Abstract
Introduction
We compared features of nerve enlargement in inherited and acquired demyelinating neuropathies using ultrasound.
Methods
We measured median and ulnar nerve cross-sectional areas in proximal and distal regions in 128 children and adults with inherited (Charcot-Marie Tooth-1 (CMT-1) (n=35)) and acquired (Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) (n=55), Guillaine-Barre Syndrome (GBS) (n=21) and Multifocal Motor Neuropathy (MMN) (n=17)) demyelinating neuropathies. We classified nerve enlargement by degree and number of regions affected. We defined patterns of nerve enlargement as: none- no enlargement; mild-nerves enlarged but never more than twice normal; regional- nerves normal at at least one region and enlarged more than twice normal at atleast one region; diffuse- nerves enlarged at all four regions with atleast one region more than twice normal size.
Results
Nerve enlargement was commonly diffuse (89%) and generally more than twice normal size in CMT-1, but not (p<0.001) in acquired disorders which mostly had either no, mild or regional nerve enlargement (CIDP (64%), GBS (95%), and MMN (100%)). In CIDP, subjects treated within three months of disease onset had less nerve enlargement than those treated later.
Discussion
Ultrasound identified patterns of diffuse nerve enlargement can be used to screen patients suspected of having CMT-1. Normal, mildly, or regionally enlarged nerves in demyelinating polyneuropathy suggests an acquired etiology. Early treatment in CIDP may impede nerve enlargement.
Keywords: ultrasound, nerve, chronic inflammatory demyelinating polyneuropathy (CIDP), multifocal motor neuropathy (MMN), Charcot-Marie-Tooth (CMT), Guillain-Barre syndrome (GBS)
INTRODUCTION
Ultrasound is a painless, inexpensive technique for imaging nerve pathology at the bedside. Ultrasound identified patterns of nerve pathology provides useful information in the diagnosis of neuropathies. In acquired mononeuropathies, ultrasound augments electrodiagnostic results in diagnostic evaluation of nerve entrapment and traumatic neuropathies [1–6], and offers superior sensitivity to magnetic resonance imaging for identifying focal nerve pathology [7]. In acquired and inherited peripheral polyneuropathies, ultrasound often detects nerve enlargement in inherited and acquired demyelinating neuropathies [8–15], including abnormal morphology and size at sites of conduction block [16]. Ultrasound is rarely abnormal in diffuse axonal polyneuropathies[9]. Recently, patterns of nerve enlargement determined by comparing size of nerves at different locations along their length have been proposed as a way of characterizing types of neuropathies [15,17].
In this study we describe a quantitative method using ultrasound to describe and compare the patterns and degree of nerve size changes in acquired and inherited demyelinating polyneuropathies. We evaluated a large series of patients with demyelinating dominantly inherited neuropathy (CMT-1) and acquired neuropathies, including chronic inflammatory demyelinating polyneuropathy (CIDP), Guillain-Barre syndrome (GBS), and multifocal motor neuropathy (MMN). We report comparisons of the patterns and degree of nerve enlargement with diagnosis and clinical features.
