hormone (iPTH) levels of 31 pg/mL (normal range, 9 to 55), 25(OH)VitD of 7.03 ng/mL (normal range, 4.8 to 52.8), and 1,25(OH)2VitD levels of 36.2 pg/mL (normal range, 25.1 to 66.1). Although 1,000 mg of calcium carbonate and 0.5 µg of alfacalcidol were given twice a day, hypocalcaemia continued with increased levels of 24 hours urine calcium (700 mg/day) and 24 hours urine creatinine (0.99 g/day); thiazide 12.5 mg was also administered to maintain the total calcium at 7.5 to 8.0 mEq/dL and ionized calcium at 1.9 to 2.2 mEq/L. Tests were performed to determine if the hemochromatosis had spread to other organs; chest X-rays showed no signs of cardiomegaly, but computed tomography (CT) before contrast enhancement showed thyroid (Hounsfield unit 157.0) and liver (Hounsfield unit 136.0), as well as a increased signal intensity associated with the cardiac muscle and enlarged inferior vena cava and hepatic veins (Fig. 1). The T2-weighted magnetic resonance imaging (MRI) of the abdomen revealed a dark signal intensity in the liver, spleen, bone marrow, and pancreas, leading to the secondary diagnosis of hemochromatosis (Fig. 2). Organs showing signs of iron impregnation were tested for function; the thyroid function test showed elevated levels of thyroid stimulating hormone (TSH, 17.18 µIU/mL; normal range, 0.4 to 4.8) with free thyroxine levels of 1.28 ng/dL (normal range, 0.8 to 1.71), and triiodothyronine levels of 1.43 ng/mL (normal range, 0.6 to 1.6). Thyroid peroxidase antibody and thyroglobulin antibody were both negative which resulted in apparent hypothyroidism caused by hemochromatosis. The patient was receiving low-dose of thyroid hormone (levothyroxine 50 µg/day), but her TSH levels were still over 10 µIU/mL at follow-up. Therefore the dose of the hormone was gradually increased. The most recent test result showed TSH levels of 8.12 µIU/mL, and the dose of thyroid hormone was increased to 150 µg/day for follow-up observation. Echocardiography revealed dilated left ventricular chamber size (left ventricular end diastolic diameter 54 mm) with global hypokinesia, and a moderate to severe left ventricular systolic dysfunction (ejection fraction 23%), which suggests congestive heart failure with dilated cardiomyopathy and restrictive diastolic dysfunction induced by hemochromatosis. The patient is medicated with digoxin 0.1875 mg, candesartan 8 mg, bisoprolol 2.5 mg, and spiractone 25 mg without any significant symptoms and is on follow-up observation as an outpatient.
Fig. 1.
Nonenhanced computed tomography scan revealed the high intensity of (A) the thyroid gland, (B) liver parenchyma with dilated inferior vena cava and intrahepatic vain, and (C) myocardium.
Fig. 2.
T2-weighted magnetic resonance imaging revealed the dark signal intensity of liver, pancreas, spleen, and bone marrow.
DISCUSSION
With this patient, secondary hemochromatosis occurred, induced by iron over-absorption due to repeated transfusion for aplastic anemia. While on follow-up observation, she was diagnosed with hypocalcaemia caused by hypoparathyroidism, subclinical hypothyroidism, hepatic failure, and cardiac dysfunction.
