Dear Sirs,
Hemophilia A is a bleeding disorder caused by the deficiency and dysfunction of factor VIII (FVIII). Administration of recombinant FVIII is the first line therapy to treat this disorder (1). A clinical complication of this therapy is the development of inhibitory antibody in 25–35% of severe hemophilia patients (2, 3). Inhibitory antibodies are primarily directed against the A3, A2 and C2 domains, which also contain sequences required for critical macromolecular interactions in clotting cascade such as interaction with von Willebrand factor (vWF), factor IXa (FIXa), phospholipid membranes, and clearance receptors (4, 5).
The macromolecular interaction between FVIII and vWF improves not only plasma survival of FVIII but also reduces its immunogenicity. Plasma derived FVIII containing endogenous vWF or recombinant FVIII preparations co-expressed with vWF have shown lower antibody response in hemophilia mice (6, 7). However, recent evidences also suggested that when FVIII was mixed in vitro with highly purified plasma derived vWF (pdvWF) in physiologically equivalent molar ratio, did not show significant decrease in inhibitor development for FVIII (8). vWF prevents endocytosis of FVIII by dendritic cells and its subsequent presentation to the specific CD4+T cells (9, 10). It may also protect exogenous FVIII from binding to existing antibodies (11). The reduction in immunogenicity of FVIII possibly involves shielding of critical epitopes in FVIII by vWF binding (12–14).
The C2 domain contains epitopes that elicit inhibitory response but also contain overlapping/proximal binding sites for vWF and phospholipids (13). Based on this, it is anticipated that phospholipid binding could provide similar protective effect that is observed with vWF. Our recent studies confirmed that phospholipid binding reduced catabolism and improved plasma survival of FVIII in vWF null mice (15). It will be interesting to investigate whether phospholipid binding could also provide similar protective effects of VWF in reducing immunogenicity by shielding the immune dominant epitopes. Hence, the immunogenicity of free FVIII and FVIII-phospholipid complex was tested in vWF null mice.
The FVIII phospholipid complex reduced immunogenicity of FVIII (16, 17) in HA mice. We have shown that complexation of FVIII with lipidic particles containing phosphatidylserine (PS), and phosphatidylinositol (PI) reduced catabolism (15, 17) and inhibitor development (17, 18) in hemophilia A (HA) mice (19). The underlying mechanism could involve sequestration of epitopes of FVIII by phospholipid binding. However, it could not be established unambiguously as vWF may bind to FVIII that is released from phospholipid complex, thus the immune dominant epitopes are continuously shielded by binding either to VWF or phospholipid. Therefore, immunogenicity of FVIII-phospholipid complex was investigated in vWF null mice to delineate whether the immunogenicity of FVIII is reduced by the sequestration of immunodomiant epitope by phospholipid binding in the absence of vWF.
FVIII-PS and FVIII-PI, were prepared as previously described (17–19). A colony of mice that are homozygous null due to the targeted deletion of vWF gene were established using a breeding pairs (JAX Strain B6.129S2-vWF<tm1Wgr>/J) from the original colony (Jackson Lab, Bar Harbour, ME). vWF knockout mice (20–25 g) of 8–12 weeks old, were used for the immunogenicity studies. The relative immunogenicity of free FVIII and FVIII lipid complexes were evaluated in vWF knockout mice. All animal handling was performed in accordance with the guidelines of institutional animal care and use committees (IACUC) at the University at Buffalo.
Relative immunogenicity differences between free FVIII and FVIII-lipid complexes were measured in vWF knockout mice as described earlier (19). All FVIII and FVIII-lipid complex preparations were confirmed endotoxin negative by limulus amoebocyte assay and were injected immediately after preparation. Groups of mice received 4 weekly i.v. injections (via penile vein, n=6) of FVIII or FVIII-Lipid complexes (10 IU/injection). Two weeks after the last injection, blood samples were collected by cardiac puncture in to acid citrate dextrose (ACD) buffer at a 10:1 (v/v) blood:ACD ratio. Plasma was separated by centrifugation at 5,000 g at 4°C for 5 min. Samples were stored at −80°C immediately after centrifugation until analysis.