MATERIALS AND METHODS
The study was approved by the Washington University institutional review board. We retrospectively compared records of ultrasound testing in patients with demyelinating CMT-1 (n=35), CIDP (n=55), GBS (n=21), and MMN (n=17) from our database of patients evaluated between June 2007 and October 2011. We referenced our results to a control group (n=90) of children and adults studied in our neuromuscular ultrasound laboratory[9]. Disease duration in chronic disorders, the time from symptom onset to the ultrasound study, was divided into duration before and after initial treatment immunomodulation agent. In treatment naïve subjects, disease duration before treatment was the disease duration. Results are reported as mean [standard deviation (SD)] unless specified. CMT-1 patients ranged in age from 2 to 71 years of age and all had clinical findings consistent with a neuropathy. We included 23 subjects with a known CMT-1 genotype (1A (20) and 1B (3)) and 12 others with a diagnosis of CMT-1 based on a family history of a first-degree relative with demyelinating polyneuropathy. All CMT-1 patients had demyelinating features consistent with an inherited polyneuropathy on our nerve conduction studies except one in whom nerve conduction studies were performed only in the first degree relative. CIDP and MMN patients all had clinical and nerve conduction features that met diagnostic criteria for the diseases[18,19]. CIDP subjects ranged from 4 to 81 years of age. Nine (16%) CIDP patients were treatment naïve, while 46 had received either plasma exchange or intravenous immunoglobulin (IVIG) (n=8), prednisone or other immunomodulation agents (n=17), or combinations of both (n=21). Disease duration was 75(89) months. Disease duration in treatment naïve subjects (n=9) was 7(7) months and in treated subjects (n=36) was 25(60) months before treatment and 64(61) months after treatment. In our cohort patients with shorter duration of disease before treatment were followed for a shorter period after treatment (rs=0.5, p=0.004). MMN subjects were aged 33 to 83 years. 11 (64%) had positive anti-GM1 and/or anti-NS6S antibodies. Six (35%) were treatment naive. Treated patients had received plasma exchange or IVIG (n=2 ), rituximab (n=8), or rituximab and prednisone (n=1). Disease duration was 122(116) months. Disease duration in the treatment naïve patients (n=6) was 19(13) months. Treated subjects (n=7) had 58(57) months before treatment and 114(71) months after treatment. GBS subjects ranged from 8 to 82 years of age. Most (16/21) were imaged within three weeks of symptom onset. Others had times from symptom onset to imaging study of 1.5–180 months.
Ultrasound examinations were performed using a Philips HD11XE or iu22 imaging system with an L12-5 or L15-8 linear array probe. Median and ulnar nerves were chosen for study, as they are easily imaged at several sites along their length. One investigator (CMZ) obtained all ultrasound images. The ultrasound probe was kept perpendicular to the nerve by maintaining an angle in which the ultrasound image of the nerve appeared smallest and brightest. Nerve cross-sectional areas (NCSA) were measured by tracing nerves just inside their hyperechoic rims. Three separate NCSA measurements, with the probe repositioned for each measurement, were averaged at each nerve site. Most subjects were seated with the entire arm anteriorly extended, supinated, and supported by a pillow on a table at approximately mid-thoracic height. Hospitalized subjects were examined in the supine position with the arm supinated, abducted, and supported at body level. Each patient had transverse images obtained from four nerve sites in one arm: the proximal and distal median and ulnar nerve, avoiding sites of possible entrapment. It is our typical protocol to exam the median and ulnar nerves in their entirety and, in the absence of localized pathology, to routinely obtain measurements at the mid-humerus, approximately 2/3 from the lateral tip of the acromion to the lateral epicondyle of the humerus (proximal) and in the forearm, approximately 3/4 from the medial epicondyle of the humerus to the ulnar styloid process (distal). Nerve enlargement limited to other locations was identified in only one subject with MMN who had enlargement of the ulnar nerve in the proximal but not distal forearm; in this case, the ulnar forearm measurement was recorded at the site of nerve enlargement.
Our prior work of 90 healthy children and adults found that nerve size varied mostly with height [9]. To allow direct comparisons between patients, a “nerve size index” (NSI) was calculated by comparing the measured NCSA to the expected NCSA based on height for each nerve site and subject [9]. The NSI was derived from the slope (m) and y intercept (b) of the simple linear regression line that related the nerve cross sectional area to height using the equation: (NCSA (mm2) / ((m x height (cm) + b)) * 100%. Corrections for height (m), derived from our labs previously published normal values [9], are + 0.041 mm2/cm for the ulnar nerve in the arm and forearm, 0.061 mm2/cm for the ulnar nerve at the elbow, 0.054 mm2/cm for the median nerve in the arm and forearm, and 0.072 mm2/cm for the median nerve at the wrist. Constants (b) were −1.5 for the ulnar nerve at the forearm and arm, −3.5 for the ulnar nerve at the elbow, −1.5 for the median nerve at the forearm and arm and −3.2 for the median nerve at the wrist.