Excessive iron absorption is known to cause various organ failures due to iron accumulation of the multiple organs, but it is rare to find clinically severe organ failure cases that involve secondary hemochromatosis induced by transfusion [3]. Hemochromatosis is a genetic or secondary disorder caused by damaged cells and organ failure after iron overload within the parenchyma of the organ [1]. Patients with symptoms and signs such as increased levels of iron concentration in the blood, iron-binding capacity, and serum ferritin concentration, hepatomegaly, pigmented skin, diabetes mellitus, heart disease, arthritis, and lower urinary tract symptoms can be diagnosed if the patient has a secondary cause that induces iron overload or if iron impregnation is confirmed by tissue biopsy-with HFE genetic mutation [4]. Normally the human body maintains iron levels at 3 to 4 g. One milligram of iron for males and 1.5 mg for menstruating females are lost every day, but equal amounts of iron are absorbed by intestinal mucosa [5]. However, in the case of hemochromatosis, 4 mg of iron are absorbed per day, which increases iron levels in the blood, leading to increased blood ferritin levels and iron-impregnation of the organs. Hereditary hemochromatosis was transmitted as an autosomal recessive trait associated with the HLA-A locus on the short arm of chromosome 6. It accounts for 80% to 90% of all cases of hereditary hemochromatosis [4]. Secondary factors include over-absorption of iron due to inadequate production of RBCs in cases of Mediterranean anemia and sideroblastic anaemia. In addition, as in this case, repeated transfusions can cause hemochromatosis [2]. When 1 unit of RBC is transfused it contains 200 to 250 mg of iron. If RBC production is inadequate because of bone marrow failure such as aplastic anemia, infused iron cannot be used to produce RBCs and instead is captured by macrophages. When this iron-capturing behavior of macrophages reaches its limit, overloaded iron is impregnated into multiple organs such as the liver, heart, spleen, pancreas, and bone marrow, which results in multiple organ failures [3].
In the early stages of hemochromatosis, fatigue or weakness can be accompanied by arthritis or skin pigmentation. Increased levels of iron impregnation in organs such as the liver, heart, pancreas, thyroid, parathyroid, and pituitary gland can cause functional failures [6]. One of the organs commonly impregnated is the liver, and impregnation of iron in the parenchyma of the liver can cause hepatomegaly, which is associated with increased levels of hepatic enzyme and can cause of liver cirrhosis and hepatocellular carcinoma. Hepatocellular carcinoma, cardiomyopathy, conduction disturbance, and congestive heart failure can increase mortality in patients with hemochromatosis. Regular check-ups with follow-up visits are necessary to prevent these issues [7,8].
Diabetes mellitus is the most common disease associated with endocrine system abnormalities, and is due to reduced insulin resistance and secretion induced by siderosis in liver and pancreas β-cells [6]. Gonadotropin deficiency commonly includes the entire pituitary hormone, resulting in impotency, menstrual irregularities, and decreased libido [9]. Siderosis can also cause functional failures in the adrenal, parathyroid, and thyroid, although these issues are less common than diabetes or gonadotropin deficiency [10,11].
There are not a sufficient number of studies on the correlation between multiple organ damage and degree and period of siderosis. In 2001, Kwon et al. [12] reported a correlation between total volume of blood transfusion and increased serum ferritin, but there was no correlation between total volume of blood transfusions and endocrine system complications in 14 pediatric patients with repeated transfusions. Chern and Lin [13] reported that 10.7% (3/28) of patients who received blood transfusions over an extended period acquired hypoparathyroidism, which is often accompanied by other issues such as hypogonadism and diabetes mellitus. In the case study being presented here, the patient was diagnosed with hypoparathyroidism from hypocalcaemia without markedly increased iPTH, and organ examinations confirmed iron accumulation to the thyroid, liver, and cardiac muscle. This indicates that hemochromatosis patients with the rare complication of hypothyroidism and hypoparathyroidism need to be tested for other organ functions and treated accordingly. In the future, larger-scale prospective studies are necessary to determine correlations between symptoms and the volume and period of blood transfusions that accompany multiple organ failures.
