Abstract
Adrenal incidentalomas are adrenal masses serendipitously detected during an imaging study performed for reasons unrelated to suspicion of adrenal disease. The incidence of adrenal incidentalomas has increased because of the widespread use of various imaging modalities. In oncology patients with adrenal incidentalomas, the characterization of the adrenal masses is challenging because nearly 50% of incidental adrenal masses are metastatic lesions that need special medical attention. Although unenhanced computed tomography (CT) densitometry, chemical shift magnetic resonance imaging (MRI), delayed contrast-enhanced CT and CT histogram analysis have been used as sensitive and specific modalities for differentiating benign from malignant adrenal masses, F-18 fluoro-2-deoxy-D-glucose positron emission tomography (F-18 FDG PET)/CT is a highly accurate imaging modality compared to CT or MRI, especially when these two imaging modalities are combined. In addition, a semiquantitative analysis using standardized uptake value ratio further improves the diagnostic accuracy of F-18 FDG PET/CT in differentiating benign from malignant adrenal masses. Thus, F-18 FDG PET/CT is very helpful for determining the best therapeutic management, especially for assessing the need for surgery.
Keywords: Adrenal incidentalomas, Fluorodeoxyglucose F18, Integrated positron emission tomography-computed tomography, Multidetector computed tomography, Magnetic resonance imaging
INTRODUCTION
Adrenal incidentalomas are adrenal masses, 1 cm or more in diameter, that are serendipitously detected during an imaging study performed for reasons unrelated to suspicion of adrenal disease [1]. The incidence of adrenal incidentalomas has increased because of the widespread use of imaging modalities, such as computed tomography (CT), ultrasound, magnetic resonance imaging (MRI), and 2-[F-18]-fluoro-2-deoxy-D-glucose positron emission tomography (F-18 FDG PET) or integrated PET/CT [2,3]. It has been estimated that the incidence of adrenal incidentalomas is 0.2% to 4.4%, increasing up to more than 10% in the elderly in imaging studies, and about 2.0% (range, 1.0% to 8.7%) in autopsy studies [2,4].
When adrenal incidentalomas are serendipitously detected during imaging studies of other organs or for nonadrenal diseases, clinical or biochemical evidence of hormonal hypersecretion should be accurately assessed [5]. On the other hand, in the absence of hormonal hypersecretion, imaging studies for risk assessment of malignancy are required, typically beginning with unenhanced CT densitometry of the abdomen [5]. When the unenhanced CT densitometry cannot differentiate between benign and malignant adrenal masses, chemical shift MRI or delayed contrast-enhanced CT can be used [1,2]. In oncology patients with adrenal incidentalomas, the characterization of adrenal masses is important in cancer staging, treatment planning and prognosis assessment because nearly 50% of adrenal incidentalomas in oncology patients have been reported as metastases [6,7,8].
PET using the radiolabeled glucose analogue, F-18 FDG, is a functional imaging modality based on the detection of increased glucose metabolism of malignant tumors. Integrated PET/CT has high accuracy in differentiating benign from malignant tumors by providing metabolic rate information, anatomic characteristics, and localization of tumors. Similarly, metabolic characterization of F-18 FDG PET or PET/CT has been highly sensitive and specific in differentiating benign from malignant adrenal masses [6,7,9]. In this review, we present a comprehensive overview of the diagnostic performance of F-18 FDG PET/CT and a brief introduction of other imaging modalities including unenhanced CT densitometry, chemical shift MRI, delayed contrast-enhanced CT and CT histogram analysis in the characterization of adrenal masses.
UNENHANCED CT DENSITOMETRY
Unenhanced CT has been the initial imaging modality of choice for the characterization of adrenal incidentalomas [3]. The size and CT features of adrenal incidentalomas are important for differentiating between benign and malignant adrenal masses [1]. Benign adrenal masses are associated with smooth, well-defined borders, diameter of 4 cm or less and an attenuation value of less than 10 Houndsfield unit (HU) on unenhanced CT [3]. On the other hand, malignant adrenal masses are associated with irregular borders, invasion into surrounding structures, diameter greater than 4 cm, and an attenuation value of more than 10 HU on unenhanced CT [3,10]. Although a cutoff of 4 cm in adrenal mass size has been reported to be a reliable parameter for diagnosis of malignant adrenal mass, recent studies have shown that HU attenuation on unenhanced CT is superior parameter to adrenal mass size in differentiating between benign and malignant adrenal masses [2,10].