Nerve enlargement was defined as a NSI greater than two standard deviations above the mean NSI in controls. Nerve enlargement greater than twice normal size was greater than twice the mean NSI in controls and was always greater than four standard deviations above the mean. Average NSI values were calculated for each subject averaging the proximal and distal median and ulnar NSI measurements. We defined three patterns of nerve enlargement (mild (type 1), regional (type 2), and diffuse (type 3)) based on a combination of the amount and anatomical extent of nerve enlargement (Table 1).
Table 1.
Patterns of Nerve Enlargement
| None | No nerve sites enlarged |
|---|---|
| Type 1 –Mild | Nerve enlargement present at one or more sites but not more than twice normal size |
| Type 2 –Regional | Nerve enlargement present with at least one site more than twice normal size and at least one site normal size |
| Type 3 –Diffuse | Nerve enlargement present at all proximal and distal sites with at least one site more than twice normal size |
Statistics were performed using PASW® Statistics GradPack 18.0. To account for outlying data, methods included Mann- Whitney U (MWU) and McNemar paired samples tests, Spearman’s rank correlational analysis (rs), Fisher exact test (FET), and when appropriate one-way ANOVA with Games-Howell post-hoc analysis.
RESULTS
Comparisons between Demyelinating Polyneuropathies, and to Controls
Nerves in both CMT-1 and acquired demyelinating polyneuropathies were larger than controls (Table 2), as previously reported [9]. Nerves were enlarged at one or more sites in: all subjects with CMT-1, 80% with CIDP; 50% of GBS during the acute phase and 60% after the acute phase, and 65% with MMN.
Table 2.
Degree of nerve enlargement in CMT-1, CIDP, GBS, and MMN
| Diagnosis | ||||
|---|---|---|---|---|
| Nerve Characteristic | CMT-1 n=35 | CIDP n=55 | GBS n=21 | MMN n=17 |
| Average NSI > twice normal (n= (%)) | 28 (80%) | 17 (31%)* | 1(5%)*,ŧ | 0* |
| Average NSI > three times normal (n= (%)) | 17 (49%) | 7 (13%)* | 0* | 0* |
| All four nerve sites > twice normal size (n= (%)) | 21 (60%) | 8 (15%)* | 0* | 0* |
| Average NSI (% mean (SD)) | 300 (111) | 200 (123)* | 130 (38)*, ŧ | 120 (23)* |
| Median Arm NSI (% mean (SD)) | 325 (154) | 236 (147)* | 152 (51)*, ŧ | 135 (61)* |
p≤0.001 compared to CMT-1;
p≤0.02 compared to CIDP
A diffuse enlargement pattern was more common in CMT-1 than CIDP, GBS, or MMN.
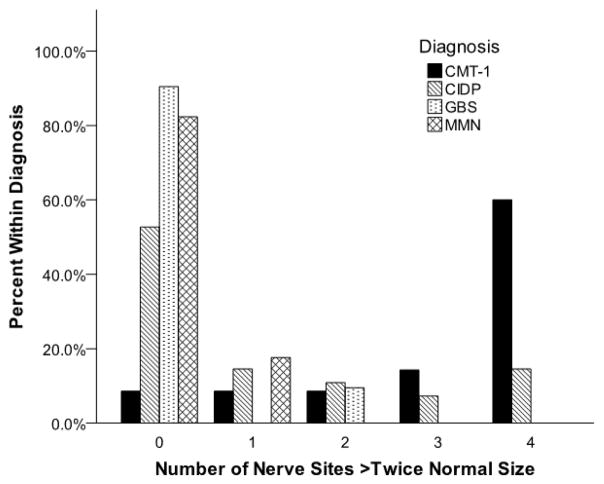
Normal sized nerves or mild (type 1) or regional (type 2) enlargement pattern were never or rarely present in CMT-1 but were common in acquired demyelinating neuropathies (Table 3). Diffuse (type 3) nerve enlargement was less common in CIDP (37%), GBS (5%) or MMN (0%) than in CMT-1 (89%). CIDP, GBS, or MMN subjects were each (p<0.001) less likely than CMT-1 subjects to have all four nerve sites more than twice normal size, or to have an average NSI more than twice normal size (Table 2, Figure 1).