Antibody titers were determined by standard antibody capture ELISA (19). Inhibitory antibody titers were determined using the Nijmegen modification of the Bethesda assay as described previously (19). As immunoglobulins are heat resistant all plasma samples were heat inactivated (90 minutes at 58°C) to minimize the interference of residual FVIII activity in the determination of inhibitory antibody levels. However, heating does not release the inhibitors already complexed with FVIII (20). T cell proliferation was determined by Thymidine (3H) incorporation. Briefly, groups of vWF deficient mice received 4 weekly injections (via penile vein, n=6) of FVIII or FVIII-Lipid complexes (10 IU/injection). Two weeks after the last injection, animals were sacrificed and spleens were collected and homogenized. Splenic T cells were isolated according to the Dynal CD4+ negative isolation kit instruction manual (Invitrogen Inc., Carlsbad, CA). Dendritic cells (DCs) were isolated from bone marrow of vWF deficient mice as described previously (21). These DCs were treated with FVIII antigen (2µg/ml) for 24 hours at 37°C and 5% CO2. Splenic T cells (2×105 cells/well) from individual mice (n = 6) were cultured in 96-well plates along with naïve or FVIII treated murine dendritic cells (DC) (7 ×105 cells/well) in T cell media. T-lymphocytes plated without antigen and with naïve Dcs served as the negative control. T-lymphocytes exposed to Concanavalin A served as the positive control. After 48 hours, 3H-thymidine (1µCi/well) was added and the cultures