Abstract
Observational evidence suggests nutritional exposures during in utero development may have long-lasting consequences for health; data from interventions are scarce. Here, we present a trial follow-up study to assess the association between prenatal food and micronutrient supplementation and childhood blood pressure and kidney function. During the MINIMat Trial in rural Bangladesh, women were randomly assigned early in pregnancy to receive an early or later invitation to attend a food supplementation program and additionally to receive either iron and folate or multiple micronutrient tablets daily. The 3267 singleton birth individuals with measured anthropometry born during the trial were eligible for a follow-up study at 4.5 y old. A total of 77% of eligible individuals were recruited and blood pressure, kidney size by ultrasound, and glomerular filtration rate (GFR; calculated from plasma cystatin c) were assessed. In adjusted analysis, early invitation to food supplementation was associated with a 0.72-mm Hg [(95% CI: 0.16, 1.28); P = 0.01] lower childhood diastolic blood pressure and maternal MMS supplementation was associated with a marginally higher [0.87 mm Hg (95% CI: 0.18, 1.56); P = 0.01] childhood diastolic blood pressure. There was also some evidence that a supplement higher in iron was associated with a higher offspring GFR. No other effects of the food or micronutrient interventions were observed and there was no interaction between the interventions on the outcomes studied. These marginal associations and small effect sizes suggest limited public health importance in early childhood.
Introduction
The impact of nutrition during development is recognized as an important underlying risk factor for an individual’s susceptibility to disease in later life. Studies have consistently demonstrated that altering the nutrient supply of pregnant animals induces profound changes in the function of body systems, including raised blood pressure, fewer kidney nephrons, and impaired pancreatic β-cell development (1–4). In humans, much of the evidence has focused on the inverse associations observed between birth weight and a range of chronic diseases in later life, including hypertension and diabetes (5, 6). These inverse associations are often interpreted as revealing the importance of nutrient supply to the developing fetus, although many other factors may underlie impaired uterine growth retardation and subsequent disease susceptibility.
A range of factors affect the supply of nutrients to the developing fetus, including placental function and maternal nutrient stores as well as the maternal diet. Of these, nutritional intake during pregnancy is the most easily manipulated and may thus have the greatest public health importance. Direct evidence from humans on the impact of diet during pregnancy on offspring cardiovascular disease (CVD)9 risk is limited and inconclusive (7). Moreover, the evidence base is dominated by observational studies that are likely to suffer from confounding due to a range of unmeasured characteristics. The follow-up of maternal supplementation trials to investigate the impact of nutrition during pregnancy on offspring CVD risk represents a powerful resource within this research field, taking advantage of the epidemiological strengths of trial design (7).
It has been suggested that the developmental origins of chronic disease may be particularly relevant to lower-income countries where nutrition and epidemiological transitions are occurring against a background of generational cycles of undernutrition and infant growth retardation (8). It is thus of particular interest to understand the impact of nutritional supplementation in pregnancy in these settings to explore the long-term consequences of these widely promoted interventions. A few studies have now published data on the impact of supplementation trials in resource-poor settings on offspring CVD risk factors. The provision of protein-energy supplements during pregnancy in The Gambia was found to be unrelated to any of the CVD risk factors studied, including body composition, blood pressure, and lipid profile in adolescents (9). No other studies to our knowledge have looked solely at protein-energy supplements during pregnancy. Multiple-micronutrient supplements (MMSs) provided in pregnancy compared with iron and folate supplements were marginally associated with lower offspring systolic, but not diastolic, blood pressure at 2 y of age in one Nepalese study (10) but were unrelated to offspring blood pressure at 6–8 y in a separate Nepalese trial where MMSs were compared with vitamin A supplementation (11). In this latter trial, there was also no impact of maternal iron, zinc, and folate supplementation on offspring blood pressure compared with vitamin A supplementation (11).
Here, we present information on blood pressure and kidney function for individuals born as part of the Maternal and Infant Nutrition Intervention in the Matlab (MINIMat; ISRCTN16581395) trial in rural Bangladesh. This trial was a large, combined food and multiple-micronutrient intervention for pregnant women aimed at improving birth weight and neonatal health (12). This paper presents follow-up data on blood pressure and kidney function of the children born during the trial when they reached 4.5 y of age.
Methods
The MINIMat trial was conducted by the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) between November 2001 and October 2003 in the rural Matlab region of Bangladesh, 57 km southeast of the capital Dhaka. Details of the trial design are available elsewhere (12–14). In brief, women were recruited early in pregnancy through regular surveillance of the demographic area covered by icddr,b. Consenting women were randomly assigned to 2 separate nutritional interventions in pregnancy: access to food supplementation and receipt of a micronutrient supplement. For the food intervention, women were randomly assigned to receive encouragement to attend government-sponsored, local community nutrition centers either early in pregnancy (around 9 wk of gestation) or at a time of their choosing (usually around 20 wk of gestation). Food supplements that provided 608 kcal/d energy and 18 g/d of vegetable protein were available to all attending women. Women participating in the MINIMat trial were also randomly assigned to receive 1 of 3 micronutrient supplements with identical appearance: 30 mg of iron and 400 μg of folate (Fe30F)/d, 60 mg of iron and 400 μg of folate (Fe60F)/d, or the UNIMAP combination of 15 micronutrients at or above the RDA (12), which contained 30 mg iron and 400 μg folate (MMS)/d. The micronutrient supplements were provided to participants on a monthly basis in special bottles (eDEM, Aprex) containing 35 capsules, which was more than sufficient for the daily tablet allowance. Compliance was assessed by the reported number of food packages received and the number of micronutrient bottle openings (recorded by the eDEM device) from enrollment to wk 30 gestation. In total, 4436 pregnant women were enrolled in the trial and there were 3560 live singleton births (Fig. 1). The primary outcomes of the original trial were birth anthropometry and neonatal survival; no effect was observed on birth size, but the combined early invitation to food supplementation and MMSs were associated with decreased childhood mortality (12).