Table 3.
Patterns of Nerve Enlargement in CMT-1 and Acquired Demyelinating Polyneuropathies
| Diagnosis (n= (%)) | ||||
|---|---|---|---|---|
| Pattern of Nerve Enlargement | CMT-1 n=35 | CIDP n=55 | GBS n=21 | MMN n=17 |
| None | 0 (0%) | 11 (20%) | 10 (48%) | 6 (35%) |
| Mild-Type 1 | 2 (6%) | 13 (24%) | 8 (38%) | 7 (41%) |
| Regional- Type 2 | 2 (6%) | 11 (20%) | 2 (10%) | 4 (24%) |
| Diffuse-Type 3 | 31 (89%) | 20 (37%)* | 1 (5%)* | 0 (0%)* |
p<0.001 compared to CMT-1
Nerves are more likely to be very enlarged on average and at multiple sites in CMT-1 than CIDP, GBS, and MMN. Average NSI in each demyelinating neuropathy group was larger (p≤0.05) than in controls.
Figure 1.
Frequencies of nerve enlargement more than twice normal size in CMT-1 and acquired demyelinating polyneuropathies.
Enlargement of nerve more than twice normal size at all sites is more common in CMT-1 (p<0.001) than in CIDP, GBS, or MMN. It occurs occasionally in CIDP.
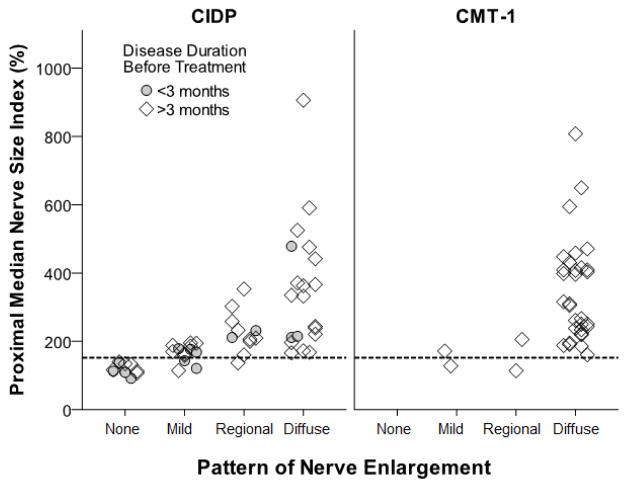
All CIDP and CMT-1 subjects with massively enlarged nerves (NSI>400%) had diffuse (type 3) nerve enlargement. CIDP subjects with less enlarged nerves could have either regional (type 2) or diffuse (type 3) nerve enlargement. CMT-1 with less enlarged nerves tended to have nerve enlargement at all sites. Comparing subjects with similar degrees of nerve enlargement (a proximal median NSI more than twice normal size but <400%), 38% (5/13) of CIDP subjects showed a regional (type 2) pattern, while all CMT-1 subjects (13/13) showed a diffuse (type 3) pattern of enlargement (p=0.04, Figure 2).
Figure 2.
Relationship between nerve size and pattern of nerve enlargement in CIDP and CMT-1.
In CIDP, massively enlarged nerves (>400%) always have diffuse (type 3) enlargement while less enlarged nerves can have either regional (type 2) or diffuse enlargement. In CMT-1, nerve enlargement is nearly always diffuse. Patients with CIDP treated within three months of symptom onset have less enlarged nerves than those with longer disease duration before treatment. The dotted line marks the upper limit of normal.
The pattern and degree of nerve enlargement differed among the acquired neuropathies (Table 2 and 3). CIDP subjects had larger (p=0.003) average NSI than GBS and were more likely to have diffuse (type 3) nerve enlargement (p=0.008).
