Abstract
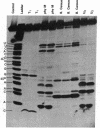
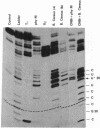
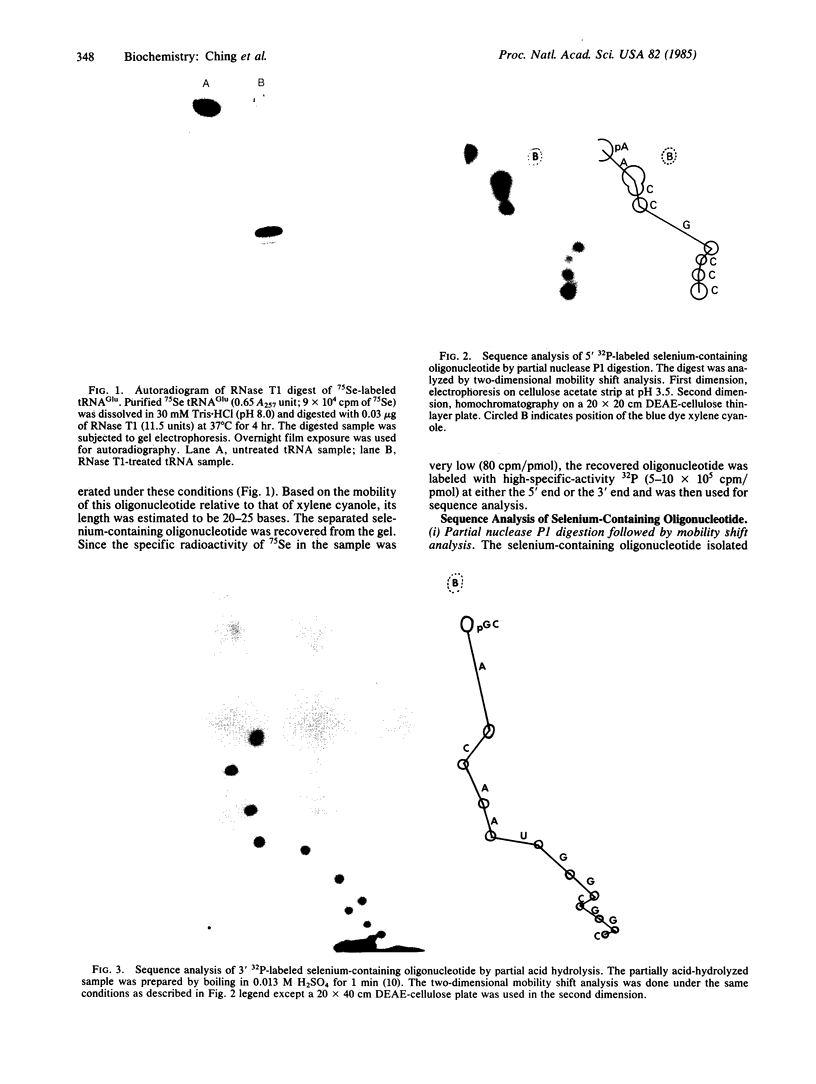
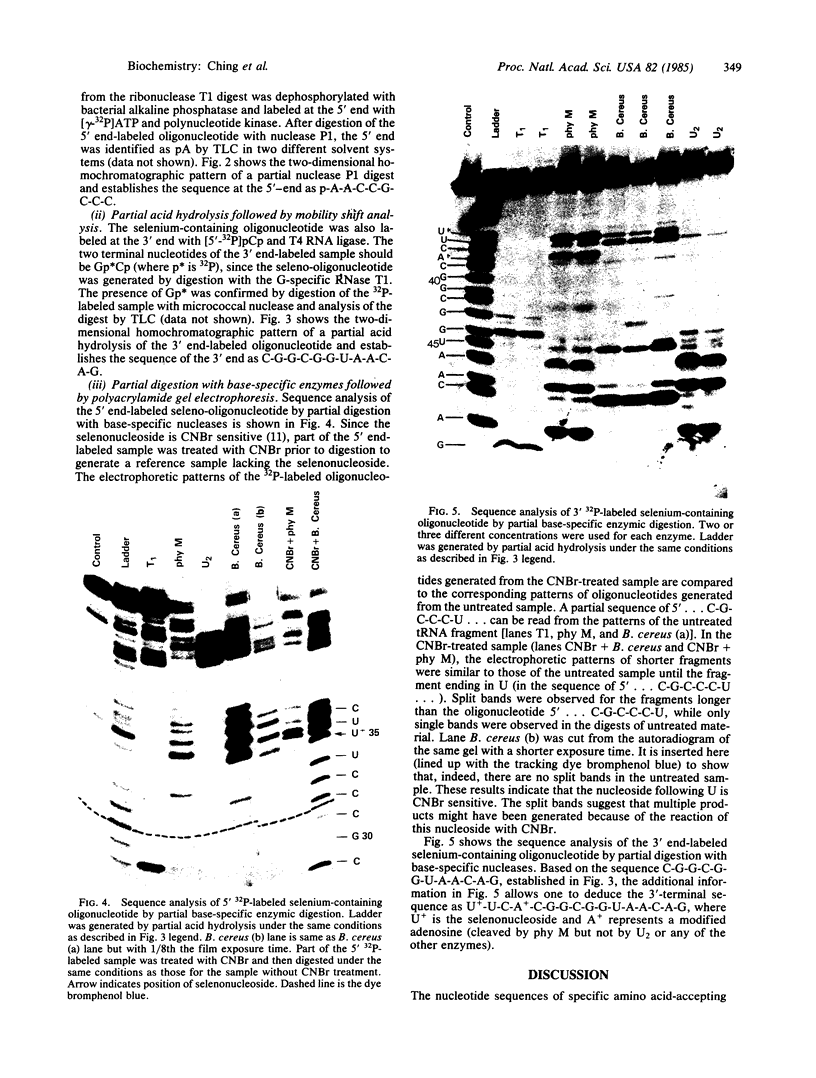
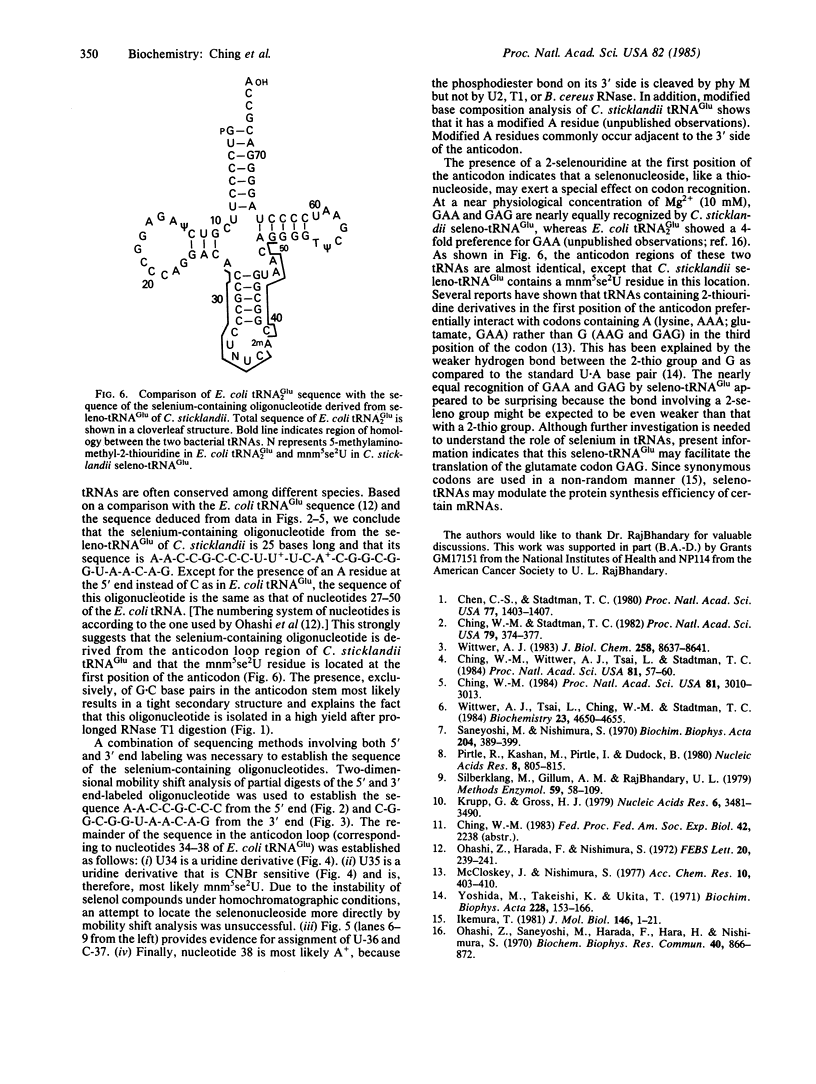
In previous studies, the single selenonucleoside component of a selenium-containing tRNAGlu isolated from Clostridium sticklandii has been shown to be 5-methyl-aminomethyl-2-selenouridine. Here, we show that this selenonucleoside is most likely located at the "wobble" position of the anticodon of the clostridial seleno-tRNAGlu. Nuclease T1 digestion of this seleno-tRNAGlu generated one major selenium-containing oligonucleotide (25 bases long). The selenium-containing residue within this oligonucleotide was located by sequence analysis of the oligonucleotide before and after removal of selenium by treatment with cyanogen bromide. The sequence of this oligonucleotide, A-A-C-C-G-C-C-C-U-U+-U-C-A+C-G-G-C-G-G-U-A-A-C-A-G, is homologous to that of the Escherichia coli tRNAGlu2 from residues 27 to 50, including the anticodon region and the variable loop, except that the E. coli tRNA has 5-methylaminomethyl-2-thiouridine instead of the selenonucleoside.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C. S., Stadtman T. C. Selenium-containing tRNAs from Clostridium sticklandii: cochromatography of one species with L-prolyl-tRNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1403–1407. doi: 10.1073/pnas.77.