Abstract
Despite 30 years of study there is no HIV-1 vaccine and until recently there was little hope for a protective immunization. Renewed optimism in this area of research comes in part from the results of a recent vaccine trial, and the use of single cell antibody cloning techniques that uncovered naturally arising, broad and potent HIV-1 neutralizing antibodies (bNAbs). These antibodies can protect against infection and suppress established HIV-1 infection in animal models. The finding that these antibodies develop in a fraction of infected individuals supports the idea that new approaches to vaccination might be developed by adapting the natural immune strategies or by structure-based immunogen design. Moreover, the success of passive immunotherapy in small animal models suggests that bNAbs may become a valuable addition to the armamentarium of anti-HIV-1 drugs.
Effective vaccines usually require potent antibody responses to block infection and/or clear the pathogen (1). For all vaccines that have been developed, the protective antibodies are directed to invariant molecular components of the pathogen. Even in the case of the Influenza virus, whose envelope spike is constantly evolving, the seasonal vaccine elicits antibodies that target parts of the spike that are shared by the dominant circulating viral species (2). In contrast, an effective HIV-1 vaccine would need to induce antibodies that target a large number of rapidly evolving contemporaneous viral strains whose envelope spikes differ by as much as 35% in their amino acid sequence (3). Besides presenting the immune system with a diverse and moving target, HIV-1 displays only a small number of functional envelope spikes per virion with a high density of rapidly shifting glycans that shield sites of potential vulnerability (4–9). Thus, a vaccine to this pathogen would have to induce high affinity antibodies that penetrate the glycan shield and bind effectively to sparsely expressed and highly diversified envelope spikes.
The possibility that such antibodies might exist was supported by clinical studies that revealed that 10–30% of individuals who had been infected for 2–4 years develop serologic activity with the ability to neutralize diverse viral isolates (10–13). Among these individuals, some (around 1%) exhibit exceptional cross-clade activity and potency, and are referred to as elite neutralizers (14). Although serum from elite neutralizers has not been tested directly, the principle that antibodies in serum could be protective against HIV-1 was established by passive transfer of pooled human serum to chimpanzees, which were then protected from infection with HIV-1IIIb (15). However, efforts to molecularly characterize the protective antibodies in human sera were stymied because of the limited nature of the techniques available for human antibody cloning.
First generation bNAbs
Despite technical limitations, several groups were able to identify anti-HIV-1 antibodies with broad neutralizing activity (bNAbs). Although not very potent, this initial group of antibodies defined some of the sites of HIV-1 vulnerability on the envelope spike which consists of three g120/gp41 heterodimers (Fig. 1). These sites included the V3 loop (e.g. 447-52D (16)), the binding site for CD4 which is primary viral receptor for entry (b12 (17)); the CD4-induced site that is the viral binding site for the co-receptor (e.g., 17b (18)); viral glycans (2G12 (19)); and the membrane proximal external region (MPER) of gp41 (4E10 and 2F5 (20, 21)). In vitro, the most broad and potent antibodies in this initial group were b12 (MeanGeo IC50 3.1 µg/ml, breadth 40%), 2G12 (3.0 µg/ml, 27%), 2F5 (3.6 µg/ml, 58%), and 4E10 (2.7 µg/ml, 96%) (22). Moreover, besides in vitro neutralization, passive transfer of b12, 2G12, 2F5 or 4E10 protected against SHIV (simian immunodeficiency viruses (SIV) that express the HIV-1 envelope glycoprotein) infection in macaques (23–29).
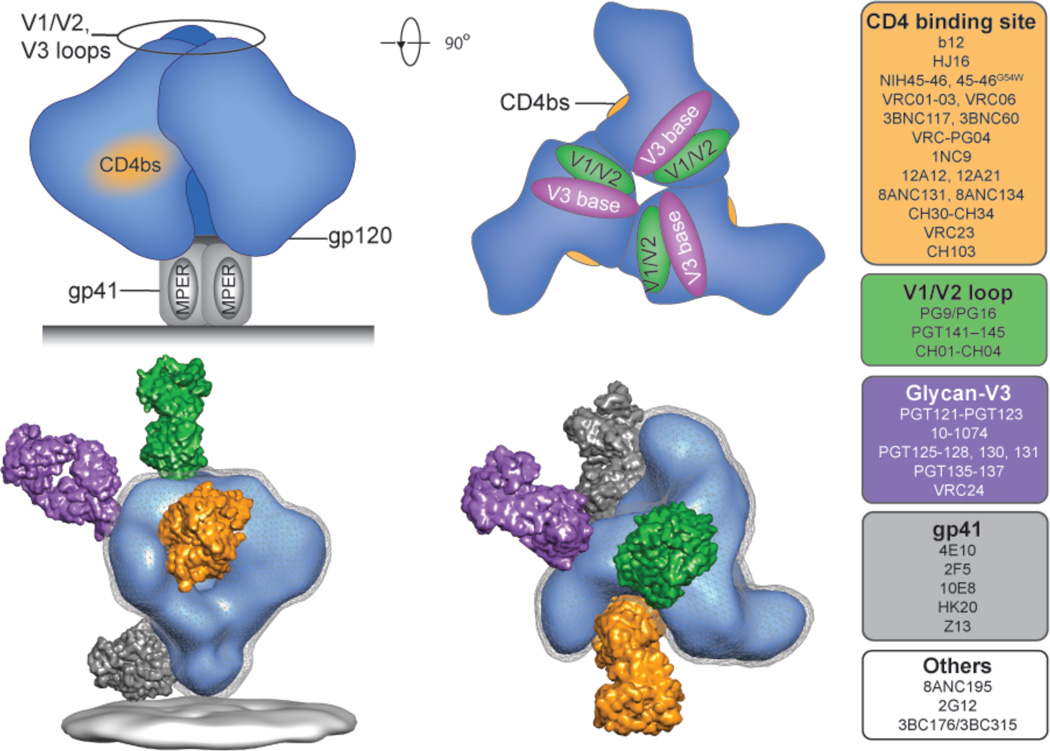
Figure 1. Antibody target sites on the HIV-1 envelope spike.