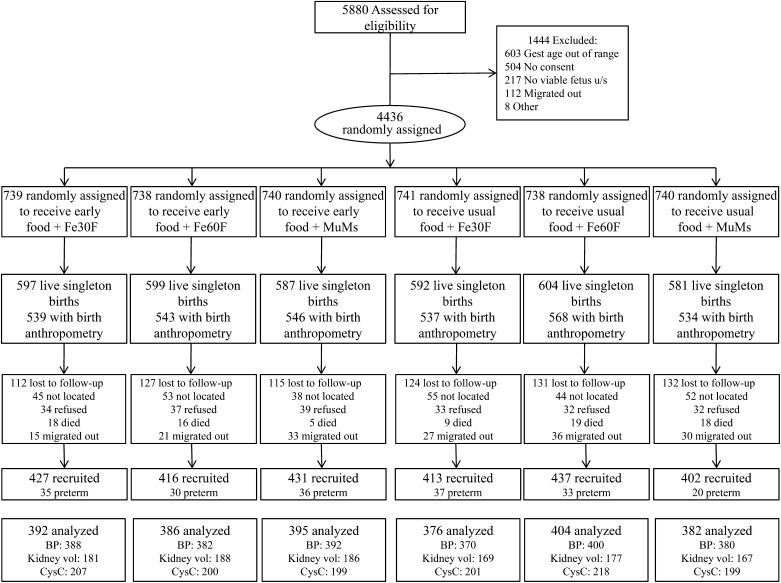
FIGURE 1.
Flow diagram of offspring born during the MINIMat trial and those recruited into the current follow-up study. Diagram represents the flow of trial participants from enrollment through birth to the current follow-up at 4.5 y of age. All individuals who were live singleton births during the trial and for whom birth anthropometry was available were eligible for the current follow-up study. Details of loss to follow-up between randomization and birth can be found elsewhere (12). BP: number of individuals recruited for whom blood pressure was measured, mm Hg. Early food: maternal randomization to access food early in pregnancy. Kidney volume: number of individuals recruited for kidney size measurements by ultrasound, cm3/m2. Preterm: individuals born before 37 wk of gestation were excluded at the analysis stage. Usual food: maternal randomization to access food at the usual time in pregnancy. CysC, number of individuals recruited for cystatin C measurements; Fe30F, 30 mg iron and 400 μg folate; Fe60F, 60 mg iron and 400 μg folate; MMS, multiple micronutrient supplement; Vol, volume.
A detailed follow-up of children born during the MINIMat trial was conducted between May 2007 and February 2009 when they were 4.5 y old. The 3267 children who were live singleton births and had measured birth anthropometry were eligible for the follow-up study and informed consent was obtained by their parents or guardians. Scientific approval was granted by the Research Review Committee of icddr,b and ethical approval from the icddr,b Ethical Review Committee as well as the ethical committees of the participating universities (London School of Hygiene and Tropical Medicine, Uppsala University, and the University of Tsukuba); separate approval was granted for the follow-up study and the original trial. A range of outcomes was assessed during the follow-up, including blood pressure and kidney function, which are presented here, and child growth and body composition, which are published elsewhere (15). Blood pressure was assessed on all individuals recruited into the 4.5-y follow-up. For cost and logistic considerations, measurements of kidney function were conducted on a subset of participants; glomerular filtration rate (GFR) assessment was restricted to individuals born during the first year of the MINIMat trial (June 2002–June 2003) and the kidney biometric study was restricted to a different subsample of individuals, born during the second half (June 2003–June 2004) of the trial to minimize participant burden.
Blood pressure was measured on all individuals in triplicate using an automated oscillometric device (Omron 705IT, Morton Medical). The first measurement was taken after the participant had been seated at rest for 5 min and there was 1 min between each subsequent measurement. Kidney function was assessed by calculation of the GFR estimated from plasma cystatin C (CysC), which was analyzed from stored, frozen samples using the immunoturbidimetric analysis (16) in Uppsala, Sweden. GFR was calculated from CysC using a prediction equation generated in Swedish patients and applicable for use in children (17). Kidney volume was assessed by ultrasonography conducted using a 3.75-MHz convex scanner (Toshiba SSA- 510A/P3, Famio-5, Toshiba Medical Systems). Two study physicians conducted all measurements after extensive training and standardization. Intra-observer error (expressed as the SD of the difference of the first and second measurement) ranged from 1.86 to 3.36 cm3 and the inter-observer error (evaluated by the SD of the difference) ranged from 1.64 to 3.83 cm3; both measurement errors were within the range reported in the literature for studies conducted by technical staff and medical doctors (18). The right and left kidney volumes were calculated by fitting a best-fit ellipsoid shape that was converted into an estimate of volume using internal software. This method was correlated (r = 0.92; P < 0.001) with the alternative length, width, depth method of assessing volume. The mean of right and left kidney volume was then adjusted for body surface area calculated from the Haycock formula (19).
