Abstract
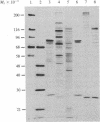
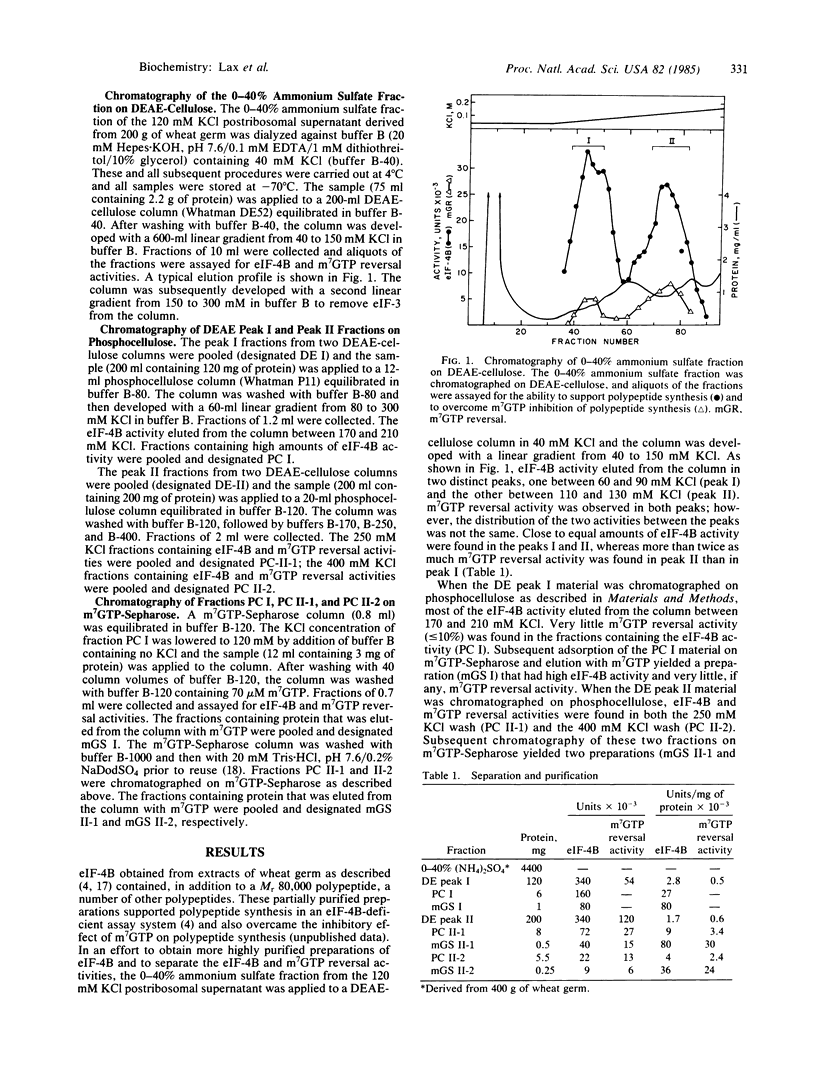
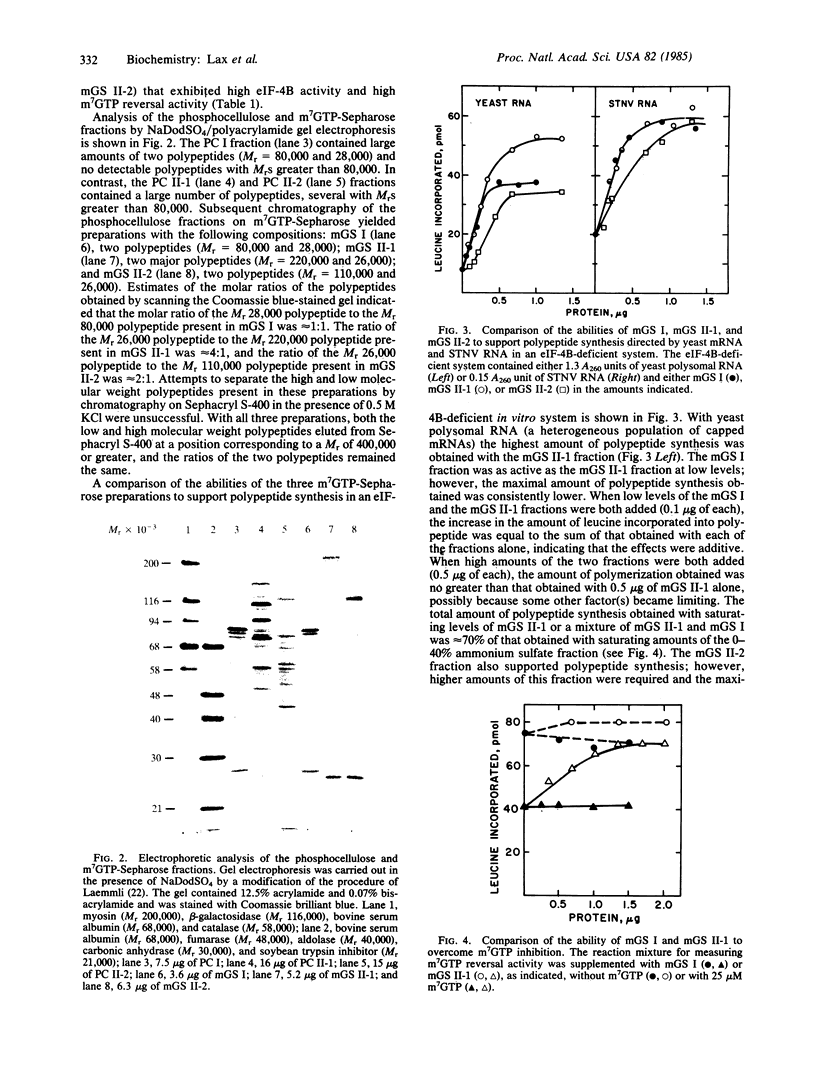
Three highly purified preparations (preparations I, II-1, and II-2) have been obtained from wheat germ and shown to support in vitro polypeptide synthesis directed by capped or uncapped mRNAs in a eukaryotic initiation factor 4B (eIF-4B)-deficient system. The three preparations differ, however, in polypeptide composition and in the ability to overcome the inhibitory effect of 7-methylguanosine 5'-triphosphate (m7GTP) on in vitro polypeptide synthesis. Preparation I contains two polypeptides (Mr = 80,000 and 28,000), which are present in a 1:1 molar ratio and are associated in a high molecular weight complex. Preparation II-1 contains two major polypeptides (Mr = 220,000 and 26,000) and preparation II-2 also contains two major polypeptides (Mr = 110,000 and 26,000). Preparations II-1 and II-2 are high molecular weight complexes; neither contains detectable amounts of a Mr 80,000 or a Mr 50,000 component. Preparations II-1 and II-2 both overcome m7GTP inhibition, whereas preparation I does not. These findings raise several questions with regard to the identity of eIF-4B and its relationship to cap recognition factors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Luedi M., Hershey J. W. Purification and characterization of initiation factors IF-E4 and IF-E6 from rabbit reticulocytes. J Biol Chem. 1977 Aug 25;252(16):5798–5803. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown-Luedi M. L., Meyer L. J., Milburn S. C., Yau P. M., Corbett S., Hershey J. W. Protein synthesis initiation factors from human HeLa cells and rabbit reticulocytes are similar: comparison of protein structure, activities, and immunochemical properties. Biochemistry. 1982 Aug 31;21(18):4202–4206. doi: 10.1021/bi00261a002. [DOI] [PubMed] [Google Scholar]
- Checkley J. W., Cooley L., Ravel J. M. Characterization of initiation factor eIF-3 from wheat germ. J Biol Chem. 1981 Feb 25;256(4):1582–1586. [PubMed] [Google Scholar]