Schematic representation of the HIV-1 envelope spike. Each of the monomer units of the trimer is composed of a gp120 (blue) and gp41 transmembrane protein (grey). The four best-characterized broadly neutralizing target sites are highlighted and include the CD4 binding site (orange); the glycan-associated epitopes on the base of the V3 loop (purple); the V1/V2 loop (green); and the MPER on gp41 (grey). EM-derived illustration of the envelope spike targeted by representative broadly neutralizing F(ab)s shown approximately to scale (bottom; NIH45–46, yellow; PG16, green; PGT128, purple; 2F5, grey). Examples of first- and second-generation bNAbs that target these and other sites are color-coded and indicated at the right hand side.
Despite these achievements there was little enthusiasm for the possibility of an antibody-based vaccine because all of the initially characterized antibodies were unusual. 2G12 has three combining sites per antigen-binding fragment (Fab) instead of the usual two (19, 30), 2F5 and 4E10 are self-reactive (31, 32) and finally, b12 is a phage-derived antibody generated by random pairing of heavy and light chains that may have never existed in nature (17). Consistent with their unusual features, innumerable attempts to elicit these antibodies by vaccination were not successful. Most importantly however, the amount of these antibodies required for complete protection in macaques was thought to be too high to be achieved by vaccination (33). Consistent with these ideas all envelope-based HIV-1 vaccines failed and as a result, much of the vaccine effort focused exclusively on T cell mediated immunity that also failed to prevent HIV-1 infection to date.
A new beginning
Two very recent developments have re-focused attention on antibody-based HIV-1 vaccines. First, single cell antibody cloning techniques were developed and introduced as a means to systematically study and obtain HIV-1 envelope-reactive antibodies (34–37)). Second, modest levels of reduced risk of infection in the RV144 human vaccine trial were correlated with antibody responses to the HIV-1 envelope spike (38, 39).
Efficient methods for human antibody cloning from single cells were initially developed to study mechanisms that govern tolerance in the B cell compartment (37). These methods were adapted in order to identify single B cells that express antibodies that bind to the HIV-1 envelope spike (34, 35, 40) or cultured B cells that were screened for the production of neutralizing activity (41, 42). When applied to individuals with high serum titers of HIV-1 neutralizing activity, these methods uncovered a large number of new antibodies that were up to 2–3 orders of magnitude more potent than those that were previously discovered (41–45). Protection experiments with one of the new antibodies in macaques showed that serum concentrations of as little as 15 µg/ml were completely protective against high dose challenges of SHIVSF162P3 (46) and similar results were also obtained in mice (47, 48) suggesting that sterilizing antibody responses might in fact be possible to achieve by vaccination.
The RV144 trial re-focused attention on antibodies because modified intention to treat analysis revealed a 31% (p=0.04) reduction in infection that correlated with the presence of antibodies that bind to the V1/V2 region of the envelope spike (38, 39). Interestingly, the antibody response induced by the vaccine did not neutralize primary HIV-1 isolates, and it has been proposed that the effects were mediated instead by antibody-dependent cellular cytotoxicity (ADCC) (49). However, this idea has not been proven directly. Although non-neutralizing antibodies showed effects in some studies (50), they failed to effectively protect against infection in mice (47) and other studies in non-human primates (51). Therefore the precise mechanism of protection in the RV144 trial is unknown.
Second generation bNAbs and their target sites
What is the molecular basis for broad and potent serologic neutralizing activity in HIV-1-infected individuals that develop these responses? Single cell antibody cloning experiments revealed that some humans produce a neutralizing response to HIV-1 by targeting two or more different sites on the envelope spike (34, 40, 44, 52, 53), whereas in others, serum neutralizing activity could be accounted for by one prominent antibody clone (41–44).
To date the new bNAbs have been found to target four major “sites of vulnerability” on the HIV-1 envelope spike (Fig. 1)(54). These include the CD4 binding site (CD4bs); the N160 glycan-dependent site associated with the V1/V2 loops; the N332 glycan dependent site at the base of the V3 loop; and the MPER on gp41 (Fig. 1). Additional sites of vulnerability are likely to be discovered as exemplified by 8ANC195 and 3BC176 that recognize yet to be defined epitopes that are distinct from the 4 major target sites (22, 40, 44).
The CD4bs is a protected site in that it is recessed and surrounded by glycans and variable regions (55). Nevertheless, this functionally conserved site is accessible to CD4 and the CD4bs antibodies. The most potent of the new bNAbs targeting the CD4bs (e.g. NIH45–46 and 3BNC117) are derived from the same germline VH-gene segment VH1–2 (44, 56–58). Structural analysis revealed that although they were isolated from different individuals, VH1–2 antibodies share a common mode of antigen recognition whereby framework residues contribute to CD4 mimicry (44, 56–58). A short complementarity determining region 3 of the light chain (CDRL3) interacts with the gp120 V5 and D loops, and a short CDRL1 circumvents clashes with glycans at Asn276 on the D loop (44, 56–58).
The N160 glycan/V1/V2 site is targeted by PG9/PG16, CH01–CH04 and PGT141–145 (Fig. 1) (41, 42). Structural analyses showed that these antibodies penetrate the glycan shield with a long anionic CDRH3 that contacts a β-strand on gp120, and interacts with N-linked glycans from two adjacent gp120 protomers in the trimeric envelope complex (59, 60). Moreover, binding of PG9 to the envelope spike seems to alter the stability of the trimer (60) and might contribute to its potent neutralizing activity.
The N332 glycan/V3 loop site is targeted by PGT121- and PGT128-like bNAbs (Fig. 1) (42, 45, 61). A crystal structure of PGT128 bound to its ligand shows that it contacts the C-terminal end of the V3 loop, Man8/9 and Man5GlcNAc2 at positions N332 and N301, respectively (61). PGT121-like antibodies also contact gp120 at the base of the V3 loop, and a high mannose- or a complex-type N-glycan at N332 (45, 62). Although the two classes of antibodies likely approach the N332 site from different angles, both seem to block infection by interfering with CD4 binding (62).