In addition to the main outcomes specified above, a variety of anthropometric measurements was conducted during the 4.5-y follow-up, including weight (digital scale: Tanita, Chasmors) and height (stadiometer: Chasmors). Nutritional status was assessed by comparison with UK reference data (20) and a cutoff of < −2 Z-scores was used to define wasting and stunting based on weight-for-height and height-for-age indices, respectively. Total body composition was assessed using a foot-to-foot bioelectrical impedance analyzer (Tanita TBF-300MA, Chasmors), applying equations previously generated in this population using deuterium dilution as the reference method in children aged 4–10 y (21). Parental socioeconomic status was assessed by a continuous wealth index previously generated in this population that included data on land ownership, the construction materials of house walls, ownership of household assets, ownership of sarees or shalwer-kameez for ceremonial use, and pairs of shoes or sandals owned (22).
Statistical analysis.
All statistical analysis was conducted using Stata 11 (Stata Corportation); P values < 0.05 were regarded as significant. Values in the text are mean ± SD or median (IQR). The effects of the prenatal interventions were assessed by intention-to-treat analysis, which focused on the effect of food and micronutrient supplementation separately before assessing any interaction between the interventions. Independent t tests and χ2 tests were used to assess any differences in characteristics from the original trial between those recruited and those lost to follow-up. Linear regression was used to investigate the effect of the maternal interventions on offspring blood pressure and kidney function. Three stages of models were used: adjusted for interventions only (model 1), adjusted for covariates unrelated to the maternal intervention but related to blood pressure/kidney function (model 2), and as model 2 but additionally adjusted for covariates that could be associated with the maternal interventions. The analysis of the food supplementation intervention compared the 2 arms: early and usual invitation to access food supplementation. The unbalanced design of the MINIMat trial allowed for the assessment of 2 important research questions: is there an impact of MMS and is there an impact of providing a high iron dose? The analysis was conducted by creating dummy variables for the MMS and Fe60F interventions and comparing these with the Fe30F group as the reference (Table 1). The linear regression models were fitted with both terms to assess their independent effects, but the coefficients are reported separately in the results. An “as-treated” analysis was conducted to assess the impact of adherence to the intervention irrespective of randomization. The reported total number of food packets consumed during pregnancy was assessed as the exposure for the food invitation intervention and the pill count obtained from the eDEM technology provided an estimate of micronutrient tablet consumption. Linear regression models were fitted adjusted for covariates relating to blood pressure and kidney function as appropriate.
TABLE 1.
Categorizing the micronutrient intervention from the MINIMat Trial for the purposes of analysis
| New code for analysis2 |
||
| Original randomization code1 | Multiple micronutrients (MMS) | High-iron dose (highFe) |
| Fe30F | 0 | 0 |
| Fe60F | 0 | 1 |
| MMS | 1 | 0 |
Original micronutrient arm of the intervention. Fe30F, 30 mg iron and 400 μg folate; Fe60F, 60 mg iron and 400 μ folate; MMS, multiple micronutrient supplement.
Variable is recoded to represent multiple micronutrients or high-iron dose: individuals were coded 0 (control) or 1 (receiving intervention) and fitted together in regression models of intention-to-treat analysis.
Results
The current follow-up study recruited 2526 children who were born during the original trial, representing 77% of the eligible cohort (Fig. 1). Recruitment rates were similar across the 6 supplement arms of the trial and the reasons for loss to follow-up were also similar in their distributions. The main cause of loss to follow-up was individuals who could not be located during the follow-up study. Among recruited individuals, maternal baseline characteristics remained equally distributed between the 6 intervention arms (Table 2). There were minor differences in characteristics of mothers whose children were recruited into the current study and those lost to follow-up (Table 2). Children lost to follow-up were more likely to have been the first born and their mothers were on average ∼9 mo younger and had spent 6 mo longer in education. Recruited individuals who were born before 37 wk of gestation (preterm, n = 191) were excluded from the analysis, leaving a sample size of 2335.
TABLE 2.