were harvested after 72 hours of incubation using a plate harvester onto a Uni-filter-96® GF/C plate (Perkin Elmer, Boston, MA). The mean counts from quadruplicate wells were measured using a Top Count scintillation counter (Perkin Elmer, Boston, MA). In order to normalize the data of each experiment and to facilitate the comparison in between experiments the activation of T-cells was expressed as stimulation index (SI). SI is the ratio of 3H thymidine incorporation in the presence of antigen [FVIII 2µg/ml] to incorporation in the absence of antigen. Statistical Analysis was conducted using Minitab 16 (Minitab Inc, State College, PA). Significant differences (p<0.05) were tested using a non parametric two-way Mann-Whitney test.
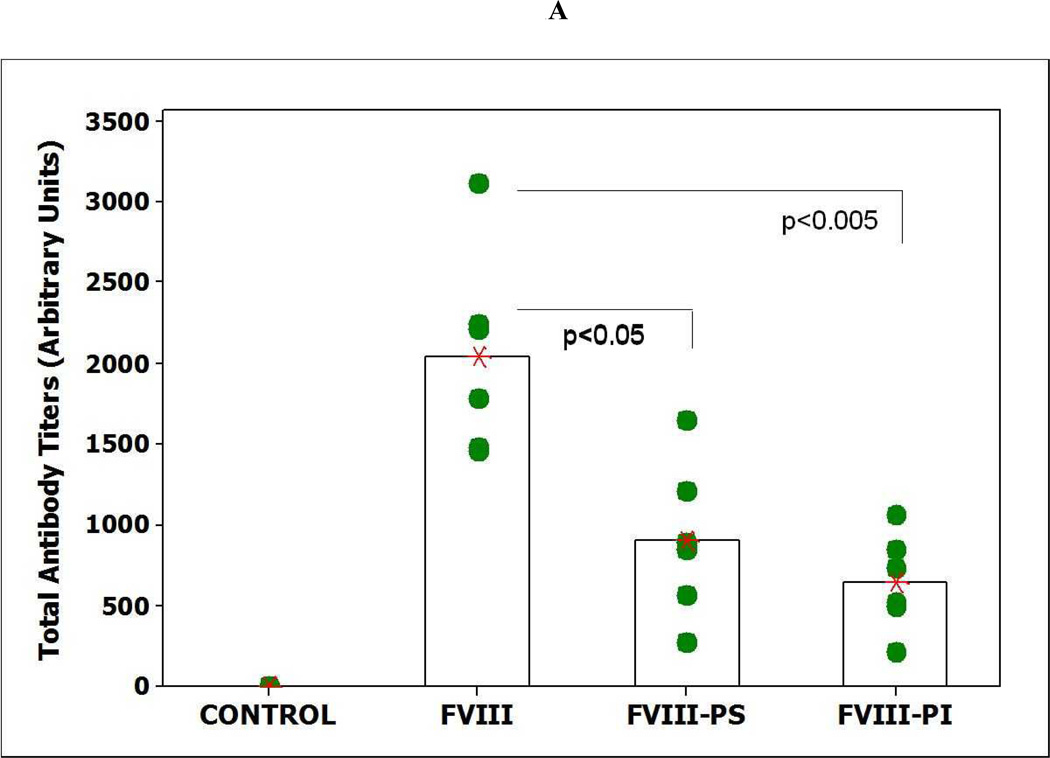
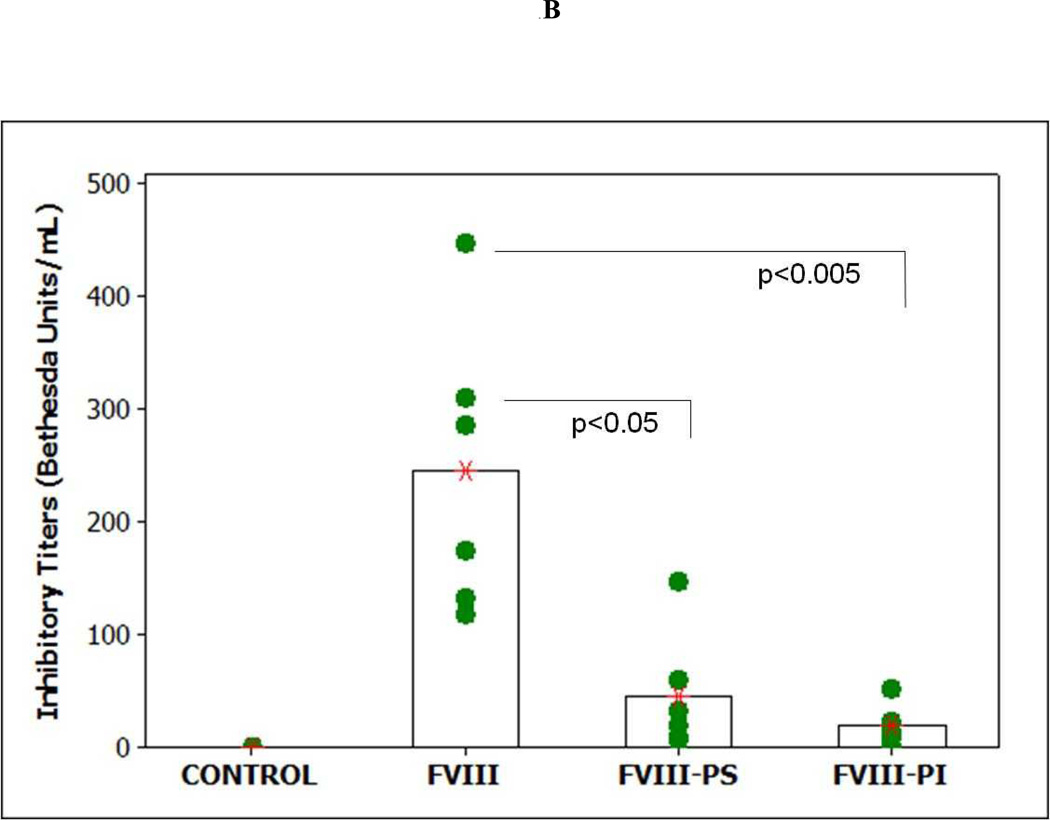
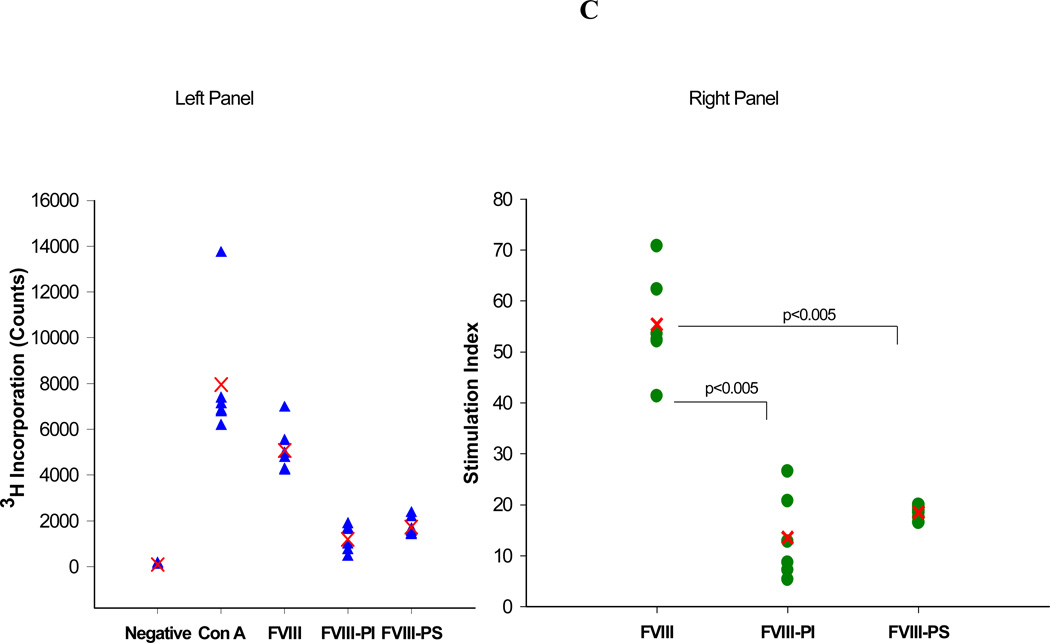
The total and inhibitory titers in mice immunized with FVIII and lipidic FVIII administered are shown in Figure 1. Following i.v. administration, of free FVIII a mean total antibody titer of 2045 ± 254 (S.E.; n=6) and an inhibitory titer of 244 ± 52 Bethesda Units (BU)/mL were observed. These titer levels are lower than those observed for free FVIII in hemophilia A mice. This decrease in immune response may be due to some central tolerance established by the endogenous mouse FVIII (low levels of circulating FVIII < 8 to 13% of normal) in vWF deficient mice that cross-reacts with the exogenous human FVIII as compared to the hemophilia mice which are non-tolerized mice model for