Most adrenal adenomas contain abundant intracellular lipids, whereas few malignant adrenal masses contain abundant intracellular lipids [11]. Previous studies have shown that a cutoff attenuation value of 10 HU or less on unenhanced CT has the best accuracy with 71% to 79% sensitivity and 96% to 98% specificity in differentiating adrenal adenomas from malignant adrenal masses [2,3,8,12]. Although 70% of adrenal adenomas contain abundant intracellular lipid and have an attenuation value of 10 HU or less on unenhanced CT, 30% of adrenal adenomas are lipid-poor masses with an attenuation value of greater than 10 HU and are considered indeterminate [2,4,12,13]. This is the fundamental principle and the major limitation for differentiating adrenal adenomas from malignant adrenal masses on both unenhanced CT densitometry and chemical shift MRI [8,12].
CHEMICAL SHIFT MRI
Chemical shift MRI, similar to unenhanced CT densitometry, depends on the detection of abundant intracellular lipids to diagnose adrenal adenomas [2]. Unlike unenhanced CT densitometry, chemical shift MRI uses different resonant frequencies of water and triglyceride molecules rather than attenuation differences [11].
Previous studies have demonstrated that quantitative or qualitative analysis of the signal intensity loss of adrenal masses in relation to the spleen on in-phase and opposed-phase images showed 84% to 100% sensitivity and 92% to 100% specificity [2]. Generally, adrenal adenomas appear as hypointense or isointense in comparison with the liver on T1-weighted images and hyperintense or isointense to the liver on T2-weighted images [2]. Korobkin et al. [14] found a negative linear relationship between the percentage of lipid-rich cells and the relative change in signal intensity on chemical shift MRI. Previous studies have shown that for lipid-rich adenomas, there is no significant difference in differentiating adrenal adenomas from malignant adrenal masses between chemical shift MRI and unenhanced CT densitometry, but chemical shift MRI might be superior when evaluating lipid-poor adenomas with an attenuation value up to 30 HU [2].
The adrenal to spleen chemical shift ratio is the lesion to spleen signal intensity ratio on opposed phase images divided by the lesion to spleen signal intensity ratio on in-phase images [11,15]. An alternative measurement, the adrenal signal intensity index, is calculated as [(SIIP×SIOP)/SIIP]×100, where SIIP and SIOP are the signal intensities measured on in-phase and opposed-phase images, respectively [11]. An adrenal to spleen chemical shift ratio of less than 0.71 or an adrenal signal intensity index of greater than 16.5% is consistent with a lipid-rich adenoma [11,15].
WASHOUT ON DELAYED CONTRAST-ENHANCED CT
The percentage washout of injected contrast media on delayed contrast-enhanced CT can differentiate adrenal adenomas from malignant adrenal masses [2]. This is because malignant adrenal masses show a slower contrast excretion, i.e., washout, due to disturbed permeability of the capillaries, in comparison to adrenal adenomas, although both malignant adrenal masses and adrenal adenomas enhance rapidly after intravenous contrast administration [2,16]. There are two measurements to estimate the percentage washout: absolute percentage washout (APW) and relative percentage washout (RPW). APW=(attenuation value at enhanced CT-attenuation value at delayed enhanced CT)/(attenuation value at enhanced CT-attenuation value at unenhanced CT)×100; and RPW=(attenuation value at enhanced CT-attenuation value at delayed enhanced CT)/attenuation value at enhanced CT×100 [2,16,17]. On a 15-minute delayed CT protocol, the use of a threshold value of 60% or higher for APW to differentiate adrenal adenomas from nonadenomas, such as metastases, adrenocortical carcinomas, and pheochromocytomas, resulted in 86% to 100% sensitivity and 83% to 92% specificity [2,16,17]. The use of a RPW threshold value of 40% to differentiate lipid-poor adenomas from nonadenomas resulted in 82% to 97% sensitivity and 92% to 100% specificity [2,17].