Difference in maternal and household characteristics between children recruited into the MINIMat trial follow-up at 4.5 y and those not recruited1
| Early food |
Usual food |
|||||||
| Fe30F | Fe60F | MMS | Fe30F | Fe60F | MMS | All recruited2 | Lost to follow-up | |
| Participants, n | 427 | 416 | 431 | 413 | 437 | 402 | 2526 | 1910 |
| Maternal variables | ||||||||
| Age, y | 26.4 ± 5.8 | 26.7 ± 6.0 | 27.1 ± 6.3 | 26.9 ± 6.0 | 26.5 ± 6.0 | 26.2 ± 5.7 | 26.6 ± 6.0 | 25.8 ± 5.9** |
| Height, cm | 149.6 ± 5.7 | 150.1 ± 5.1 | 150.0 ± 5.2 | 150.0 ± 5.4 | 149.8 ± 5.0 | 149.7 ± 5.4 | 149.9 ± 85.3 | 149.7 ± 5.4 |
| Weight,3 kg | 45.1 ± 6.7 | 45.5 ± 7.0 | 45.1 ± 6.7 | 45.2 ± 7.3 | 45.2 ± 6.8 | 45.4 ± 6.4 | 45.3 ± 6.8 | 45.5 ± 7.0 |
| BMI,3 kg/m2 | 20.2 ± 2.6 | 20.2 ± 2.8 | 20.0 ± 2.7 | 20.0 ± 2.7 | 20.2 ± 2.7 | 20.3 ± 2.4 | 20.1 ± 2.7 | 20.3 ± 2.7* |
| Hb,3 g/L | 117 ± 12.7 | 116 ± 13.1 | 117 ± 12.7 | 117 ± 11.9 | 116 ± 12.6 | 118 ± 13.1 | 117 ± 12.7 | 116 ± 13.1 |
| Education, y | 7.2 ± 2.6 | 7.4 ± 2.7 | 7.1 ± 2.7 | 7.2 ± 2.8 | 7.2 ± 2.7 | 7.3 ± 2.6 | 7.2 ± 2.7 | 7.7 ± 2.8** |
| Primiparity, % | 31.4 | 31.7 | 28.5 | 29.5 | 28.7 | 32.1 | 30.3 | 38.8** |
| Household variables | ||||||||
| Wealth index | −0.4 ± 2.3 | 0.0 ± 2.2 | −0.2 ± 2.5 | 0.1 ± 2.3 | −0.1 ± 2.3 | 0.1 ± 2.2 | −0.0 ± 2.3 | 0.1 ± 2.4 |
Values are means ± SDs or percentage where indicated. Asterisks indicate different from those recruited: *P < 0.05, **P < 0.01. Early food: maternal randomization to access food early in pregnancy. Usual food: maternal randomization to access food at the usual time in pregnancy; Fe30F, 30 mg iron and 400 μg folate; Fe60F, 60 mg iron and 400 μ folate; MMS, multiple micronutrient supplement.
All individuals recruited into current follow-up study.
Measured during wk 8 of gestation.
The mean age at follow-up was 4.6 ± 0.1 y and 50.5% of the recruited individuals were boys. At follow-up, the average BMI-for-age Z-score was −1.7 ± 1.0 and the height-for-age Z-score was −1.5 ± 1.0; 28% of boys and 33% of girls were classified as stunted (Table 3). All 3 blood pressure measurements were correlated (r > 0.65; P = < 0.001) and the mean of 3 readings was used as an estimate of average blood pressure. The mean systolic blood pressure was 91.1 ± 7.5 mm Hg for girls and 91.4 ± 7.7 mm Hg for boys. Mean diastolic blood pressure was 55.0 ± 6.4 mm Hg for girls and 53.8 ± 6.5 mm Hg for boys. The mean kidney volume adjusted for body surface area was 104.0 ± 15.5 cm3/m2 for boys and 104 ± 15.9 cm3/m2 for girls. Mean GFR was 158.2 ± 35.1 mL/(min · 1.73 m2) with no difference between boys and girls. Neither kidney volume nor GFR was associated with blood pressure (data not shown).
TABLE 3.
Anthropometry and body composition of offspring born during the MINIMat trial, Bangladesh at 4.5 y of age1
| Girls | Boys | |
| Participants, n | 1157 | 1178 |
| Age, y | 4.6 ± 0.1 | 4.6 ± 0.1 |
| Height, cm | 99.9 ± 26.8 | 100.5 ± 4.3 |
| Weight, kg | 13.4 ± 1.6 | 14.1 ± 1.7 |
| BMI, kg/m2 | 13.6 ± 1.0 | 13.9 ± 1.0 |
| Fat-free mass,2 kg | 10.7 ± 1.0 | 11.8 ± 1.1 |
| Fat mass,2 kg | 2.9 ± 0.7 | 2.4 ± 0.8 |
| Height-for-age Z-score3 | −1.6 ± 0.9 | −1.4 ± 1.0 |
| BMI-for-age Z-score3 | −1.6 ± 0.9 | −1.7 ± 1.0 |
The food invitation intervention successfully produced 2 groups of women who received different amounts of food during pregnancy. The median reported food packet consumption in the early invitation group was 103 packets (IQR: 67, 128) compared with 70 packets (IQR: 39, 92) in the usual invitation arm. The invitation to early food supplementation was associated with lower offspring diastolic blood pressure by a mean of 0.74 mm Hg [(95% CI: 0.18, 1.30); P = 0.01] in the fully adjusted analysis (model 3) compared with the offspring of women in the usual invitation arm (Table 4). There was no association of this intervention with systolic blood pressure or with either kidney volume or GFR.
TABLE 4.