10E8 targets the membrane proximal region in gp41 (Fig. 1). This antibody recognizes an α-helix, and unlike 2F5 and 4E10, which were less potent first generation antibodies targeting a closely related site, 10E8 is reported not to be auto- or phospholipid-reactive (63).
Vaccine design efforts have focused on independent targeting of the four major sites of vulnerability on the envelope spike, but it is still unclear how frequently each of these sites are targeted by antibodies that develop during natural infection. For example, the most recent estimates based on serologic studies and computational methods indicate that only a fraction of the infected individuals that produce broadly neutralizing antibodies do so by producing a dominant CD4bs-specific monoclonal antibody with exceptional breadth and potency (53, 64). However, only a few individuals have been studied comprehensively and additional sites of vulnerability could still be discovered. It may therefore still be too early to make firm conclusions about the relative frequency with which antibodies to specific viral targets or their combinations contribute to the development of anti-HIV-1 serologic breadth and potency in infected individuals. Although the process of obtaining this information is costly, it may be particularly valuable in informing vaccine approaches because neutralizing antibodies that develop naturally with greater frequency may also be easier to elicit by vaccination.
Characteristics of bNAbs
One of the surprising findings that emerged during the initial molecular characterization of the human HIV-1 antibody response is that envelope-directed antibodies are highly somatically mutated (34). Human IgG antibodies typically carry 10–20 VH gene somatic mutations (65). In contrast, HIV-1-specific IgG antibodies with limited neutralizing activity carry nearly twice as many mutations (34, 66) whereas the heavy chain genes of the new generation of broad and potent neutralizers frequently carry over 80 VH mutations (44, 67–69).
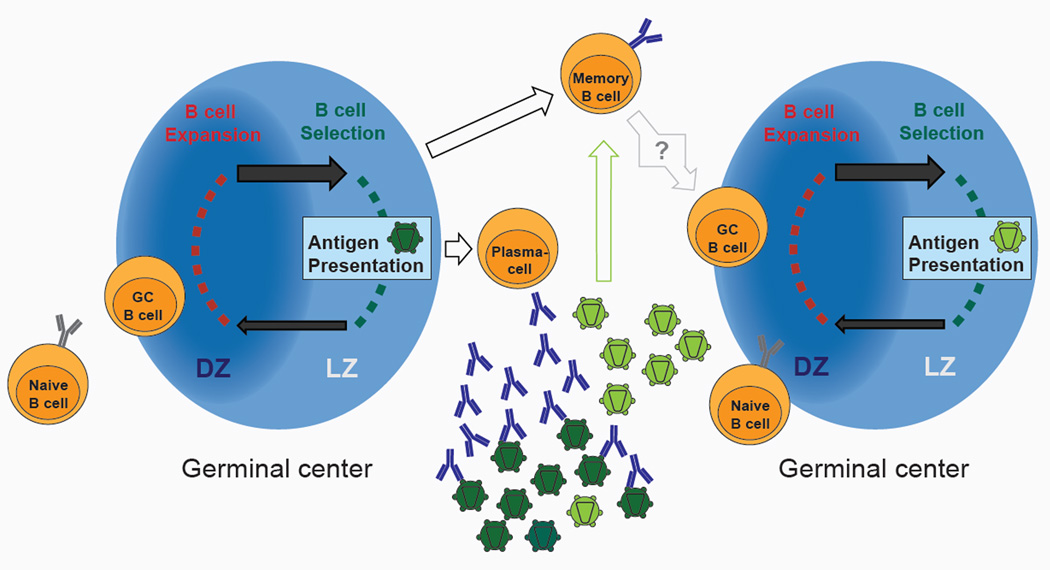
Somatic mutations are introduced into antibody genes during the immune responses to antigen in a defined microanatomical compartment called the germinal center that is located within secondary lymphoid organs (Fig. 2) (70). Following interaction with cognate antigen and T cells, B cells enter the germinal center where they undergo clonal expansion, somatic mutation and selection for B cells expressing high affinity antibody variants (70). Mutation is focused on the CDRs of the antibody that typically contact the antigen. This maximizes the probability of altering the binding properties of the antibody and minimizes changes in the framework regions (FWRs) that form the conserved structural support for the binding site (71–73). B cells that develop high affinity antibodies are favored in the germinal center, because they capture greater amounts of antigen, which is then processed and presented to cognate T-follicular helper cells that deliver a positive selection signal (70). The selection mechanism is subject to an affinity ceiling. Affinity is directly related to the “on” and “off” rates of the antibody: the higher the “on” rate, and the slower the “off” rate, the higher the affinity. Affinity selection is limited because the “on” rate is limited by diffusion, and the “off” rate is limited by the rate of antigen internalization (74, 75). Once the antigen is inside the cell and being degraded, there is no added value to a slower “off” rate. Thus, any B cell with an antibody that has an off rate that is slower than the rate of antigen internalization is equally selected. Once the affinity ceiling is reached there is no further value to additional somatic mutation and B cells exit the germinal center as plasma cells or memory B cells (70). In humans, this affinity ceiling is usually reached by 10–15 nucleotide mutations in the VH. Thus, the high level of mutation in HIV-1 antibodies are unikely to be the product of a single round of affinity maturation in the germinal center and high levels of mutation imply multiple rounds of selection in response to a continually evolving pathogen (Fig. 2).
Figure 2. The HIV-1 envelope-specific B cell response.