FVIII. However, responses observed in both animal models are much higher than clinically observed in hemophilia patients and may be due to administration of human protein in a mouse model. The administration of FVIII-PI and FVIII-PS lipid particles resulted in significantly lower immune response at the end of the 6 weeks as compared to the free FVIII in vWF null mice. The mean total antibody titer for mice immunized with FVIII PI was 644 ± 294 (S.E.; n=6) and the inhibitory titer was 18.6 ± 7.2 (BU)/mL (n=6) respectively. Similarly, The mean total antibody titer and inhibitory antibody titer for mice immunized with FVIII-PS was 902 ± 120 (S.E.; n=6) and 45.6 ± 21.6 (BU)/mL (n=6) respectively. The reduction in median total and inhibitory immune response was significant (Mann–Whitney, nonparametric test) for FVIII-PS (P<0.05) and FVIII-PI (P<0.005) as compared to the free FVIII group. The control group receiving saline did not show any immune response. This reduction in titers is consistent with our T-cell proliferation studies that showed PS and PI significantly reduced the activation of FVIII specific T cells. The T cell stimulation index for the PS treatment group was (18.5 ± 1.3 S.E) significantly lower than that observed for co-cultures challenged with free FVIII (55.4±10.1 S.E.). Similarly, the mean SI for PI treatment group was (13.6 ± 8.4 S.E.) about 4 fold lower than free FVIII group. The reduction in median stimulation index was significant (Mann–Whitney, nonparametric test) for both FVIII-lipidic complexes (P<0.005).