Effect of maternal food intervention on offspring blood pressure and kidney function at 4.5 y in Bangladesh1
| n | Model 12 | P value R2 (%) | n | Model 23 | P value R2 (%) | n | Model 34 | P value R2 (%) | |
| Systolic pressure (mm Hg) | 2312 | 0.46 (−0.16, 1.08) | 0.15 (0.05) | 2196 | 0.42 (−0.21, 1.05) | 0.19 (0.03) | 1969 | 0.57 (−0.08, 1.21) | 0.09 (0.09) |
| Diastolic pressure (mm Hg) | 2312 | 0.58 (0.06, 1.11) | 0.03 (0.16) | 2196 | 0.59 (0.05, 1.13) | 0.03 (0.16) | 1969 | 0.72 (0.16, 1.28) | 0.01 (0.26) |
| Kidney volume (cm3/m2) | 1068 | −0.09 (−1.98, 1.79) | 0.92 (−0.09) | 968 | 0.01 (−1.90, 1.91) | 0.99 (−0.09) | 861 | 0.45 (−1.55, 2.45) | 0.66 (−0.08) |
| GFR5 [mL/(min · 1.73 m2)] | 1224 | −1.86 (−5.79, 2.08) | 0.36 (−0.01) | 1222 | −1.92 (−5.77, 1.94) | 0.33 (−0.00) | 1093 | −1.23 (−5.27, 2.80) | 0.55 (−0.05) |
Values are regression coefficients (β) showing the difference in mean blood pressure (95% CI) for individuals born to women invited to receive food supplements early in pregnancy (coded 0) compared with the usual time (coded 1), derived from linear regression analysis. R2 refers to adjusted partial R2 for all models. GFR, glomerular filtration rate; MMS, multiple micronutrient supplement.
Model 1, adjusted MMS and iron dummy variables only.
Model 2, additionally adjusted for age, sex, wealth index, tertiles of maternal wk 8 of gestation blood pressure (blood pressure models only), and season of birth fitted as Fourier terms (36).
Model 3, as model 2 but additionally adjusted for height, BMI, fat free mass, diarrhea in the past 2 wk, and feeling well on the study day.
GFR calculated from plasma cystatin C (17).
The mean number of tablets consumed by women in the 3 micronutrient intervention groups was 81 (Fe30F), 80 (Fe60F), and 76 (MMS), respectively. In the unadjusted intention-to-treat analysis, there was no association between the micronutrient intervention and offspring blood pressure or kidney function (Table 5). However, in the adjusted analysis, there was an effect of the micronutrient intervention on diastolic blood pressure, with a mean of 0.65 mm Hg [(95% CI: 0.06, 1.24); P = 0.03] higher diastolic blood pressure for children whose mothers received MMSs during pregnancy compared with iron and folate. There was no effect of the high- compared with low-iron intervention on offspring blood pressure (Table 6). There was also no effect of the iron intervention on offspring kidney function in unadjusted analysis, but in the adjusted analysis, individuals whose mothers had received 60 mg of iron during pregnancy had a mean 4.98 mL/(min · 1.73 m2) [(95% CI: 0.30, 9.67); P = 0.04] higher GFR at 4.5 y of age than those whose mothers had received 30 mg of iron during pregnancy.
TABLE 5.
Effect of maternal multiple micronutrient supplementation on offspring blood pressure and kidney function at 4.5 y in Bangladesh1
| n | Model 12 | P value R2 (%) | n | Model 23 | P value R2 (%) | n | Model 34 | P value R2 (%) | |
| Systolic pressure (mm Hg) | 2312 | 0.05 (−0.71, 0.81) | 0.90 (−0.04) | 2196 | 0.05 (−0.73, 0.82) | 0.90 (−0.04) | 1969 | 0.22 (−0.58, 1.02) | 0.58 (−0.03) |
| Diastolic pressure (mm Hg) | 2312 | 0.55 (−0.10, 1.20) | 0.09 (0.08) | 2196 | 0.60 (−0.07, 1.26) | 0.08 (0.09) | 1969 | 0.87 (0.18, 1.56) | 0.01 (0.25) |
| Kidney volume (cm3/m2) | 1069 | −0.98 (−3.30, 1.34) | 0.41 (−0.03) | 968 | −1.75 (−4.09, 0.60) | 0.14 (0.11) | 861 | −1.45 (−3.89, 1.00) | 0.25 (0.04) |
| GFR5 [mL/(min · 1.73 m2)] | 1224 | 1.30 (−3.55, 6.14) | 0.60 (−0.06) | 1222 | 2.08 (−2.66, 6.83) | 0.39 (−0.02) | 1093 | 3.43 (−1.56, 8.42) | 0.18 (0.07) |
Values are regression coefficients (β) showing the difference in mean blood pressure (95% CI) for individuals born to women in the MMS compared with the Fe30F intervention group, derived from linear regression analysis. R2 refers to adjusted partial R2 for all models. Fe30F, 30 mg iron and 400 µg of folate; GFR, glomerular filtration rate; MMS, multiple micronutrient supplement.
Model 1, adjusted for the iron intervention dummy and food intervention variables only.
Model 2, additionally adjusted for age, sex, wealth index, tertiles of maternal blood pressure (blood pressure models only), and season of birth.
Model 3, as model 2 but additionally adjusted for height, BMI, fat-free mass, diarrhea in past 2 wk, and feeling well on the study day.
GFR calculated from plasma cystatin C (17).
TABLE 6.