CT HISTOGRAM ANALYSIS
CT histogram analysis measures the number of negative-attenuation pixels, i.e., pixels with an attenuation value of less than 0 HU within a region of interest (ROI) and percentage fraction of negative pixels in each ROI [13,16]. The pixels in each ROI are processed with a histogram tool that reveals a plot of pixel attenuation along the X-axis versus the frequency of pixels at each attenuation value on the Y-axis [13]. A threshold of more than 10% negative pixels is generally used with unenhanced CT for differentiation of benign from malignant mass. Ho et al. [18] revealed that CT histogram analysis using a threshold of more than 10% negative pixels with 84% sensitivity and 100% sensitivity was more accurate than unenhanced CT densitometry using an attenuation value of less than 10 HU with 68% sensitivity and 100% specificity for the diagnosis of adrenal adenoma. Because 68% of adrenal adenomas were lipid-rich and all of the lipid-rich adenomas had more than 10% negative pixels, CT histogram analysis showed no false-positive results. A previous study also demonstrated that CT histogram analysis was very sensitive for lipid detection, particularly in lipid-poor adrenal adenomas with an attenuation value of more than 10 HU [13]. Indeterminate adrenal masses classified on unenhanced CT densitometry can be confidently diagnosed as lipid-poor adenomas using CT histogram analysis [18].
F-18 FDG PET/CT
PET using the radiolabeled glucose analogue, F-18 FDG, is a functional imaging modality based on the detection of increased glucose metabolism of malignant tumors. Integrated PET/CT using F-18 FDG has high accuracy in differentiating benign from malignant tumors by providing metabolic rate information by F-18 FDG, in addition to anatomic characteristics and localization of tumors by either unenhanced or enhanced CT. It is widely accepted that F-18 FDG PET or PET/CT is highly accurate in differentiating benign from malignant adrenal masses. Blake et al. [19] reported that F-18 FDG PET/CT demonstrated 100% sensitivity and 93.8% specificity for the characterization of 41 adrenal masses, and only two of 32 benign adrenal lesions (6.3%) showed false-positive results. After incorporation of the washout analysis with contrast-enhanced CT, F-18 FDG PET/CT increased specificity to 100% because two adrenal lesions with false-positive PET finding for malignancy had been characterized as benign [19]. Boland et al. [20] revealed that F-18 FDG PET/CT had a sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of 99%, 100%, 100%, 93%, and 99%, respectively, for detecting benign adrenal masses and 100%, 99%, 93%, 100%, and 99%, respectively, for detecting malignant adrenal masses. Yun et al. [21] also demonstrated that F-18 FDG PET showed excellent diagnostic performance in the characterization of adrenal masses detected on CT or MRI with 100% sensitivity, 94% specificity and 96% accuracy. A systematic review of meta-analysis results showed that most adrenal masses can be characterized using F-18 FDG PET or PET/CT with high sensitivity (97%), specificity (91%), and accuracy (area under the receiver operating characteristic (ROC) curve [AUC], 0.98) [6]. In addition, qualitative (visual) analysis alone can suffice for differentiating benign from malignant adrenal masses [6].
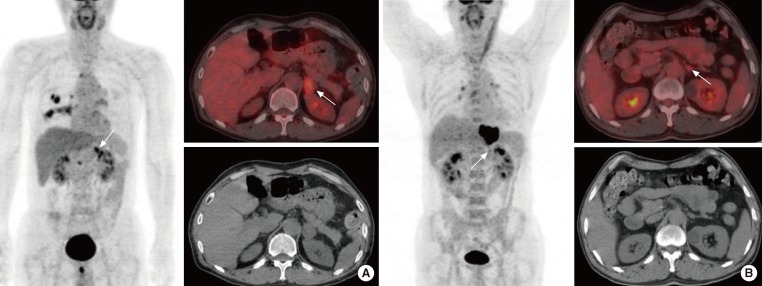
We have previously reported an excellent diagnostic accuracy of F-18 FDG PET/CT in differentiating malignant from benign lesions. In our study for oncology patients who underwent F-18 FDG PET/CT for cancer staging and therapeutic monitoring, we used a standardized uptake values (SUV)ratio, a semiquantitative parameter, for the characterization of adrenal masses. Maximum SUV (SUVmax) of the adrenal mass divided by maximum SUV of the segment 8 of the liver was defined as SUVratio [22]. We have reported that the size, HU, SUVmax, and SUVratio of 45 benign adrenal masses were 1.7±0.8, 6.5±11.8, 0.9±1.2, and 0.9±0.4 cm, respectively, and of 44 malignant adrenal masses, 2.6±1.6, 22.0±11.9, 7.7±5.0, and 2.5±1.8 cm, respectively [22]. The various parameters of malignant adrenal masses were significantly higher than that of benign adrenal masses. In ROC curve analysis, SUVratio (AUC, 0.892) was the most accurate parameter from among the size, HU, SUVmax, and SUVratio and SUVratio, and a cutoff of 1.0 could differentiate benign from malignant adrenal adenomas with 90.9% sensitivity and 75.6% specificity [22]. Fig. 1 illustrates a true-positive result and a true-negative result of F-18 FDG PET/CT for the characterization of adrenal masses in oncology patients.