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M. Occurrence of selenium-containing tRNAs in mouse leukemia cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3010–3013. doi: 10.1073/pnas.81.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Stadtman T. C. Selenium-containing tRNAGlu from Clostridium sticklandii: correlation of aminoacylation with selenium content. Proc Natl Acad Sci U S A. 1982 Jan;79(2):374–377. doi: 10.1073/pnas.79.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Wittwer A. J., Tsai L., Stadtman T. C. Distribution of two selenonucleosides among the selenium-containing tRNAs from Methanococcus vannielii. Proc Natl Acad Sci U S A. 1984 Jan;81(1):57–60. doi: 10.1073/pnas.81.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Krupp G., Gross H. J. Rapid RNA sequencing: nucleases from Staphylococcus aureus and Neurospora crassa discriminate between uridine and cytidine. Nucleic Acids Res. 1979 Aug 10;6(11):3481–3490. doi: 10.1093/nar/6.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi Z., Saneyoshi M., Harada F., Hara H., Nishimura S. Presumed anticodon structure of glutamic acid tRNA from E. coli: a possible location of a 2-thiouridine derivative in the first position of the anticodon. Biochem Biophys Res Commun. 1970 Aug 24;40(4):866–872. doi: 10.1016/0006-291x(70)90983-6. [DOI] [PubMed] [Google Scholar]