Effect of maternal iron supplementation on offspring blood pressure and kidney function at 4.5 y in Bangladesh1
| n | Model 12 | P value R2 (%) | n | Model 23 | P value R2 (%) | n | Model 34 | P value R2 (%) | |
| Systolic pressure (mm Hg) | 2312 | −0.04 (−0.80, 0.72) | 0.91 (−0.04) | 2196 | 0.06 (−0.71, 0.84) | 0.87 (−0.04) | 1969 | 0.04 (−0.76, 0.84) | 0.93 (−0.05) |
| Diastolic pressure (mm Hg) | 2312 | 0.26 (−0.39, 0.90) | 0.44 (−0.02) | 2196 | 0.33 (−0.33, 1.00) | 0.33 (0.00) | 1969 | 0.42 (−0.27, 1.11) | 0.23 (0.02) |
| Kidney volume (cm3/m2) | 1068 | −0.10 (−2.40, 2.21) | 0.93 (−0.09) | 968 | −0.54 (−2.88, 1.80) | 0.65 (−0.08) | 861 | −0.91 (−3.38, 1.57) | 0.47 (−0.05) |
| GFR5 [mL/(min · 1.73 m2)] | 1224 | 4.47 (−0.32, 9.26) | 0.07 (0.19) | 1222 | 5.05 (0.36, 9.74) | 0.04 (0.27) | 1093 | 4.68 (−0.22, 9.58) | 0.06 (0.22) |
Values are regression coefficients (β) showing the difference in mean blood pressure and kidney function (95% CI) for individuals born to women in the Fe60F compared to Fe30F intervention group, derived from linear regression analysis. R2 refers to adjusted partial R2 for all models. Fe60F, 60 mg of iron and 400 µg of folate; GFR, glomerular filtration rate; MMS, multiple micronutrient supplement.
Model 1, adjusted for the MMS intervention dummy and food intervention variables only.
Model 2, additionally adjusted for age, sex, wealth index, tertiles of maternal blood pressure (blood pressure models only), and season of birth.
Model 3, as model 2 but additionally adjusted for height, BMI, fat-free mass, diarrhea in past 2 wk, and feeling well on the study day.
GFR calculated from plasma cystatin C (17).
The combined effect of the food and micronutrient interventions was assessed by introducing 2 interactions terms, one between the food and MMS intervention and one between the food and Fe intervention. There appeared to be no interaction between the interventions in relation to offspring blood pressure or kidney function (Supplemental Table 1). There were no interactions between any of the potential effect modifiers tested (sex, wealth index, or maternal baseline BMI) and any of the 3 interventions on either child blood pressure or kidney function (data not shown).
The analyses of both the food and micronutrient interventions were repeated using an as-treated rather than intention-to-treat design. There was no association between the number of food packets consumed and offspring blood pressure, kidney volume, or GFR (Supplemental Table 2). Similarly, there was no association between tablet consumption as measured by bottle opening and offspring blood pressure, kidney volume, or GFR (Supplemental Table 3).
Discussion
This study is one of the first to report follow-up data on offspring blood pressure and kidney function as a result of a combined pregnancy nutritional intervention. There is evidence that invitation to access food supplements early in pregnancy was associated with a slightly lower offspring diastolic blood pressure at 4.5 y compared with the standard care in later pregnancy. In contrast, MMSs were associated with slightly higher diastolic blood pressure compared with iron and folate supplementation alone and a high- compared with low-iron supplement was associated with a higher offspring GFR. No other effects of the food or micronutrient interventions on offspring blood pressure, kidney volume, or GFR were observed and there was no interaction between the interventions on the outcomes studied.
Previous studies of protein-energy supplementation trials do not provide a direct comparison with the MINIMat intervention, partly because they lack a temporal variation in food supplementation. The protein-energy supplements provided from 20 wk of gestation to pregnant women in The Gambia were not associated with offspring blood pressure at 10–17 y of age (23), and those provided during pregnancy and early childhood in Guatemala were similarly not associated with offspring blood pressure at 20–29 y of age (24). In India, although a community-based cereal meal intervention was not associated with offspring blood pressure in adolescence, children born in intervention areas had a lower measure of global arterial stiffness (the augmentation index) (25). In the current study, no association was observed between food packet consumption and offspring diastolic blood pressure irrespective of the treatment arm, which cautions against the overinterpretation of these findings. In addition, the relatively small effect size and the lack of an effect on systolic blood pressure may question the validity of the findings. However, these data do suggest that researchers may wish in future studies to assess the timing as well as the type of pregnancy intervention.
Multiple-micronutrient supplementation in pregnancy was associated with a marginal increase in offspring diastolic blood pressure, although the association was only strongly apparent in the fully adjusted analysis. Two other trials, both from Nepal, of multiple-micronutrient supplementation in pregnancy have published data on offspring blood pressure. In contrast to the MINIMat trial, both the Nepalese trials reported an increase in birth weight in the multiple-micronutrient arms of the trial (26, 27). The follow-up studies have shown inconsistent results, however. Vaidya et al. (10) reported lower systolic blood pressure at 2.5 y of age for children born to women who had received multiple micronutrients in pregnancy. In contrast, Stewart et al. (11) found no effect of MMSs on child blood pressure at 6–8 y, consistent with the results presented here. One interpretation of the first Nepalese trial is a detrimental effect of the high-iron dose in the comparison group (10). The design of the MINIMat trial allows for this to be tested and the analysis presented here has shown no effect of high- compared with low-iron dose on offspring blood pressure in this population.
It has been suggested that a reduction in nephron number as a result of inadequate fetal nutrition represents a potential mechanism linking low birth weight and hypertension, with supportive evidence from animal models (3, 28–30). In humans, low birth weight has been associated with lower kidney volume (by ultrasound) (31) and chronic kidney disease in later life (32). Few human studies have investigated the association between the maternal diet during pregnancy and offspring kidney function. In the second Nepalese trial reported above, the control group (vitamin A supplements only) had a higher risk of urinary microalbuminuria (microalbumin:creatinine ratio ≥3.4 mg/mmol) compared with individuals whose mothers received folic acid or folic acid, iron, and zinc in addition to vitamin A (11). Here, we found weak evidence for an increased GFR in children exposed to high-iron supplementation during in utero development, which is challenging to interpret. Some authors have suggested that fewer nephrons will initially be associated with compensatory hyperfiltration (33), which could be consistent with a higher GFR. In later life, these same individuals may demonstrate relatively low GFR, which is a standard marker of kidney disease (34), but further studies will be required to unravel this association and assess validity.