Nerve characteristics within diagnoses
In CMT-1 the diffuse (type 3) nerve enlargement pattern was common even in children (88%; 8 of 9), with the youngest (age 2.6 years) subject’s nerves measuring over three times normal size (average NSI = 327%). Average NSI did not vary with age (p=0.8) and was similar in children (317(141) and adults (295(101), p=0.7). Average NSI did not differ (p=0.7) between CMT-1A (312(122) and CMT-1 without genetic evaluation (308(92)). Average NSI trended (p=0.06) smaller in the three subjects with CMT-1B (193(22)) than in CMT-1A. The four (11%) CMT-1 subjects without diffuse (type 3) nerve enlargement did not have other distinguishing characteristics; they were ages 6 (one nerve site mildly enlarged, type 1 enlargement, genotype unknown), 29 (two sites enlarged, type 2 enlargement, CMT-1A), 40 (four sites mildly enlarged, type 1 enlargement, CMT-1B) and 65 (three sites enlarged, type 2 enlargement, CMT-1A) years.
In CIDP subjects with mild (type 1) or regional (type 2) patterns, nerve enlargement more frequently affected the proximal than distal median (p<0.001) (83% (20/24) vs. 17% (4/24) and ulnar (p=0.04) (75% (18/24) vs. 37% (9/24)) nerves. Only one CIDP subject had enlargement of the distal nerve without enlargement of the proximal median or ulnar nerve.
In GBS, the most common enlarged nerve site was the proximal median nerve (43%); in MMN, it was the proximal ulnar nerve (47%). In both GBS and MMN with mild (type 1) or regional (type 2) nerve enlargement, the frequency of nerve enlargement was similar (p≥0.1) in the proximal and distal nerve.
Effects of Disease Duration and Treatment on Nerve Enlargement
In CIDP, subjects with longer disease duration before treatment had more nerve enlargement (Figure 2). Average NSI increased with longer disease duration before (rs=0.4, p=0.002) but not after (rs=0.3, p=0.1) treatment. CIDP subjects with less than three months of symptoms before treatment had smaller (p=0.009) average NSI (150(83) vs. 226(146)) and were less likely (p=0.004) to have any nerve measure more than twice normal size (2/15 vs. 18/30) than those with longer periods before treatment. Diffuse (type 3) nerve enlargement occurred with similar frequency (p=0.5) in treated (39% (18/46)) and treatment naïve (22% (2/9)) subjects with CIDP; each was less frequent (p<0.001) than in CMT-1.
In GBS patients, the degree and presence of nerve enlargement was similar in the acute and non-acute phase. There was no difference (p>0.1) between subjects imaged in the acute (n=16) and non-acute phase (n=5) in the average (124(35) vs. 152(43)%) or proximal median (145(47)) vs. 172(63)%) NSIs or the likelihood of having any site with nerve enlargement (50% (8/16) vs. 60% (3/5), p=0.7). No subject imaged in the acute phase, and only one imaged in the non-acute phase, had diffuse (type 3) nerve enlargement. Neither average nor proximal median NSI varied significantly with time after GBS onset (both p=0.5).
In MMN, there was no difference (p≥0.5) between patients with or without any nerve enlargement in disease duration before treatment (37(40) vs. 44(59) months) or total disease duration (124(118) vs. 118(123) months). Neither average nor proximal ulnar NSIs varied significantly with disease duration before or after treatment (p≥0.09). There was no difference (p≥0.5) between treatment naive and treated subjects with MMN in the average (122(22) vs. 120(25))% or proximal ulnar (139(57) vs. 148(39))% NSI or the likelihood of having any site with nerve enlargement (4/6 vs. 7/11 subjects, p=1). There was no difference (p>0.2) between antibody positive and negative subjects with MMN in the average nerve size, number of abnormal nerve sites, or likelihood of having any nerve site enlarged.