- Ohashi Ziro, Harada Fumio, Nishimura Susumu. Primary sequence of glutamic acid tRNA II from Escherichia coli. FEBS Lett. 1972 Feb 1;20(2):239–241. doi: 10.1016/0014-5793(72)80804-4. [DOI] [PubMed] [Google Scholar]
- Pirtle R., Kashdan M., Pirtle I., Dudock B. The nucleotide sequence of a major species of leucine tRNA from bovine liver. Nucleic Acids Res. 1980 Feb 25;8(4):805–815. [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi M., Nishimura S. Selective modification of 4-thiouridylate residue in Escherichia coli transfer RNA with cyanogen bromide. Biochim Biophys Acta. 1970 Apr 15;204(2):389–399. doi: 10.1016/0005-2787(70)90158-9. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J. Specific incorporation of selenium into lysine- and glutamate- accepting tRNAs from Escherichia coli. J Biol Chem. 1983 Jul 25;258(14):8637–8641. [PubMed] [Google Scholar]
- Wittwer A. J., Tsai L., Ching W. M., Stadtman T. C. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino)methyl]-2-selenouridine. Biochemistry. 1984 Sep 25;23(20):4650–4655. doi: 10.1021/bi00315a021. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takeishi K., Ukita T. Structural studies on a yeast glutamic acid tRNA specific to GAA codon. Biochim Biophys Acta. 1971 Jan 1;228(1):153–166. doi: 10.1016/0005-2787(71)90555-7. [DOI] [PubMed] [Google Scholar]