There are a number of important strengths of the current study, including the large sample size and good retention of participants; 77% of eligible individuals born during the maternal trial were successfully recruited at 4.5 y of age. Loss to follow-up is a well-recognized issue for long-term follow-up studies within this research field and these rates of loss are relatively low (35). The lack of differential follow-up rates between the intervention groups and the marginal differences between recruited individuals and those lost to follow-up suggests limited selection bias for the current study. A related issue is the reduction in sample size as a consequence of participant attrition, which affects study power. In this study, the precision estimate for the effect of maternal MMS supplementation on offspring systolic blood pressure ranged from −0.57 to 1.02 mm Hg and effects within this range cannot therefore be discounted. However, it is arguable whether effect sizes of this magnitude would be meaningful from a public health standpoint. Cost and logistic considerations resulted in the measurements of kidney volume and GFR being conducted on a subset of individuals at the 4.5-y follow-up, but the sample size remained large and the precision of effect estimates high. Although it was a limitation that only a small number of individuals had both kidney function measurements, this would not be expected to have influenced the analysis of the intervention effect; the sample size for both outcomes remained large and there is no reason to think that the intervention would have a different effect in these subgroups.
The main limitation with the original study design is that women were only randomly assigned to encouragement to access food provision rather than being provided with the food supplement itself. Some women would therefore be unlikely to access the supplement at all, although only 54 individuals reported not having had a single food packet during their pregnancy and the majority (76%) of these were in the usual food invitation arm. The intervention was designed to evaluate the existing government nutritional provision, but as a result lacked a true control arm. However, data on food packet consumption during pregnancy did demonstrate that the intervention successfully created 2 distinct groups; women in the early invitation arm accessed the service much earlier in pregnancy and consumed on average 30 more packets during pregnancy. The micronutrient intervention benefitted from being a double-blind randomized trial but again lacked a control group that could be provided with a placebo owing to the ethics of withdrawing FeFol from this antenatal population, which receives 60 mg of iron and 400 μg folate as standard. This intervention is further compromised by its unbalanced design; there is no multiple micronutrient arm that also contains 60 mg of iron. As a consequence, any effect of the MMS arm could be confounded by the iron treatment arm and it is not possible to look at the interaction between these different treatments. However, because only limited intervention effects have been observed, this limitation is of minor concern. A final important limitation relates to the age of study participants who may be too young for differences in CVD risk factors to emerge. It will be important to study this cohort into the future to assess any prolonged effects of the pregnancy intervention, particularly as groups of offspring become exposed to different environments as they age.
In conclusion, this is one of the first studies to include a follow-up of a combined food and micronutrient intervention in pregnancy. There is some evidence that access to food supplements early in pregnancy is associated with reduced offspring diastolic blood pressure in childhood, albeit with a relatively small effect size. Weaker evidence was also found for an impact of MMSs on increased offspring diastolic blood pressure and of high- compared with low-iron supplements on increase offspring GFR. Overall, there was limited evidence for long-lasting impacts of pregnancy supplementation on offspring blood pressure or markers of kidney function in this population.
Supplementary Material
Acknowledgments
L.-Å.P., S.E.M., S.-E.A., and Y.W. designed the study; S.H., A.I.K., and M.D.H.H. conducted the fieldwork; Y.W. provided ultrasound expertise and training; A.J.C.F. advised on the statistical analysis; and S.H. wrote the manuscript with guidance from S.E.M. and A.J.C.F. All authors read and approved the final manuscript.
Footnotes
Abreviations used: CVD, cardiovascular disease; Fe30F, 30 mg iron and 400 µg of folate; Fe60F, 60 mg of iron and 400 µg of folate; GFR, glomerular filtration rate; icddr,b, International Centre for Diarrheal Disease Research, Bangladesh; MMS, multiple micronutrient supplement.