Naïve B cells selected by antigen interact with cognate T cells and are recruited to the germinal center. In the dark zone (DZ) B cells proliferate and express Activation-induced deaminase (AID) resulting in the acquisition of somatic mutations. The cells of this expanded and diversified B cell clone migrate to the light zone (LZ) where they encounter the antigen (e.g. viral envelope protein; dark green) presented as immune complexes on the surface of follicular dendritic cells. High affinity antigen-binding B cells that capture and present antigen to T cells are selected to return to the dark zone to proliferate or differentiate into memory B or plasma cells. High affinity envelope-directed antibodies produced by the plasma cells exert selection pressure on the virus population, but viral escape variants emerge and expand (light green). Envelope antigens expressed by the selected virus clone are presented to either naive B cells or to memory B cells that have already encountered an HIV-1 envelope antigen thereby re-initiating the cycle of clonal selection and somatic mutation.
bNAbs are exceptionally highly mutated including insertions and deletions, which are uncommon in other antibodies (42, 44,45, 67–69). When reverted to their germline sequences, the new bNAbs lose their neutralizing activity and even fail to bind to most HIV-1 envelope proteins, raising the question of how B cells bearing these antibodies were stimulated to join the germinal center reaction to begin with (44, 56, 76, 77). One possibility is that the germline antibody expressing B cell was initially stimulated by a non-HIV-1 antigen and developed reactivity to HIV-1 by chance mutation (78). Another possibility would be that only some HIV-1 envelope spikes are able to activate B cells expressing the appropriate germline antibodies. This idea is supported by the finding that some SHIVs induce bNAbs whereas others do not (79), and by a prospective study on an HIV-1 infected individual who developed a broad and potent CD4bs-specific antibody (see below) (80).
The exceptionally high number of somatic mutations in bNAbs was analyzed by investigating the role of mutations in the antibodies’ FWRs. Unlike other antibodies, including HIV-1-reactive antibodies with limited neutralizing activity, bNAbs were found to require FWR mutations for their potent activity (81). In contrast to most other antibodies, mutations in the FWRs of bNAbs can contribute to neutralization breadth and potency by altering the antibody’s flexibility and by enhancing direct contact with the antigen (81). This is of particular importance, since IgV genes have evolved to be more resistant against somatic mutations that alter the coding sequence of the FWR. Moreover, these mutations are normally selected against because they tend to destabilize the antibody scaffold that is required to support the CDRs. Therefore, the requirement for FWR mutation may in part explain why large numbers of mutations are required to generate most bNAbs.
A second unusual feature shared by some but not all of the new bNAbs is a long CDRH3, which can be used to penetrate the glycan shield (59). For example, PG9/PG16 and PGT145 which recognize a combination of carbohydrates and the V1/V2 loop, show exceptionally long CDRH3s. Moreover, long CDRH3s are found in antibodies that recognize a complex epitope composed of carbohydrates and protein at the base of the V3 loop, as well as in some of the antibodies to the CD4 binding site, and in 10E8 that targets the MPER (82).
Finally, antibodies cloned from HIV-1 specific memory B cells are frequently polyreactive (66, 76). Polyreactivity refers to an antibody’s ability to bind to a variety of different antigens with low affinity (83). Although polyreactivity is a normal feature of the human antibody system, it is selected against during B cell development (37). Polyreactivity increases, however, in the germinal center as a result of somatic mutation and selection such that 23% of all memory B cells normally produce these antibodies (65). Memory B cells producing antibodies specific for the HIV-1 envelope spike are unusual in that approximately 70% are polyreactive (66, 76). This property can be positively selected at two different steps in the development of HIV-1 specific B cells. Mature B-cells expressing polyreactive anti-HIV-1 receptors are preferentially recruited into the germinal center reaction and this property is often retained despite somatic mutation and affinity maturation (76). In addition, non-polyreactive HIV-1-reactive B cells that enter germinal centers and undergo somatic mutation can also be selected for polyreactivity (78, 80).
Why HIV-1 antibodies are selected for polyreactivity is unknown, but one potential explanation is that this property can increase the valency and therefore the avidity of these antibodies. It has been estimated that HIV-1 displays only ~ 15 functional envelope spikes per virion (8), and therefore an antibody would have to bind monovalently unless it can bind to two separate monomers on the same trimer (6–8). In theory, polyreactivity would allow heterotypic binding by combining the recognition of the envelope spike with one Fab arm and a nearby polyreactive, or specific low affinity ligand with the other (45, 76, 84). This general strategy, which can be referred to as heteroligation, may be used by antibodies such as 2F5 and 4E10 combining binding of gp41 and the viral membrane (85, 86), and antibody 21c, which binds to both gp120 and CD4 (84).
Despite the prevalence of polyreactivity among all HIV-1 envelope-directed antibodies, most of the new bNAbs are not highly poly- or self-reactive. Exceptions include the less potent first generation bNAbs 2F5 and 4E10 that target the gp41 MPER (31, 87), a few antibodies to the CD4bs (i.e. NIH45–46 and CH103/CH104/CH106) and to the glycanassociated V1/2 loops (i.e. CH01–CH04) (44, 80, 88).
In conclusion, a low level of polyreactivity is a normal feature of the antibody system that appears to be used by HIV-1 antibodies to increase their affinity/avidity. It should not necessarily be viewed as an obstacle to the development of an HIV-1 vaccine.
New approaches to HIV-1 vaccination
Although passive transfer experiments in macaques and mice (46, 48) strongly suggest that a vaccine that induces potent bNAbs would be protective against HIV-1, these antibodies have never been induced by immunization in humans (89). Characterization of the human antibody response to HIV-1 is starting to reveal some of the reasons why this has been so difficult and is providing some initial clues to rational approaches to this problem.