- Clark J. M., Jr, Klein W. H. In vitro translation of STNV-RNA. Methods Enzymol. 1974;30:754–761. doi: 10.1016/0076-6879(74)30074-2. [DOI] [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Edery I., Lee K. A., Sonenberg N. Functional characterization of eukaryotic mRNA cap binding protein complex: effects on translation of capped and naturally uncapped RNAs. Biochemistry. 1984 May 22;23(11):2456–2462. doi: 10.1021/bi00306a021. [DOI] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hellmann G. M., Chu L. Y., Rhoads R. E. A polypeptide which reverses cap analogue inhibition of cell-free protein synthesis. Purification and binding to capped oligonucleotides. J Biol Chem. 1982 Apr 25;257(8):4056–4062. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Purification of protein synthesis initiation factors from rabbit reticulocytes. Methods Enzymol. 1979;60:101–108. doi: 10.1016/s0076-6879(79)60010-1. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Seal S. N., Schmidt A., Marcus A. Fractionation and partial characterization of the protein synthesis system of wheat germ. I. Resolution of two elongation factors and five initiation factors. J Biol Chem. 1983 Jan 25;258(2):859–865. [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Trachsel H., Hecht S., Shatkin A. J. Differential stimulation of capped mRNA translation in vitro by cap binding protein. Nature. 1980 May 29;285(5763):331–333. doi: 10.1038/285331a0. [DOI] [PubMed] [Google Scholar]
- Spremulli L. L., Walthall B. J., Lax S. R., Ravel J. M. Partial purification of the factors required for the initiation of protein synthesis in wheat germ. J Biol Chem. 1979 Jan 10;254(1):143–148. [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Braun L., Staehelin T. Purification of seven protein synthesis initiation factors from Krebs II ascites cells. Biochim Biophys Acta. 1979 Feb 27;561(2):484–490. doi: 10.1016/0005-2787(79)90156-4. [DOI] [PubMed] [Google Scholar]
- Walthall B. J., Spremulli L. L., Lax S. R., Ravel J. M. Isolation and purification of protein synthesis initiation factors from wheat germ. Methods Enzymol. 1979;60:193–204. doi: 10.1016/s0076-6879(79)60016-2. [DOI] [PubMed] [Google Scholar]
- Webb N. R., Chari R. V., DePillis G., Kozarich J. W., Rhoads R. E. Purification of the messenger RNA cap-binding protein using a new affinity medium. Biochemistry. 1984 Jan 17;23(2):177–181. doi: 10.1021/bi00297a001. [DOI] [PubMed] [Google Scholar]