Literature Cited
- 1.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98 [DOI] [PubMed] [Google Scholar]
- 2.McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats: a review. Placenta. 2006;27 Suppl A:S56–60 [DOI] [PubMed] [Google Scholar]
- 3.Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol. 2005;16:2545–56 [DOI] [PubMed] [Google Scholar]
- 4.Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. J Intern Med. 2007;261:437–52 [DOI] [PubMed] [Google Scholar]
- 5.Adair L, Dahly D. Developmental determinants of blood pressure in adults. Annu Rev Nutr. 2005;25:407–34 [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ. The developmental origins of insulin resistance. Horm Res. 2005;64 Suppl 3:2–7 [DOI] [PubMed] [Google Scholar]
- 7.Hawkesworth S. Conference on "Multidisciplinary approaches to nutritional problems". Postgraduate Symposium. Exploiting dietary supplementation trials to assess the impact of the prenatal environment on CVD risk. Proc Nutr Soc. 2009;68:78–88 [DOI] [PubMed] [Google Scholar]
- 8.Prentice AM, Moore SE. Early programming of adult diseases in resource poor countries. Arch Dis Child. 2005;90:429–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkesworth S, Walker CG, Sawo Y, Fulford AJ, Jarjou LM, Goldberg GR, Prentice A, Prentice AM, Moore SE. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in The Gambia. Am J Clin Nutr. 2011;94:S1853–60 [DOI] [PubMed] [Google Scholar]
- 10.Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371:492–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP, Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139:1575–81 [DOI] [PubMed] [Google Scholar]
- 12.Persson LÅ, Arifeen S, Ekstrom EC, Rasmussen KM, Frongillo EA, Yunus M. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in bangladesh: The MINIMat Randomized Trial. JAMA. 2012;307:2050–9 [DOI] [PubMed] [Google Scholar]
- 13.Tofail F, Persson LA, El Arifeen S, Hamadani JD, Mehrin F, Ridout D, Ekstrom EC, Huda SN, Grantham-McGregor SM. Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. Am J Clin Nutr. 2008;87:704–11 [DOI] [PubMed] [Google Scholar]
- 14.Khan AI, Kabir I, Ekstrom EC, Asling-Monemi K, Alam DS, Frongillo EA, Yunus M, Arifeen S, Persson LA. Effects of prenatal food and micronutrient supplementation on child growth from birth to 54 months of age: a randomized trial in Bangladesh. Nutr J. 2011;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AI, Kabir I, Hawkesworth S, Ekstrom E-C, Arifeen S, Frongillo EA, Persson LA. Early invitation to food and/or multiple micronutrient supplementation in pregnancy does not affect body composition in offspring at 54 months: follow-up of the MINIMat randomized trial, Bangladesh. Matern Child Nutr. Epub 2012 Dec 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flodin M, Jonsson AS, Hansson LO, Danielsson LA, Larsson A. Evaluation of Gentian cystatin C reagent on Abbott Ci8200 and calculation of glomerular filtration rate expressed in mL/min/1.73 m(2) from the cystatin C values in mg/L. Scand J Clin Lab Invest. 2007;67:560–7 [DOI] [PubMed] [Google Scholar]
- 17.Grubb A, Nyman U, Bjork J, Lindstrom V, Rippe B, Sterner G, Christensson A. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–31 [DOI] [PubMed] [Google Scholar]
- 18.Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, Beek FJ. Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology. 1999;211:623–8 [DOI] [PubMed] [Google Scholar]
- 19.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6 [DOI] [PubMed] [Google Scholar]
- 20.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–29 [PubMed] [Google Scholar]
- 21.Kahn AI, Hawkesworth S, Hossain D, Arifeen SE, Moore SE, Hills AP, Wells JC, Persson LA, Kabir I. Body composition of Bangaldeshi children: comparison and development of leg-to-leg bioelectrical impedance equation. J Health Popul Nutr. 2012;30:281–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha KK, Frongillo EA, Alam DS, Arifeen SE, Persson LA, Rasmussen KM. Appropriate infant feeding practices result in better growth of infants and young children in rural Bangladesh. Am J Clin Nutr. 2008;87:1852–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkesworth S, Prentice AM, Fulford AJ, Moore SE. Maternal protein-energy supplementation does not affect adolescent blood pressure in The Gambia. Int J Epidemiol. 2009;38:119–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb AL, Conlisk AJ, Barnhart HX, Martorell R, Grajeda R, Stein AD. Maternal and childhood nutrition and later blood pressure levels in young Guatemalan adults. Int J Epidemiol. 2005;34:898–904 [DOI] [PubMed] [Google Scholar]
- 25.Kinra S, Rameshwar Sarma KV, Ghafoorunissa , Mendu VV, Ravikumar R, Mohan V, Wilkinson IB, Cockcroft JR, Davey Smith G, Ben-Shlomo Y. Effect of integration of supplemental nutrition with public health programmes in pregnancy and early childhood on cardiovascular risk in rural Indian adolescents: long term follow-up of Hyderabad nutrition trial. BMJ. 2008;337:a605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, Filteau S, Tomkins A, Costello AM. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365:955–62 [DOI] [PubMed] [Google Scholar]
- 27.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, Adhikari RK, Sommer A, West KP., Jr Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. J Nutr. 2007;137:1066–72 [DOI] [PubMed] [Google Scholar]
- 29.Merlet-Bénichou C, Gilbert T, Muffat-Joly M, Lelievre-Pegorier M, Leroy B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 1994;8:175–80 [DOI] [PubMed] [Google Scholar]
- 30.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer J, Wang Z, Hoy W. Low birth weight and reduced renal volume in Aboriginal children. Am J Kidney Dis. 2001;37:915–20 [DOI] [PubMed] [Google Scholar]
- 32.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, et al. Is low birth weight an antecedent of CKD in later life? A Systematic Review of Observational Studies. Am J Kidney Dis. 2009;54:248–61 [DOI] [PubMed] [Google Scholar]
- 33.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–47 [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83 [DOI] [PubMed] [Google Scholar]
- 35.Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, Koletzko B, Lucas A. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child. 2008;93:458–61 [DOI] [PubMed] [Google Scholar]
- 36.Fulford AJ, Rayco-Solon P, Prentice AM. Statistical modelling of the seasonality of preterm delivery and intrauterine growth restriction in rural Gambia. Paediatr Perinat Epidemiol. 2006;20:251–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.