Hemochromatosis can be correctly diagnosed if siderosis is confirmed from a liver tissue biopsy. However, as shown in this case, for a patient with low platelet levels, even with repeated platelet transfusions, an invasive examination is difficult to perform. Instead of a liver tissue biopsy, CT before contrast enhancement and MRI can be useful to examine iron impregnation. Bell et al. [14] reported a correlation between blood ferritin levels and signal intensity of the liver in CT, and suggested that if serum ferritin levels are above 1,000 ng/mL. CT can replace invasive biopsy and be helpful in diagnosing hemochromatosis. However, although CT has a high specificity (96%), its sensitivity is relatively low (63%) for diagnosing secondary hemochromatosis [15,16]. Meanwhile, MRI has both high specificity and sensitivity for diagnosing hemochromatosis and evaluating the treatment progress [17]. In this case study, CT before contrast enhancement showed markedly elevated Hounsfield units in the liver (136.0) compared to the normal range (61.0±9.0) [14], and a T2-weighted liver MRI revealed darker sections of liver, spleen, and bone marrow, which also suggest hemochromatosis. In the case presented here, hypothyroidism related to other factors was excluded since the patient had multiple functional failures of the organs caused by hemochromatosis. The CT results included elevated Hounsfield units of the thyroid (157) compared to normal range (118.1±12.2), Graves disease level of 69.5±17.6, and Hashimoto thyroiditis of 61.4±9.1 [18]. The elevated Hounsfield units led to the patient's diagnosis of siderosis-induced hypothyroidism. In terms of hypoparathyroidism, previous studies also reported hemochromatosis in the study subjects. Due to difficulties with imaging and biopsy examinations, hypoparathyroidism induced by hemochromatosis was diagnosed although the patients presented with hypocalcemia without increased iPTH [10,11,13,19,20].
Patients with secondary hemochromatosis induced from repeated transfusions may have irreversible functional disorders with severe impacts or consequences on their daily life [13]. Endocrine failure can cause delayed development and secondary sexual characteristics in children. Therefore, it is critical to restrict transfusions and iron chelate agents as needed, and schedule patients for regular follow-up visits. Furthermore, it is necessary to establish screening examinations each period, with a larger-scale study requiring repeated transfusions to clarify the effects of the amount and period of blood transfusions on endocrine failure.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Burt MJ, George DK, Powell LW. Haemochromatosis: a clinical update. Med J Aust. 1996;164:348–351. [PubMed] [Google Scholar]
- 2.Kim MK, Lim DJ, Baek KH, Song KH, Kang MI, Lee KW, Lee JW. A case of transfusion-associated hemochromatosis involving multiple organs. Korean J Med. 2008;75:709–713. [Google Scholar]
- 3.Schafer AI, Cheron RG, Dluhy R, Cooper B, Gleason RE, Soeldner JS, Bunn HF. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med. 1981;304:319–324. doi: 10.1056/NEJM198102053040603. [DOI] [PubMed] [Google Scholar]
- 4.Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD, Anderson GJ, Southey MC, Giles GG, English DR, Hopper JL, Olynyk JK, Powell LW, Gertig DM. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221–230. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]
- 5.Beard JL, Dawson H, Pinero DJ. Iron metabolism: a comprehensive review. Nutr Rev. 1996;54:295–317. doi: 10.1111/j.1753-4887.1996.tb03794.x. [DOI] [PubMed] [Google Scholar]
