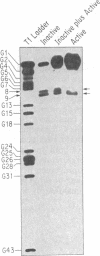
Abstract
Previous work indicates that aminoacyl-tRNA synthetases make a transient covalent adduct with cognate tRNAs, through Michael addition of an enzyme nucleophile to the carbon-6 position of uridine 8. We report the selective reduction of the 5,6 double bond of 4-thiouridine at position 8 in Escherichia coli tyrosine tRNA, so as to prevent formation of the presumed covalent enzyme-nucleic acid adduct. The completely reduced tRNA molecules are inactivated for aminoacylation. With partial reduction, a mixed pool of active and inactive molecules is created and the degree of inactivation exactly matches the extent of 4-thiouridine reduction. The active molecules recovered from this mixed pool are specifically unaltered at position 8. The results are consistent with the view that the covalent enzyme-RNA adduct is an obligatory intermediate for aminoacylation of this tRNA.
Full text
PDF

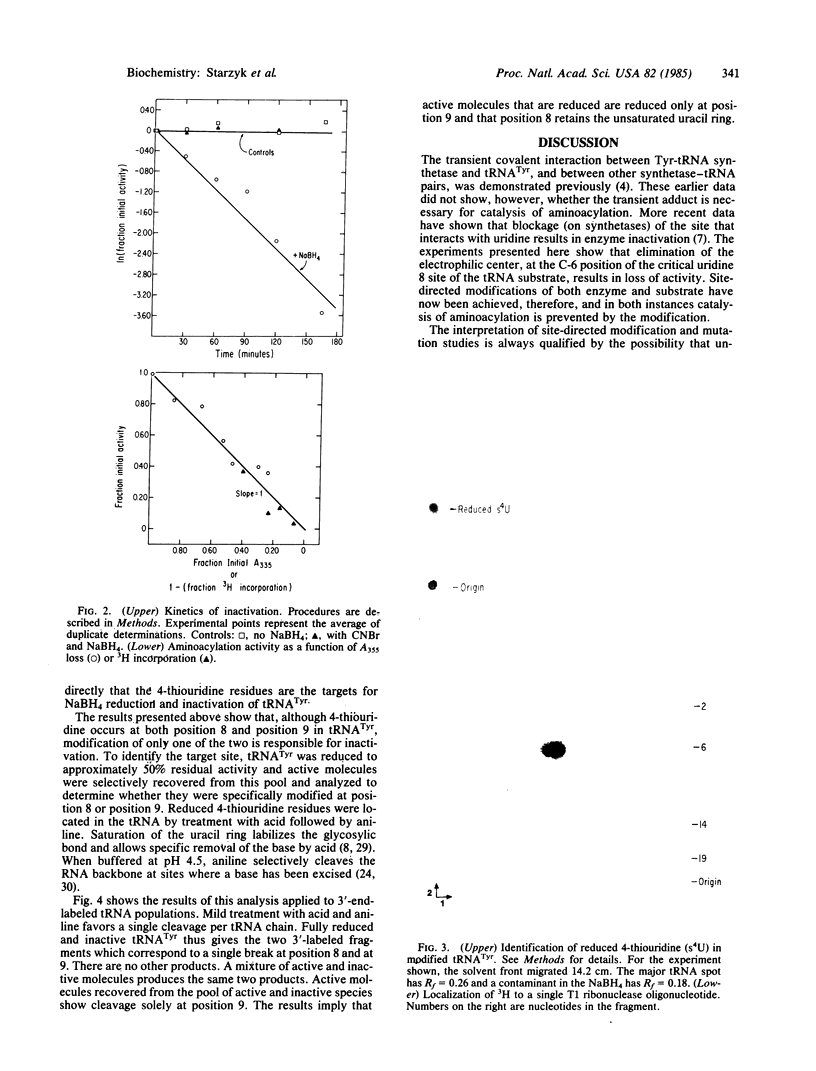

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthelot F., Gros F., Favre A. Biological activity of cross-linked Escherichia coli tRNA f Met. Eur J Biochem. 1972 Sep 18;29(2):343–347. doi: 10.1111/j.1432-1033.1972.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré D. S., Thomas G., Favre A. Conformation and functioning of tRNAs: cross-linked tRNAs as substrate for tRNA nucleotidyl-transferase and aminoacyl synthetases. Biochimie. 1974;56(8):1089–1101. doi: 10.1016/s0300-9084(74)80097-0. [DOI] [PubMed] [Google Scholar]
- Cerutti P., Holt J. W., Miller N. Detection and determination of 5,6-dihydrouridine and 4-thiouridine in transfer ribonucleic acid from different sources. J Mol Biol. 1968 Jun 28;34(3):505–518. doi: 10.1016/0022-2836(68)90176-9. [DOI] [PubMed] [Google Scholar]
- Cerutti P., Miller N. Selective reduction of yeast transfer ribonucleic acid with sodium borohydride. J Mol Biol. 1967 May 28;26(1):55–66. doi: 10.1016/0022-2836(67)90260-4. [DOI] [PubMed] [Google Scholar]
- Daniel W. E., Jr, Cohn M. Changes in tertiary structure accompanying a single base change in transfer RNA. Proton magnetic resonance and aminoacylation studies of Escherichia coli tRNAMet f1 and tRNAMet f3 and their spin-labeled (s4U8) derivatives. Biochemistry. 1976 Sep 7;15(18):3917–3924. doi: 10.1021/bi00663a003. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre A., Michelson A. M., Yaniv M. Photochemistry of 4-thiouridine in Escherichia coli transfer RNA1Val. J Mol Biol. 1971 May 28;58(1):367–379. doi: 10.1016/0022-2836(71)90252-x. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J. N., Landy A., Zadrazil S., Smith J. D. The nucleotide sequences of tyrosine transfer RNAs of Escherichia coli. Eur J Biochem. 1970 Apr;13(3):461–483. doi: 10.1111/j.1432-1033.1970.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Hara H., Horiuchi T., Saneyoshi M., Nishimura S. 4-Thiouridine-specific spin-labeling of E. coli transfer RNA. Biochem Biophys Res Commun. 1970 Jan 23;38(2):305–311. doi: 10.1016/0006-291x(70)90713-8. [DOI] [PubMed] [Google Scholar]
- Lipsett M. N., Doctor B. P. Studies on tyrosine transfer ribonucleic acid, a sulfur-rich species from Escherichia coli. J Biol Chem. 1967 Sep 25;242(18):4072–4077. [PubMed] [Google Scholar]
- Lipsett M. N. The isolation of 4-thiouridylic acid from the soluble ribonucleic acid of Escherichia coli. J Biol Chem. 1965 Oct;240(10):3975–3978. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T., Silverman S., Kohli J., Söll D. Nucleotide sequence of phenylalanine transfer RNA from Schizosaccharomyces pombe: implications for transfer RNA recognition by yeast phenylalanyl-tRNA synthetase. Biochemistry. 1978 May 2;17(9):1622–1628. doi: 10.1021/bi00602a007. [DOI] [PubMed] [Google Scholar]