Figure 1.
Effect of PI and PS on immunogenicity of FVIII. A) The mean of total antibody titers (bars) and individual (closed circles) antibody titers against FVIII as determined by antibody capture ELISA in vWF mice immunized via i.v. administration of free FVIII, FVIII-PI, and FVIII-PS. B) The mean of inhibitory titers (bars) and individual (closed circles) antibody titers against FVIII as determined by the Nijmegen modification of the Bethesda assay in vWF knockout mice immunized via i.v. administration of free FVIII, FVIII-PI, FVIII-PS. C) Left panel shows the raw data for Thymidine (3H) incorporation (counts per minute) of different groups of splenocytes. The mean count (cross) and splenocytes from individual spleen (n=6) are shown (filled triangles). Typically negative group showed counts less than 300. Right panel shows the stimulation indices of different groups of splenocytes. The mean stimulation index (cross) and individual (filled circles) stimulation indices from individual spleens (n=6) of different treatment groups as determined by thymidine (3H) incorporation T cell proliferation assay.
The underlying mechanism of lipid-mediated reduction in antibody titers could be complex. Based on the studies, it appears that it would involve sequestration of epitopes similar to that observed for FVIII-vWF complex, in addition to the immunomodulatory effects of these phospholipids.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01 HL-70227) to SVB. Dipak S. Pisal received predoctoral fellowship from Pfizer Inc. We would like to thank Dr. William J. Jusko, Department of Pharmaceutical Sciences, University at Buffalo, for letting us use plate harvester and Top-count scintillation counter instruments in his lab and Nancy Pyszczynski for her technical help with these instruments.
REFERENCES
- 1.Klinge J, Ananyeva NM, Hauser CA, et al. Hemophilia A--from basic science to clinical practice. Semin Thromb Hemost. 2002 Jun;28(3):309–322. doi: 10.1055/s-2002-32667. [DOI] [PubMed] [Google Scholar]
- 2.Pratt KP, Thompson AR. B-cell and T-cell epitopes in anti-factor VIII immune responses. Clin Rev Allergy Immunol. 2009 Oct;37(2):80–95. doi: 10.1007/s12016-009-8120-7. [DOI] [PubMed] [Google Scholar]
- 3.Ananyeva NM, Lacroix-Desmazes S, Hauser CA, et al. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coagul Fibrinolysis. 2004 Mar;15(2):109–124. doi: 10.1097/00001721-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jacquemin MG, Saint-Remy JM. Factor VIII immunogenicity. Haemophilia. 1998 Jul;4(4):552–557. doi: 10.1046/j.1365-2516.1998.440552.x. [DOI] [PubMed] [Google Scholar]
- 5.Skupsky J, Zhang AH, Su Y, et al. A role for thrombin in the initiation of the immune response to therapeutic factor VIII. Blood. 2009 Nov;114(21):4741–4748. doi: 10.1182/blood-2008-10-186452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrmann M, Pasi J, Saint-Remy JMR, et al. Von Willebrand factor modulates factor VIII immunogenicity: Comparative study of different factor VIII concentrates in a haemophilia A mouse model. Thrombosis and Haemostasis. 2002 Aug;88(2):221–229. [PubMed] [Google Scholar]