DISCUSSION
Ultrasound, a painless procedure, can be used to screen children, and others, suspected of having CMT-1 who do not tolerate electrodiagnostic studies. Nerves are frequently (100%) and often greatly enlarged in dominant demyelinating hereditary neuropathies (CMT-1), a finding similar to other smaller studies of CMT-1 [11,20,15]. Ultrasound detection of nerve enlargement in CMT-1 is present to a similar degree in both children and adults and occurs in children as young as two years old. The presence and degree of nerve enlargement in inherited neuropathies may vary among genotypes. Nerves trended (p=0.06) to be smaller in our three patients with CMT-1b compared to those with CMT-1A. There may be less nerve enlargement in CMT-X and CMT-2 than in CMT-1A[11,20]. With suspected inherited peripheral neuropathy, normal nerve size argues against CMT-1 but cannot exclude other inherited etiologies.
The pattern of nerve enlargement may depend on both the type of demyelinating disease and the degree of nerve enlargement. Normal nerves or mild (type 1) or regional (type 2) nerve enlargement is rare in CMT-1 and suggests an acquired demyelinating polyneuropathy. Diffuse (type 3) nerve enlargement of the medan and ulnar nerves is typical of CMT-1 but also occur in 37% patients with CIDP, particularly when nerves are massively (more than four times normal size) enlarged. Diffuse nerve enlargement was not seen in MMN or during the acute phase of GBS. In more moderately enlarged nerves, the regional pattern of nerve enlargement was not present in CMT-1 but was in acquired demyelinating neuropathies. In patients with moderately enlarged proximal median nerves, regional nerve enlargement was seen in CIDP (5 of 13) but not in CMT-1 (0 of 13). The qualitative occurrence and location of “focal” nerve enlargement limited to discrete segments of nerves has been reported in cases of acquired demyelinating neuropathies [10,16,21] and requires further comparative study.
Our study suggests that nerve enlargement in chronic acquired demyelinating neuropathies is associated with disease duration, particularly of “active” disease prior to treatment. In CIDP, patients with less than three months of symptoms before treatment initiation had smaller nerves and were less likely to have any nerve twice larger than normal than those with a longer time from symptom onset to initial treatment. In GBS, with less than four weeks of active disease duration, there is less nerve enlargement than in CIDP. Our retrospective study did not assess the adequacy of treatment in individual patients but suggests that the treatments may impede nerve enlargement. Improvement in nerve enlargement over time has been reported in a child with GBS [8]. In some cases, however, changes in nerve size can be long lasting. We found nerve enlargement in patients during the non-acute phase of GBS and in treated patients with CIDP and MMN. As nerve enlargement can persist in acquired demyelinating neuropathies after treatment, ultrasound can augment electrodiagnostic evaluations which may lose sensitivity following treatment.
In CIDP, proximal nerve regions may be more susceptible to enlargement. We found that regional or mild nerve enlargement in CIDP most often affected the median and ulnar nerve in the proximal arm. Measures of nerve size from additional locations, including more proximal sites in the cervical roots[14], brachial plexus, or axilla, could detect additional enlargement and might be more sensitive to the presence of disease but are unlikely to change our findings regarding differences in patterns of enlargement between CIDP and CMT-1. A recent study of nerve size measured from 24 sites in the neck and arm showed no difference in the degree of cervical nerve root enlargement between CIDP and CMT-1 and conluded that only two measurements from both the median and ulnar nerve in the arm and forearm, similar to the protocol in our study, were required to identify patterns of nerve enlargement that differentiated CIDP from CMT-1[15]. A prior study of MMN found nerve enlargement slightly more often in the brachial plexus than the proximal or distal median or ulnar nerves [10]. Our study measured median and ulnar nerve size at the level of the mid-humerus and distal forearm. Additional studies are required to determine if nerve enlargement at other locations differs between the acquired demyelinating neuropathies.