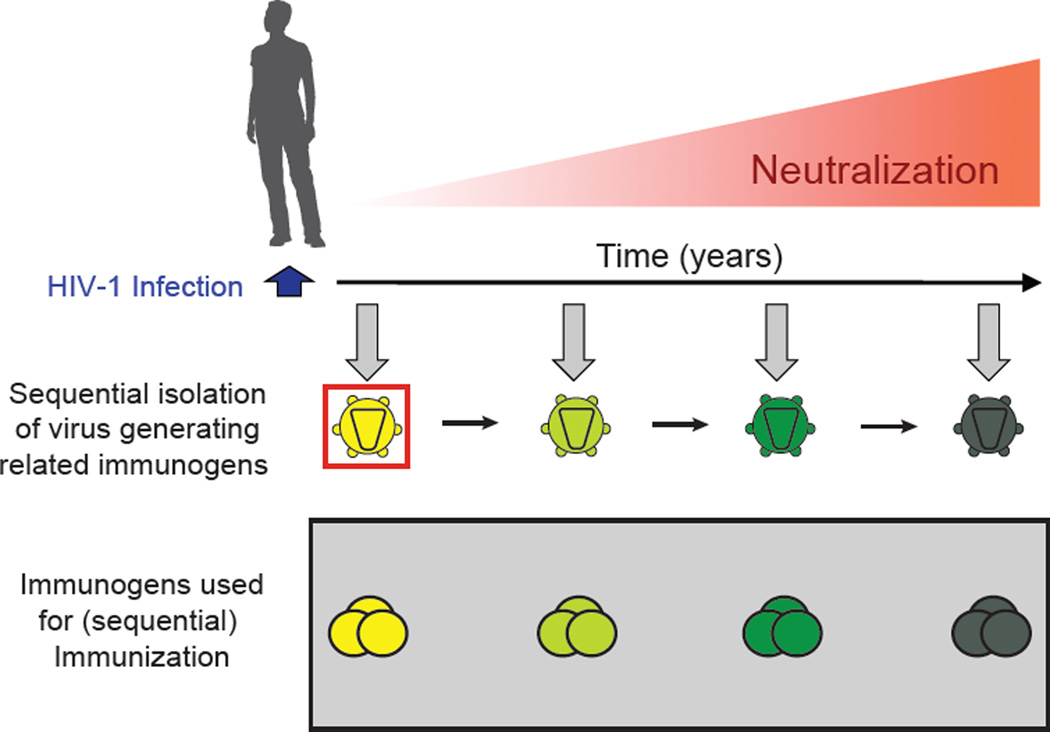
Given the unusual structural properties of bNAbs, it appears likely that HIV-1 vaccination will require alternatives to the traditional vaccine approaches. One possibility is to try to reproduce the natural sequence of events that led to the development of a potent bNAb using HIV-1 envelope proteins obtained from infected individuals that developed such antibodies (5, 44,67, 80, 90). This approach necessitates understanding the co-evolution of the virus and the antibodies in individuals that develop breadth and potency. Only one such study has been published to date (80). The authors of this study used single cell antibody cloning techniques (34, 37) to isolate the antibody CH103, the corresponding transmitted founder virus, and multiple intermediates from an African donor who developed serologic breadth and potency (80). Sequence analysis revealed extensive epitope evolution by the virus, and somatic mutation in the antibody preceding the development of breadth and potency (55% of HIV-1 strains were neutralized with a mean IC50 of 4.54 µg/ml). Most importantly, although the germline precursor of CH103 did not bind heterologous envelope proteins, it bound to the transmitted founder virus (80). Therefore it seems unlikely that other envelope proteins would initiate the response that led to the development of the CH103 antibody. Mimicking envelope evolution by sequential immunization is therefore one approach to activate and then shepherd B cells expressing the correct germline antibody to evolve into bNAbs (Fig. 3).
Figure 3. Implications for vaccine design by studying HIV-1 and antibody co-evolution in an individual with bNAbs.
Longitudinal analysis of an HIV-1-infected individual was performed from the time of infection up to the development of bNAbs (Liao et al., 2013). The evolution of the HIV-1 envelope on the virus drives the diversification of the antibody response. Isolation and sequences analysis of the HIV-1 envelope on the founder virus (red square) and on viruses at later stages (green to dark green) provides crucial information for generation of antigens that can potentially elicit a broadly neutralizing antibody response. Mimicking the evolution of antigens in an HIV-1 vaccine approach is a promising strategy to elicit bNAbs in humans.
A second non-mutually exclusive approach is to design the immunogen to specifically activate B cells expressing the germline precursors of bNAbs (91, 92). For example, combinations of computational and genetic selection methods have been used to design antigens that can activate tissue culture B cells expressing the VH1–2 germline precursors of the most potent CD4bs-specific antibodies (91, 92). Used alone, these designer antigens may not induce bNAbs but they could be potent stimulators of clonal expansion of the cells that can develop such antibodies, which might be a first step in a more complex immunization scheme (91, 92).
An important impediment to developing an HIV-1 vaccine has been the use of large animal models that necessitate low throughput and are not amenable to genetic experimentation. Systematic testing of new ideas and new HIV-1 vaccination approaches will necessitate the development of small animal models that can allow rapid iterative experimentation and a mechanistic understanding of the immune responses required to induce protection by an HIV-1 vaccine.
HIV-1 immunotherapy
Kitasato and von Behring first used serum antibodies as therapeutics for treating tetanus and diphtheria infection over a century ago. Today, serum therapy is still in use for snake and spider bites as well as rabies post exposure prophylaxis. Furthermore, there are over 30 FDA-approved monoclonal antibodies for cancer, autoimmune and infectious diseases (93). Despite these successes, it was generally believed that antibodies would have no role in HIV-1 therapy because of the rapid viral escape and the failure of the initial attempts at combination antibody therapy in humanized mice (94) and in humans (95, 96).
In an early study, humanized mice infected with HIV-1JR-CSF or HIV-1SF162 were treated with a cocktail of the antibodies b12, 2G12 and 2F5. Although there was a transient decline in the viral load, viremia returned to baseline within 5–7 days and therefore it was concluded that even cocktails of bNAbs could not effectively control viremia (94).