Fig. 1.
(A) A 63-year-old man with lung cancer underwent F-18 fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET)/computed tomography (CT). F-18 FDG PET/CT showed focal FDG uptake (maximum standardized uptake value, SUVmax 5.9) in the left adrenal mass (arrows) that was 2.7 cm in diameter. The SUVratio (SUVmax of the adrenal mass divided by SUVmax of the segment 8 of the liver) and Houndsfield unit (HU) of the adrenal mass were 1.7 and 5.5, respectively. On unenhanced abdominal CT, the adrenal mass was misinterpreted as benign, but this mass was considered metastatic based on the F-18 FDG PET/CT finding. After adrenalectomy, this adrenal mass proved to be metastatic. (B) A 50-year-old man with esophageal cancer underwent F-18 FDG PET/CT. F-18 FDG PET/CT showed focal FDG uptake (SUVmax 3.8) in the left 1.4-cm adrenal mass (arrows). SUVratio and HU of the adrenal mass were 0.9 and 23.0, respectively. On unenhanced abdominal CT, this adrenal mass was misinterpreted as a malignancy, but the mass was considered benign based on the F-18 FDG PET/CT finding. It was stable in size for the next 9 months and proved to be benign.
Previous studies have demonstrated that the incidence of adrenal incidentalomas in patients with a history of extra-adrenal malignancy ranges from 27% to 50%, whereas in patients with no history of malignancy or biochemical abnormality, almost all adrenal incidentalomas have been benign [6,23]. The likelihood that an adrenal incidentaloma is malignant is also associated with whether or not the patient has a history of extra-adrenal malignancy.
In the characterization of adrenal masses by F-18 FDG PET/CT, a false-positive rate of 5% is reported due to significant FDG uptake in some adrenal adenomas, adrenal endothelial cysts, inflammatory, and infectious lesions [21]. False-negative results may be seen in adrenal metastatic lesions with hemorrhage or necrosis, small (<10 mm) metastatic nodules, and metastases from non-FDG avid malignancies including carcinoid and pulmonary bronchoalveolar carcinoma [24].
Metser et al. [7] reported that combined information from integration of F-18 FDG PET (a SUV cutoff of 3.1) with unenhanced CT (an attenuation value cutoff of 10 HU) is more specific than information from F-18 FDG PET alone for differentiating benign from malignant adrenal masses in oncology patients. When using PET/CT criteria of SUV of 3.1 or less for any HU and SUV greater than 3.1 for masses less than 10 HU, the sensitivity, specificity, PPV and NPV were 100%, 98%, 97%, and 100%, respectively [7]. Although it is known that PET is less sensitive and less specific for the characterization of smaller lesions, F-18 FDG PET has been shown to correctly classify all small adrenal masses (1.5 cm or less in diameter) using a SUV cutoff of 3.1 [7]. Caoili et al. [25] demonstrated that unenhanced CT in combination with SUV ratio (adrenal/liver) with a cutoff of 1.0 differentiated adrenal adenomas from nonadenomas with 89% sensitivity and 75% specificity. F-18 FDG uptake in the adrenal mass that was visibly less than that of the liver was more specific for adenoma, whereas F-18 FDG uptake by the adrenal mass that was visibly greater than that of the liver was specific for malignancy [25]. We have also reported that only SUVratio (adrenal/liver) with a cutoff of 1.0 could differentiate benign from malignant adrenal masses with 80.0% sensitivity and 86.4% specificity for the characterization of small adrenal masses (1.5 cm or less in diameter) [25].