This ultrasound study, the largest of nerve pathology in acquired and inherited demyelinating neuropathies, describes the characteristics of nerve enlargement in the median and ulnar nerves of a diverse population and includes patients both with and without active disease. Other factors including disease duration, severity, or response to treatment could affect results and limits the generalizability of our findings. The association between disease duration before and after treatment in our subjects with CIDP could bias the effects of disease duration on nerve enlargement seen in our study. Prospective, longitudinal studies of treatment naïve patients are required to best determine how nerves respond to disease and treatment.
In conclusion, median and ulnar nerve enlargement in CMT-1, a common type of inherited neuropathy [22], is nearly always present and is typically diffuse (type 3). Ultrasound can be used to screen patients when an inherited demyelinating neuropathy is suspected, especially as both children and adults with CMT-1 have nerve enlargement. Normal, mildly (type 1), or regionally (type 2) enlarged nerves in the arm in demyelinating polyneuropathy suggests an acquired etiology. In CIDP, early treatment may prevent subsequent nerve enlargement. Longitudinal studies focused on the relationship between nerve enlargement and disease activity are required to determine if the pattern and degree of nerve enlargement can be used to monitor long term disease activity and efficacy of treatment.
Acknowledgments
The study was supported by the Washington University Neuromuscular Research Fund and the National Institute of Health Neurological Sciences Academic Development Award K12 NS00169009.
Footnotes
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Padua L, Aprile I, Pazzaglia C, Frasca G, Caliandro P, Tonali P, Martinoli C. Contribution of ultrasound in a neurophysiological lab in diagnosing nerve impairment: A one-year systematic assessment. Clin Neurophysiol. 2007;118 (6):1410–1416. doi: 10.1016/j.clinph.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Padua L, Di Pasquale A, Liotta G, Granata G, Pazzaglia C, Erra C, Briani C, Coraci D, De Franco P, Antonini G, Martinoli C. Ultrasound as a useful tool in the diagnosis and management of traumatic nerve lesions. Clin Neurophysiol. 2013 doi: 10.1016/j.clinph.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Volpe A, Rossato G, Bottanelli M, Marchetta A, Caramaschi P, Bambara LM, Bianconi C, Arcaro G, Grassi W. Ultrasound evaluation of ulnar neuropathy at the elbow: correlation with electrophysiological studies. Rheumatology (Oxford) 2009;48 (9):1098–1101. doi: 10.1093/rheumatology/kep167. [DOI] [PubMed] [Google Scholar]
- 4.Beekman R, Visser LH, Verhagen WI. Ultrasonography in ulnar neuropathy at the elbow: a critical review. Muscle Nerve. 2011;43 (5):627–635. doi: 10.1002/mus.22019. [DOI] [PubMed] [Google Scholar]
- 5.Mhoon JT, Juel VC, Hobson-Webb LD. Median nerve ultrasound as a screening tool in carpal tunnel syndrome: Correlation of cross-sectional area measures with electrodiagnostic abnormality. Muscle & nerve. 2012;46 (6):861–870. doi: 10.1002/mus.23426. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright MS, Hobson-Webb LD, Boon AJ, Alter KE, Hunt CH, Flores VH, Werner RA, Shook SJ, Thomas TD, Primack SJ, Walker FO. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012;46 (2):287–293. doi: 10.1002/mus.23389. [DOI] [PubMed] [Google Scholar]
- 7.Zaidman CM, Seelig MJ, Baker JC, Mackinnon SE, Pestronk A. Detection of Peripheral Nerve Pathology: Comparison of Ultrasound and Magnetic Resonance Imaging. Neurology. doi: 10.1212/WNL.0b013e3182904f3f. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida V, Mariotti P, Veltri S, Erra C, Padua L. Nerve ultrasound follow-up in a child with Guillain-Barre syndrome. Muscle & nerve. 2012;46 (2):270–275. doi: 10.1002/mus.23325. [DOI] [PubMed] [Google Scholar]