- Pochon F., Pascal Y., Pitha P., Michelson A. M. Photochimie des polynucléotides. IV. Photochimie de quelques nucléosides puriques méthylés. Biochim Biophys Acta. 1970 Aug 8;213(2):273–281. [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R. Understanding the recognition of transfer RNAs by aminoacyl transfer RNA synthetases. Adv Enzymol Relat Areas Mol Biol. 1979;49:187–222. doi: 10.1002/9780470122945.ch5. [DOI] [PubMed] [Google Scholar]
- Schoemaker H. J., Schimmel P. R. Effect of aminoacyl transfer RNA synthetases on H-5 exchange of specific pyrimidines in transfer RNAs. Biochemistry. 1977 Dec 13;16(25):5454–5460. doi: 10.1021/bi00644a009. [DOI] [PubMed] [Google Scholar]
- Schoemaker H. J., Schimmel P. R. Inhibition of an aminoacyl transfer RNA synthetase by a specific trinucleotide derived from the sequence of its cognate transfer RNA. Biochemistry. 1977 Dec 13;16(25):5461–5464. doi: 10.1021/bi00644a010. [DOI] [PubMed] [Google Scholar]
- Schreier A. A., Schimmel P. R. Transfer ribonucleic acid synthetase catalyzed deacylation of aminoacyl transfer ribonucleic acid in the absence of adenosine monophosphate and pyrophosphate. Biochemistry. 1972 Apr 25;11(9):1582–1589. doi: 10.1021/bi00759a006. [DOI] [PubMed] [Google Scholar]
- Schwartz I., Ofengand J. Photo-affinity labeling of tRNA binding sites in macromolecules. I. Linking of the phenacyl-p-azide of 4-thiouridine in (Escherichia coli) valyl-tRNA to 16S RNA at the ribosomal P site. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3951–3955. doi: 10.1073/pnas.71.10.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugart L. Effect of selective chemical modification of 4-thiouridine of phenylalanine transfer ribonucleic acid on enzyme recognition. Arch Biochem Biophys. 1972 Feb;148(2):488–495. doi: 10.1016/0003-9861(72)90167-1. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Starzyk R. M., Koontz S. W., Schimmel P. A covalent adduct between the uracil ring and the active site of an aminoacyl tRNA synthetase. Nature. 1982 Jul 8;298(5870):136–140. doi: 10.1038/298136a0. [DOI] [PubMed] [Google Scholar]
- Thomas N. S., Butcher P. D., Arnstein H. R. Polyribosome binding of rabbit globin messenger RNA and messenger ribonucleoprotein labelled with bacteriophage-T4 RNA ligase and 5'-[32P] phosphocytidine 3'-phosphate. Nucleic Acids Res. 1983 Jan 11;11(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Studies on polynucleotides. CI. Escherichia coli tyrosine and formylmethionine transfer ribonucleic acids: effect of chemical modification of 4-thiouridine to uridine on their biological properties. J Biol Chem. 1972 Aug 10;247(15):4879–4892. [PubMed] [Google Scholar]
- Wetzel R., Söll D. Analogs of methionyl-tRNA synthetase substrates containing photolabile groups. Nucleic Acids Res. 1977;4(5):1681–1694. doi: 10.1093/nar/4.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. A specific chemical chain scission of tRNA at 7-methylguanosine. FEBS Lett. 1970 Dec;11(3):160–164. doi: 10.1016/0014-5793(70)80518-x. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Söll D. Studies of transfer RNA tertiary structure of singlet-singlet energy transfer. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2838–2842. doi: 10.1073/pnas.71.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv M., Chestier A., Gros F., Favre A. Biological activity of irradiated tRNA Val containing a 4-thiouridine-cytosine dimer. J Mol Biol. 1971 May 28;58(1):381–388. doi: 10.1016/0022-2836(71)90253-1. [DOI] [PubMed] [Google Scholar]