- 6.Hahn JU, Steiner M, Bochnig S, Schmidt H, Schuff-Werner P, Kerner W. Evaluation of a diagnostic algorithm for hereditary hemochromatosis in 3,500 patients with diabetes. Diabetes Care. 2006;29:464–466. doi: 10.2337/diacare.29.02.06.dc05-1417. [DOI] [PubMed] [Google Scholar]
- 7.Adams PC, Deugnier Y, Moirand R, Brissot P. The relationship between iron overload, clinical symptoms, and age in 410 patients with genetic hemochromatosis. Hepatology. 1997;25:162–166. doi: 10.1002/hep.510250130. [DOI] [PubMed] [Google Scholar]
- 8.Rivers J, Garrahy P, Robinson W, Murphy A. Reversible cardiac dysfunction in hemochromatosis. Am Heart J. 1987;113:216–217. doi: 10.1016/0002-8703(87)90039-1. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa I, Morikawa M, Nakano Y, Konishi J. Hemochromatosis of the pituitary gland: MR imaging. Radiology. 1988;168:213–214. doi: 10.1148/radiology.168.1.3380960. [DOI] [PubMed] [Google Scholar]
- 10.Himoto Y, Kanzaki S, Nomura H, Araki T, Takahashi Y, Seino Y. Hypothyroidism and hypoparathyroidism in an 11 year old boy with hemochromatosis secondary to aplastic anemia. Acta Paediatr Jpn. 1995;37:534–536. doi: 10.1111/j.1442-200x.1995.tb03371.x. [DOI] [PubMed] [Google Scholar]
- 11.Shirota T, Shinoda T, Aizawa T, Mizukami T, Katakura M, Takasu N, Yamada T. Primary hypothyroidism and multiple endocrine failure in association with hemochromatosis in a long-term hemodialysis patient. Clin Nephrol. 1992;38:105–109. [PubMed] [Google Scholar]
- 12.Kwon HJ, Joo SW, Kook JH, Rha JY, Kook H, Woo YJ, Hwang TJ. Endocrinopathy in hemochromatosis patients multi-transfused for aplastic anemia. Korean J Pediatr Hematol Oncol. 2001;8:181–188. [Google Scholar]
- 13.Chern JP, Lin KH. Hypoparathyroidism in transfusion-dependent patients with beta-thalassemia. J Pediatr Hematol Oncol. 2002;24:291–293. doi: 10.1097/00043426-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Bell H, Rostad B, Raknerud N, Try K. Computer tomography in the detection of hemochromatosis. Tidsskr Nor Laegeforen. 1994;114:1697–1699. [PubMed] [Google Scholar]
- 15.Howard JM, Ghent CN, Carey LS, Flanagan PR, Valberg LS. Diagnostic efficacy of hepatic computed tomography in the detection of body iron overload. Gastroenterology. 1983;84:209–215. [PubMed] [Google Scholar]
- 16.Guyader D, Gandon Y, Deugnier Y, Jouanolle H, Loreal O, Simon M, Bourel M, Carsin M, Brissot P. Evaluation of computed tomography in the assessment of liver iron overload. A study of 46 cases of idiopathic hemochromatosis. Gastroenterology. 1989;97:737–743. doi: 10.1016/0016-5085(89)90646-x. [DOI] [PubMed] [Google Scholar]
- 17.Alustiza JM, Artetxe J, Castiella A, Agirre C, Emparanza JI, Otazua P, Garcia-Bengoechea M, Barrio J, Mujica F, Recondo JA Gipuzkoa Hepatic Iron Concentration by MRI Study Group. MR quantification of hepatic iron concentration. Radiology. 2004;230:479–484. doi: 10.1148/radiol.2302020820. [DOI] [PubMed] [Google Scholar]
- 18.Iida Y, Konishi J, Harioka T, Misaki T, Endo K, Torizuka K. Thyroid CT number and its relationship to iodine concentration. Radiology. 1983;147:793–795. doi: 10.1148/radiology.147.3.6844615. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto K, Okubo Y, Harada C, Saito H, Sata A, Nishikawa A, Ohwada R, Tsuiki M, Yamamoto M, Hashimoto E, Sato K, Takano K. Latent hypoparathyroidism in an osteoporotic patient with multiple endocrinopathies and secondary hemochromatosis due to multiple blood transfusions, unmasked by alendronate and glucocorticoid at adrenal crisis. Intern Med. 2008;47:515–520. doi: 10.2169/internalmedicine.47.0642. [DOI] [PubMed] [Google Scholar]
- 20.Mautalen CA, Kvicala R, Perriard D, Bugnard E, Rossi E, Duhart J. Case report: hypoparathyroidism and iron storage disease. Treatment with 25-hydroxy-vitamin D3. Am J Med Sci. 1978;276:363–368. doi: 10.1097/00000441-197811000-00015. [DOI] [PubMed] [Google Scholar]