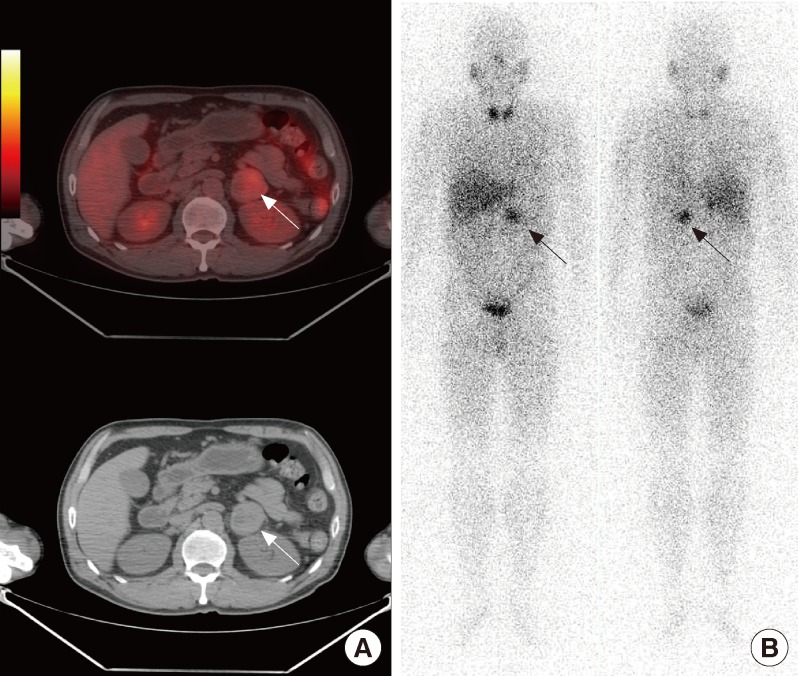
Perri et al. [13] reported that combined SUV and CT histogram analysis from F-18 FDG PET/CT showed 100% sensitivity, 97.3% specificity, 95.7% PPV, and 100% NPV for differentiating benign from malignant adrenal masses in oncology patients. In addition, combined SUV and CT histogram analysis (AUC, 0.996) was more accurate than simple SUV analysis (AUC, 0.961), and the combination of SUV analysis and unenhanced CT densitometry (AUC, 0.987) showed few false-positive results including one lipid-poor adenoma and one pheochromocytoma [13]. Pheochromocytomas are commonly observed tumors of the adrenal glands and a variety of nuclear medicine imaging modalities have been used to correctly localize pheochromocytomas. F-18 FDG PET imaging was not sufficient for differentiating metastatic adrenal mass from pheochromocytoma because both lesions take up F-18 FDG [26]. Fig. 2 shows a true-positive result and a true-negative result of F-18 FDG PET/CT for the characterization of adrenal masses in oncology patients. F-18 fluorodihydroxyphenylalanine PET and F-18 fluorodopamine PET are promising imaging modalities for the localization of pheochromocytomas [27].
Fig. 2.
A 57-year-old man with left adrenal mass underwent F-18 fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET)/computed tomography (CT) and I-131 metaiodobenzylguanidine (MIBG) scintigraphy. (A) F-18 FDG PET/CT showed a 4.0-cm hypermetabolic mass with maximum standardized uptake value of 4.1 (arrows). (B) I-131 MIBG scintigraphy also showed focal tracer uptake in the left adrenal gland (arrows). After adrenalectomy, this adrenal mass proved to be a pheochromocytoma.