- 9.Zaidman CM, Al-Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle & nerve. 2009;40 (6):960–966. doi: 10.1002/mus.21431. [DOI] [PubMed] [Google Scholar]
- 10.Beekman R, van den Berg LH, Franssen H, Visser LH, van Asseldonk JT, Wokke JH. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology. 2005;65 (2):305–307. doi: 10.1212/01.wnl.0000169179.67764.30. [DOI] [PubMed] [Google Scholar]
- 11.Martinoli C, Schenone A, Bianchi S, Mandich P, Caponetto C, Abbruzzese M, Derchi LE. Sonography of the median nerve in Charcot-Marie-Tooth disease. AJR American journal of roentgenology. 2002;178 (6):1553–1556. doi: 10.2214/ajr.178.6.1781553. [DOI] [PubMed] [Google Scholar]
- 12.Cartwright MS, Brown ME, Eulitt P, Walker FO, Lawson VH, Caress JB. Diagnostic nerve ultrasound in Charcot-Marie-Tooth disease type 1B. Muscle & nerve. 2009;40 (1):98–102. doi: 10.1002/mus.21292. [DOI] [PubMed] [Google Scholar]
- 13.Imamura K, Tajiri Y, Kowa H, Nakashima K. Peripheral nerve hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy detected by ultrasonography. Intern Med. 2009;48 (7):581–582. doi: 10.2169/internalmedicine.48.1924. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka N, Kohriyama T, Ochi K, Nishitani M, Sueda Y, Mimori Y, Nakamura S, Matsumoto M. Detection of cervical nerve root hypertrophy by ultrasonography in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci. 2004;219 (1–2):15–21. doi: 10.1016/j.jns.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto T, Ochi K, Hosomi N, Takahashi T, Ueno H, Nakamura T, Nagano Y, Maruyama H, Kohriyama T, Matsumoto M. Ultrasonographic nerve enlargement of the median and ulnar nerves and the cervical nerve roots in patients with demyelinating Charcot-Marie-Tooth disease: distinction from patients with chronic inflammatory demyelinating polyneuropathy. J Neurol. 2013 doi: 10.1007/s00415-013-7021-0. [DOI] [PubMed] [Google Scholar]
- 16.Granata G, Pazzaglia C, Calandro P, Luigetti M, Martinoli C, Sabatelli M, Padua L. Ultrasound visualization of nerve morphological alteration at the site of conduction block. Muscle & nerve. 2009;40 (6):1068–1070. doi: 10.1002/mus.21449. [DOI] [PubMed] [Google Scholar]
- 17.Padua L, Martinoli C, Pazzaglia C, Lucchetta M, Granata G, Erra C, Briani C. Intra- and internerve cross-sectional area variability: new ultrasound measures. Muscle & nerve. 2012;45 (5):730–733. doi: 10.1002/mus.23252. [DOI] [PubMed] [Google Scholar]
- 18.Van den Bergh PY, Pieret F. Electrodiagnostic criteria for acute and chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2004;29 (4):565–574. doi: 10.1002/mus.20022. [DOI] [PubMed] [Google Scholar]
- 19.European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society--first revision. J Peripher Nerv Syst. 2010;15 (4):295–301. doi: 10.1111/j.1529-8027.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber S, Oldag A, Kornblum C, Kollewe K, Kropf S, Schoenfeld A, Feistner H, Jakubiczka S, Kunz WS, Scherlach C, Tempelmann C, Mawrin C, Dengler R, Schreiber F, Goertler M, Vielhaber S. Sonography of the median nerve in CMT1A, CMT2A, CMTX, and HNPP. Muscle & nerve. 2012 doi: 10.1002/mus.23681. [DOI] [PubMed] [Google Scholar]
- 21.Scheidl E, Bohm J, Simo M, Rozsa C, Bereznai B, Kovacs T, Aranyi Z. Ultrasonography of MADSAM neuropathy: focal nerve enlargements at sites of existing and resolved conduction blocks. Neuromuscular disorders : NMD. 2012;22 (7):627–631. doi: 10.1016/j.nmd.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol. 2011;69 (1):22–33. doi: 10.1002/ana.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]