Infected humans were also treated with an overlapping cocktail of three antibodies, namely 2G12, 2F5, and 4E10 (95, 96). Eight chronically and six acutely HIV-1-infected individuals on anti-retroviral therapy (ART) with undetectable viral loads were selected for infection with viruses that were sensitive to at least 2 of the three components of the antibody cocktail in in vitro assays. ART was stopped one day after starting a course of 11 weeks of treatment with the antibody combination. Antibody therapy failed entirely in 6 out the 8 chronically infected subjects, and only one showed prolonged suppression of viremia (95). The results with the acutely infected subjects were more difficult to interpret since they had not been subjected to control treatment interruption experiments. However, again a single individual remained controlled with antibody alone for the entire treatment period and beyond. Of note, the only antibody that produced escape variants was 2G12 suggesting that only 2G12 exerted selective pressure on the virus (95). Very similar results were observed in an independent study with the same cocktail of antibodies given weekly for 12 weeks after ART interruption (96). Of the 10 acutely or early infected individuals enrolled in this study, 2 remained controlled throughout the treatment period and again the viruses that emerged during therapy were only resistant to 2G12 (96). Together, the two human studies suggest that, if there was an effect of immunotherapy, it was probably due to a single antibody with relatively low levels of activity compared to the recently discovered more potent bNAbs.
The overall conclusion of both the original mouse and human studies was that antibodies were not able to control HIV-1 infection, because the virus rapidly escapes by selection of resistant variants (94–96). The availability of the more potent new generation bNAbs prompted a re-evaluation of immunotherapy in HIV-1-infected humanized mice (97). The mice used in these studies were immunodeficient and reconstituted with human haematopoetic stem cells (98, 99). They were infected with HIV-1YU2, a CCR5-tropic virus that is difficult to neutralize. Mice remained infected for over 100 days, showed a decrease in the CD4/CD8 T cell ratio as well as virus diversification. However, these mice do not produce strong T or B cell immune responses to the antigen and they have impaired immune effector responses (97, 100). The antibodies that were initially tested were: 45–46G54W, a CD4 binding site antibody (57); 10–1074 and PGT128, two N332 glycan dependent antibody (42, 45); PG16, a N160 glycan dependent antibody (41) and 3BC176, which targets a yet to be defined conformational epitope (40). Although each of the antibodies was highly active against HIV-1YU2 in vitro, therapy with any single antibody caused only a transient decrease in viremia followed by a rapid emergence of escape variants (97). However, escape from the antibodies was restricted to a limited set of mutations in the antibody target sites suggesting that combinations of antibodies directed to different sites on the envelope spike might be difficult to overcome (97). Indeed, long-term control of viremia was achieved when as few as three antibodies were combined (97, 101). Moreover, due to the long half-life of the antibodies, viremia remained suppressed for an average of 60 days after cessation of treatment, corresponding to the length of time that the antibody levels remained therapeutic (97). Thus, combination HIV-1 immunotherapy differs from combination anti-retroviral therapy, because the longer half-life of the antibodies maintains viremic control for a long period of time after stopping antibody administration. In addition, antibodies interfere with different aspects of the viral life cycle. ART prevents new infection by interfering with reverse transcription, integration, proteolytic processing, and/or viral entry. The precise mechanism by which antibodies suppress infection is unknown, but may involve antibody-mediated increase in the rate of viral decay (102), killing of infected cells by antibody mediated cytototxicity (ADCC) (103), blocking infection by interference with CD4 binding (44, 56, 57), and blocking cell-to-cell and cell free virus transmission (104). Whether the two modes of therapy might be complementary or even synergistic remains to be determined.
The HIV-1 immunotherapy experiments in humanized mice established the concept that antibodies can be effective in suppressing HIV-1 infection, but they also raised a number of important questions that remain to be resolved. For example, can a vaccine that induces potent bNAbs be used therapeutically in individuals controlled by ART? Can antibody-based therapy be used instead of ART to provide long-term HIV-1 control without daily medication? Can vectors such as Adeno-associated virus (AAV) that have been used to direct antibody synthesis by the host and prevent infection (48, 105) also be used to produce a long-term single shot HIV-1 therapy? Will immunotherapy interfere with HIV-1 transmission?
To evaluate the promising results obtained in experiments with humanized mice we will first have to determine whether they can be reproduced in non-human primates. SHIV-infected macaques support a far higher overall viral burden and diversity than mice, and the disease resembles HIV-1 in that it is a chronic infection that can lead to immunodeficiency. Moreover and most importantly, macaques have an adaptive immune system and an intact effector cell compartment whereas humanized mice do not. These immune system components cannot eliminate the virus but they do contribute to regulating the viral load and are essential to maintaining the level of setpoint viremia. We speculate that adaptive and innate components of the immune system will facilitate viremic control by antibodies therapy in intact hosts. Should the mouse immunotherapy experiments be confirmed in macaques, iterative experimentation in mice and macaques will expedite the development of new immunotherapeutic approaches.
Experiments in macaques should precede human studies, but they may not be entirely predictive because SHIV infection in macaques differs from HIV-1 infection in humans in a number of important ways. Whether and how antibodies can be used to control infection in humans can only be resolved in clinical trials (Fig. 4).