It is known that surgery should be considered in patients who have adrenal masses with radiological features suggesting malignancy or hormonal hypersecretion, whereas patients who have adrenal masses with nonsecretion of hormone and radiological features suggesting benignity should undergo biochemical and imaging follow-up [28]. Ansquer et al. [28] demonstrated that F-18 FDG PET/CT was negative in 31 of 32 (97%) nonsurgical adrenal masses and positive in 35 of 49 (73%) potentially surgical adrenal masses including 24 of 27 (89%) malignant lesions and 12 of 22 (55%) benign, secreting lesions. Only one nonsecreting adrenal adenoma and all the malignant lesions, except three metastases from renal cell carcinoma, were F-18 FDG positive.
Thus, F-18 FDG uptake is a good indicator of malignant and/or secreting adrenal masses and should lead one to consider surgery. In contrast, in the absence of previous poorly FDG-avid cancer (especially renal cell carcinoma), low F-18 FDG uptake on adrenal mass allow patients to avoid unnecessary surgical removal of benign adrenal mass [28].
CONCLUSIONS
F-18 FDG PET/CT is a highly accurate imaging modality for differentiating benign from malignant adrenal masses compared to CT or MRI, especially after incorporation of other CT image analyses, such as unenhanced CT densitometry, delayed contrast-enhanced CT and CT histogram analysis. In addition, using the SUVratio (adrenal/liver), a semiquantitative parameter, can further improve diagnostic accuracy of F-18 FDG PET/CT in differentiating benign from malignant adrenal masses. Highly accurate F-18 FDG PET/CT might help for making a decision on the best therapeutic management in clinical practice, especially in determining whether to perform surgery.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Young WF., Jr Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–610. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 2.Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, Reimondo G, Pia A, Toscano V, Zini M, Borretta G, Papini E, Garofalo P, Allolio B, Dupas B, Mantero F, Tabarin A Italian Association of Clinical Endocrinologists. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164:851–870. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 3.Kanagarajah P, Ayyathurai R, Manoharan M, Narayanan G, Kava BR. Current concepts in the management of adrenal incidentalomas. Urol Ann. 2012;4:137–144. doi: 10.4103/0974-7796.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaltsas G, Chrisoulidou A, Piaditis G, Kassi E, Chrousos G. Current status and controversies in adrenal incidentalomas. Trends Endocrinol Metab. 2012;23:602–609. doi: 10.1016/j.tem.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Tessonnier L, Sebag F, Palazzo FF, Colavolpe C, De Micco C, Mancini J, Conte-Devolx B, Henry JF, Mundler O, Taieb D. Does 18F-FDG PET/CT add diagnostic accuracy in incidentally identified non-secreting adrenal tumours? Eur J Nucl Med Mol Imaging. 2008;35:2018–2025. doi: 10.1007/s00259-008-0849-3. [DOI] [PubMed] [Google Scholar]
- 6.Boland GW, Dwamena BA, Jagtiani Sangwaiya M, Goehler AG, Blake MA, Hahn PF, Scott JA, Kalra MK. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. 2011;259:117–126. doi: 10.1148/radiol.11100569. [DOI] [PubMed] [Google Scholar]
- 7.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47:32–37. [PubMed] [Google Scholar]
- 8.Vikram R, Yeung HD, Macapinlac HA, Iyer RB. Utility of PET/CT in differentiating benign from malignant adrenal nodules in patients with cancer. AJR Am J Roentgenol. 2008;191:1545–1551. doi: 10.2214/AJR.07.3447. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, Kung J, Zhuang H, Alavi A. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058–2062. [PubMed] [Google Scholar]
- 10.Zeiger MA, Siegelman SS, Hamrahian AH. Medical and surgical evaluation and treatment of adrenal incidentalomas. J Clin Endocrinol Metab. 2011;96:2004–2015. doi: 10.1210/jc.2011-0085. [DOI] [PubMed] [Google Scholar]
- 11.Fujiyoshi F, Nakajo M, Fukukura Y, Tsuchimochi S. Characterization of adrenal tumors by chemical shift fast low-angle shot MR imaging: comparison of four methods of quantitative evaluation. AJR Am J Roentgenol. 2003;180:1649–1657. doi: 10.2214/ajr.180.6.1801649. [DOI] [PubMed] [Google Scholar]
- 12.Korobkin M. CT characterization of adrenal masses: the time has come. Radiology. 2000;217:629–632. doi: 10.1148/radiology.217.3.r00dc52629. [DOI] [PubMed] [Google Scholar]
- 13.Perri M, Erba P, Volterrani D, Guidoccio F, Lazzeri E, Caramella D, Mariani G. Adrenal masses in patients with cancer: PET/CT characterization with combined CT histogram and standardized uptake value PET analysis. AJR Am J Roentgenol. 2011;197:209–216. doi: 10.2214/AJR.10.5342. [DOI] [PubMed] [Google Scholar]
- 14.Korobkin M, Giordano TJ, Brodeur FJ, Francis IR, Siegelman ES, Quint LE, Dunnick NR, Heiken JP, Wang HH. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996;200:743–747. doi: 10.1148/radiology.200.3.8756925. [DOI] [PubMed] [Google Scholar]
- 15.Outwater EK, Siegelman ES, Huang AB, Birnbaum BA. Adrenal masses: correlation between CT attenuation value and chemical shift ratio at MR imaging with in-phase and opposed-phase sequences. Radiology. 1996;200:749–752. doi: 10.1148/radiology.200.3.8756926. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PT, Horton KM, Fishman EK. Adrenal imaging with multidetector CT: evidence-based protocol optimization and interpretative practice. Radiographics. 2009;29:1319–1331. doi: 10.1148/rg.295095026. [DOI] [PubMed] [Google Scholar]
- 17.Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, Raghupathi KI. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002;222:629–633. doi: 10.1148/radiol.2223010766. [DOI] [PubMed] [Google Scholar]
- 18.Ho LM, Paulson EK, Brady MJ, Wong TZ, Schindera ST. Lipid-poor adenomas on unenhanced CT: does histogram analysis increase sensitivity compared with a mean attenuation threshold? AJR Am J Roentgenol. 2008;191:234–238. doi: 10.2214/AJR.07.3150. [DOI] [PubMed] [Google Scholar]
- 19.Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, Boland GW. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy: initial experience. Radiology. 2006;238:970–977. doi: 10.1148/radiol.2383042164. [DOI] [PubMed] [Google Scholar]
- 20.Boland GW, Blake MA, Holalkere NS, Hahn PF. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative versus quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956–962. doi: 10.2214/AJR.08.1431. [DOI] [PubMed] [Google Scholar]
- 21.Yun M, Kim W, Alnafisi N, Lacorte L, Jang S, Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42:1795–1799. [PubMed] [Google Scholar]
- 22.Lee HJ, Song BI, Kang SM, Jeong SY, Seo JH, Lee SW, Yoo J, Ahn BC, Lee J. Usefulness of F-18 FDG PET/CT in adrenal incidentaloma: differential diagnosis of adrenal metastasis in oncologic patients. Nucl Med Mol Imaging. 2009;43:421–428. [Google Scholar]
- 23.Lenert JT, Barnett CC, Jr, Kudelka AP, Sellin RV, Gagel RF, Prieto VG, Skibber JM, Ross MI, Pisters PW, Curley SA, Evans DB, Lee JE. Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery. 2001;130:1060–1067. doi: 10.1067/msy.2001.118369. [DOI] [PubMed] [Google Scholar]
- 24.Chong S, Lee KS, Kim HY, Kim YK, Kim BT, Chung MJ, Yi CA, Kwon GY. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics. 2006;26:1811–1824. doi: 10.1148/rg.266065057. [DOI] [PubMed] [Google Scholar]
- 25.Caoili EM, Korobkin M, Brown RK, Mackie G, Shulkin BL. Differentiating adrenal adenomas from nonadenomas using (18)F-FDG PET/CT: quantitative and qualitative evaluation. Acad Radiol. 2007;14:468–475. doi: 10.1016/j.acra.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC. Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET. Radiology. 1999;212:35–41. doi: 10.1148/radiology.212.1.r99jl3035. [DOI] [PubMed] [Google Scholar]
- 27.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansquer C, Scigliano S, Mirallie E, Taieb D, Brunaud L, Sebag F, Leux C, Drui D, Dupas B, Renaudin K, Kraeber-Bodere F. 18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. Eur J Nucl Med Mol Imaging. 2010;37:1669–1678. doi: 10.1007/s00259-010-1471-8. [DOI] [PubMed] [Google Scholar]