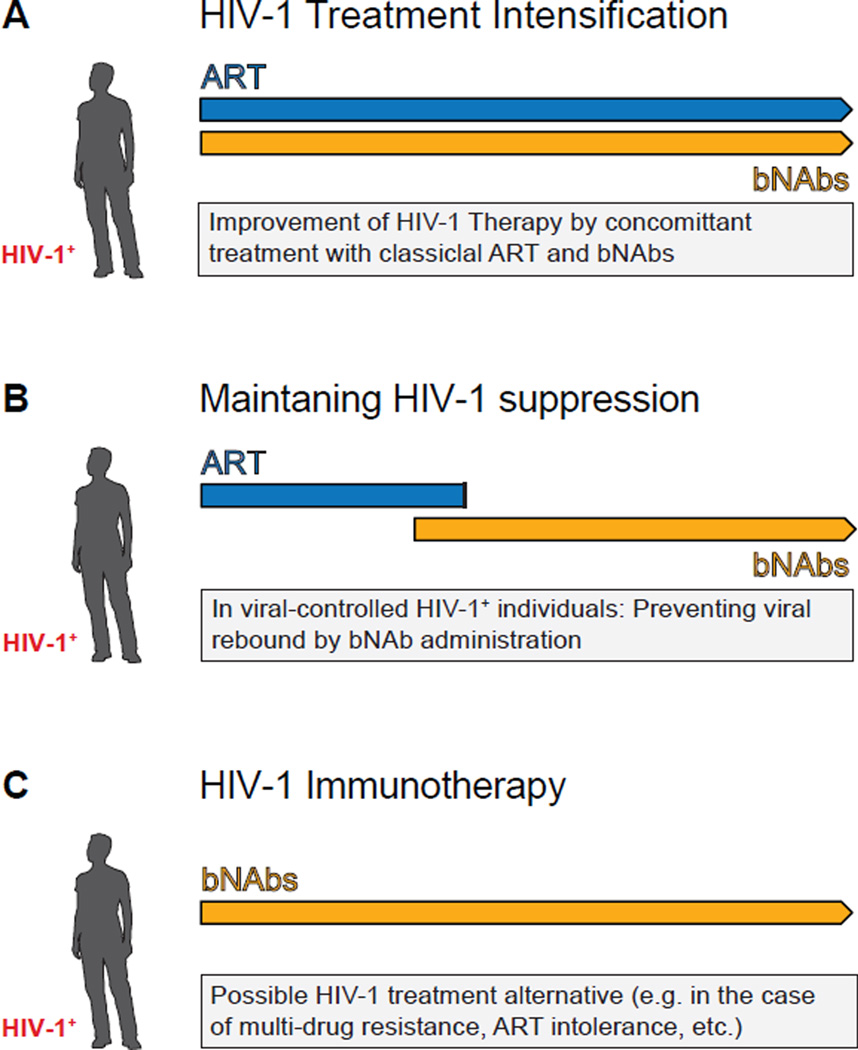
Figure 4. Potential use of bNAbs in HIV-1 therapy.
Suggested settings for the evaluation of bNAbs in clinical trials. A.) Different modes of action in anti-retroviral drugs (ART) and HIV-1 neutralizing antibodies might result in significant HIV-1 treatment intensification in humans. B.) Prevention of viral rebound while ART is halted might be accomplished by HIV-1 antibody therapy. In order to reduce the likelihood of viral escapes from bNAbs, HIV-1-infected individuals should have fully suppressed viral loads before antibody therapy is started. C.) Treatment with a combination of bNAbs could be of particular interest, especially if individuals either do not tolerate (e.g. drug-drug interaction, severe side-effects) ART or are resistant to it.
In conclusion, over the last four years, the landscape for HIV-1 immunotherapy and vaccine development has changed dramatically as a result of antibody cloning efforts. The next years should reveal whether these results can be effectively translated into the clinic.
References
- 1.Plotkin SA. Correlates of protection induced by vaccination. Clinical and vaccine immunology : CVI. 2010 Jul;17:1055. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur K, Sullivan M, Wilson PC. Targeting B cell responses in universal influenza vaccine design. Trends in immunology. 2011 Nov;32:524. doi: 10.1016/j.it.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaschen B, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002 Jun 28;296:2354. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 4.Wei X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003 Mar 20;422:307. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 5.Moore PL, et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012 Nov;18:1688. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein JS, et al. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proceedings of the National Academy of Sciences of the United States of America. 2009 May 5;106:7385. doi: 10.1073/pnas.0811427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein JS, Bjorkman PJ. Few and far between: how HIV may be evading antibody avidity. PLoS pathogens. 2010 May;6:e1000908. doi: 10.1371/journal.ppat.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu P, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006 Jun 15;441:847. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 9.Doores KJ, et al. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proceedings of the National Academy of Sciences of the United States of America. 2010 Aug 3;107:13800. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doria-Rose NA, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. Journal of virology. 2009 Jan;83:188. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikell I, et al. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS pathogens. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray ES, et al. HIV-1 neutralization breadth develops incrementally over 4 years and is associated with CD4+ T cell decline and high viral load during acute infection. Journal of virology. 2011 Mar 9; doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sather DN, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. Journal of virology. 2009 Jan;83:757. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. Journal of virology. 2009 Jul;83:7337. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichberg JW, Murthy KK, Ward RH, Prince AM. Prevention of HIV infection by passive immunization with HIVIG or CD4-IgG. AIDS research and human retroviruses. 1992 Aug;8:1515. doi: 10.1089/aid.1992.8.1515. [DOI] [PubMed] [Google Scholar]
- 16.Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993 Jan 15;150:635. [PubMed] [Google Scholar]
- 17.Burton DR, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994 Nov 11;266:1024. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 18.Thali M, et al. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. Journal of virology. 1993 Jul;67:3978. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trkola A, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. Journal of virology. 1996 Feb;70:1100. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchacher A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS research and human retroviruses. 1994 Apr;10:359. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 21.Muster T, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. Journal of virology. 1993 Nov;67:6642. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West AP., Jr Computational analysis of anti-HIV-1 antibody neutralization panel data to identify potential functional epitope residues. PNAS. 2013 doi: 10.1073/pnas.1309215110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascola JR, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. Journal of virology. 1999 May;73:4009. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000 Feb;6:207. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 25.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000 Feb;6:200. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 26.Parren PW, et al. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunology letters. 1997 Jun 1;57:105. doi: 10.1016/s0165-2478(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 27.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009 Aug;15:951. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hessell AJ, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS pathogens. 2009 May;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. Journal of virology. 2010 Feb;84:1302. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003 Jun 27;300:2065. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 31.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005 Jun 24;308:1906. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med. 2013 Feb 11;210:241. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mascola JR, Nabel GJ. Vaccines for the prevention of HIV-1 disease. Current opinion in immunology. 2001 Aug;13:489. doi: 10.1016/s0952-7915(00)00246-6. [DOI] [PubMed] [Google Scholar]
- 34.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009 Apr 2;458:636. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 35.Scheid JF, et al. A method for identification of HIV gp140 binding memory B cells in human blood. Journal of immunological methods. 2009 Apr 15;343:65. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiller T, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. Journal of immunological methods. 2008 Jan 1;329:112. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003 Sep 5;301:1374. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 38.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361:2209. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 39.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012 Apr 5;366:1275. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein F, et al. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J Exp Med. 2012 Jul 30;209:1469. doi: 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009 Oct 9;326:285. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011 Sep 22;477:466. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010 Aug 13;329:856. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheid JF, et al. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science. 2011 Jul 14; doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2012 Nov 20;109:E3268. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012 Nov 13;109:18921. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietzsch J, et al. A mouse model for HIV-1 entry. Proceedings of the National Academy of Sciences of the United States of America. 2012 Sep 25;109:15859. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012 Jan 5;481:81. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonsignori M, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. Journal of virology. 2012 Nov;86:11521. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bomsel M, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011 Feb 25;34:269. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Burton DR, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proceedings of the National Academy of Sciences of the United States of America. 2011 Jul 5;108:11181. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonsignori M, et al. Two Distinct Broadly Neutralizing Antibody Specificities of Different Clonal Lineages in a Single HIV-1-infected Donor: Implications for Vaccine Design. Journal of virology. 2012 Feb 1; doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgiev IS, et al. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science. 2013 May 10;340:751. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 54.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012 Sep 21;37:412. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou T, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007 Feb 15;445:732. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 Aug 13;329:811. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diskin R, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011 Dec 2;334:1289. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West AP, Jr, Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jul 24;109:E2083. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011 Dec 15;480:336. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Julien JP, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proceedings of the National Academy of Sciences of the United States of America. 2013 Mar 12;110:4351. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pejchal R, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011 Nov 25;334:1097. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Julien JP, et al. Broadly Neutralizing Antibody PGT121 Allosterically Modulates CD4 Binding via Recognition of the HIV-1 gp120 V3 Base and Multiple Surrounding Glycans. PLoS pathogens. 2013 May;9:e1003342. doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang J, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012 Nov 15;491:406. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker LM, et al. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS pathogens. 2010;6 doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tiller T, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007 Feb;26:205. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mouquet H, et al. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PloS one. 2011;6:e24078. doi: 10.1371/journal.pone.0024078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011 Sep 16;333:1593. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corti D, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PloS one. 2010;5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao X, Chen W, Feng Y, Dimitrov DS. Maturation Pathways of Cross-Reactive HIV-1 Neutralizing Antibodies. Viruses. 2009 Dec;1:802. doi: 10.3390/v1030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Victora GD, Nussenzweig MC. Germinal Centers. Annu Rev Immunol. 2011 Mar 24; [Google Scholar]
- 71.Wu TT, Kabat EA. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132:211. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amzel LM, Poljak RJ. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- 73.Wagner SD, Milstein C, Neuberger MS. Codon bias targets mutation. Nature. 1995 Aug 31;376:732. doi: 10.1038/376732a0. [DOI] [PubMed] [Google Scholar]
- 74.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998 Jun;8:751. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 75.Foote J, Milstein C. Kinetic maturation of an immune response. Nature. 1991 Aug 8;352:530. doi: 10.1038/352530a0. [DOI] [PubMed] [Google Scholar]
- 76.Mouquet H, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010 Sep 30;467:591. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao X, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochemical and biophysical research communications. 2009 Dec 18;390:404. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao HX, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011 Oct 24;208:2237. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shingai M, et al. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proceedings of the National Academy of Sciences of the United States of America. 2012 Nov 27;109:19769. doi: 10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liao HX, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013 Apr 25;496:469. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein F, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013 Mar 28;153:126. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 83.Mouquet H, Nussenzweig MC. Polyreactive antibodies in adaptive immune responses to viruses. Cellular and molecular life sciences : CMLS. 2012 May;69:1435. doi: 10.1007/s00018-011-0872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diskin R, Marcovecchio PM, Bjorkman PJ. Structure of a clade C HIV-1 gp120 bound to CD4 and CD4-induced antibody reveals anti-CD4 polyreactivity. Nature structural & molecular biology. 2010 May;17:608. doi: 10.1038/nsmb.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alam SM, et al. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2009 Dec 1;106:20234. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ofek G, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. Journal of virology. 2004 Oct;78:10724. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alam SM, et al. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007 Apr 1;178:4424. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonsignori M, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. Journal of virology. 2011 Oct;85:9998. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCoy LE, Weiss RA. Neutralizing antibodies to HIV-1 induced by immunization. J Exp Med. 2013 Feb 11;210:209. doi: 10.1084/jem.20121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nature biotechnology. 2012 May;30:423. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGuire AT, et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med. 2013 Apr 8;210:655. doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jardine J, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013 May 10;340:711. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott LJ, Lamb HM. Palivizumab. Drugs. 1999 Aug;58:305. doi: 10.2165/00003495-199958020-00009. [DOI] [PubMed] [Google Scholar]
- 94.Poignard P, et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999 Apr;10:431. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 95.Trkola A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005 Jun;11:615. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 96.Mehandru S, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. Journal of virology. 2007 Oct;81:11016. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012 Dec 6;492:118. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brehm MA, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010 Apr;135:84. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Traggiai E, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004 Apr 2;304:104. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 100.Baenziger S, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−gamma c−/− mice. Proceedings of the National Academy of Sciences of the United States of America. 2006 Oct 24;103:15951. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diskin R, et al. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J Exp Med. 2013 May 27; doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Igarashi T, et al. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat Med. 1999 Feb;5:211. doi: 10.1038/5576. [DOI] [PubMed] [Google Scholar]
- 103.Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Current opinion in HIV and AIDS. 2009 Sep;4:388. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abela IA, et al. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS pathogens. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson PR, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009 Aug;15:901. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]