- 7.Delignat S, Dasgupta S, Andre S, et al. Comparison of the immunogenicity of different therapeutic preparations of human factor VIII in the murine model of hemophilia A. Haematologica. 2007 Oct;92(10):1423–1426. doi: 10.3324/haematol.11438. [DOI] [PubMed] [Google Scholar]
- 8.Qadura M, Waters B, Burnett E, et al. Recombinant and plasma-derived factor VIII products induce distinct splenic cytokine microenvironments in hemophilia A mice. Blood. 2009 Jul 23;114(4):871–880. doi: 10.1182/blood-2008-09-174649. [DOI] [PubMed] [Google Scholar]
- 9.Dasgupta S, Repesse Y, Bayry J, et al. VWF protects FVIII from endocytosis by dendritic cells and subsequent presentation to immune effectors. Blood. 2007 Jan 15;109(2):610–612. doi: 10.1182/blood-2006-05-022756. [DOI] [PubMed] [Google Scholar]
- 10.Navarrete AM, Dasgupta S, Teyssandier M, et al. Endocytic receptor for pro-coagulant factor VIII: relevance to inhibitor formation. Thromb Haemost. 2010 Dec;104(6):1093–1098. doi: 10.1160/TH10-05-0294. [DOI] [PubMed] [Google Scholar]
- 11.Lacroix-Desmazes S, Repesse Y, Kaveri SV, et al. The role of VWF in the immunogenicity of FVIII. Thrombosis Research. 2008;122:S3–S6. doi: 10.1016/S0049-3848(08)70002-1. [DOI] [PubMed] [Google Scholar]
- 12.Meeks SL, Healey JF, Parker ET, et al. Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. 2007 Dec 15;110(13):4234–4242. doi: 10.1182/blood-2007-06-096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt KP, Qian J, Ellaban E, et al. Immunodominant T-cell epitopes in the factor VIII C2 domain are located within an inhibitory antibody binding site. Thromb Haemost. 2004 Sep;92(3):522–528. doi: 10.1160/TH03-12-0755. [DOI] [PubMed] [Google Scholar]
- 14.Saenko EL, Scandella D. The acidic region of the factor VIII light chain and the C2 domain together form the high affinity binding site for von willebrand factor. J Biol Chem. 1997 Jul 18;272(29):18007–18014. doi: 10.1074/jbc.272.29.18007. [DOI] [PubMed] [Google Scholar]
- 15.Pisal DS, Balu-Iyer SV. Phospholipid binding improves plasma survival of factor VIII. Thromb Haemost. 2010 Nov 3;104(5):1073–1075. doi: 10.1160/TH10-06-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaveri SV, Dasgupta S, Andre S, et al. Factor VIII inhibitors: role of von Willebrand factor on the uptake of factor VIII by dendritic cells. Haemophilia. 2007 Dec;13:61–64. doi: 10.1111/j.1365-2516.2007.01575.x. [DOI] [PubMed] [Google Scholar]
- 17.Peng A, Straubinger RM, Balu-Iyer SV. Phosphatidylinositol containing lipidic particles reduces immunogenicity and catabolism of factor VIII in hemophilia a mice. AAPS J. 2010 Sep;12(3):473–481. doi: 10.1208/s12248-010-9207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramani K, Miclea RD, Purohit VS, et al. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J Pharm Sci. 2008 Apr;97(4):1386–1398. doi: 10.1002/jps.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purohit VS, Ramani K, Sarkar R, et al. Lower inhibitor development in hemophilia A mice following administration of recombinant factor VIII-O-phospho-L-serine complex. J Biol Chem. 2005 May 6;280(18):17593–17600. doi: 10.1074/jbc.M500163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbruggen B, van Heerde WL, Laros-van Gorkom BA. Improvements in factor VIII inhibitor detection: From Bethesda to Nijmegen. Semin Thromb Hemost. 2009 Nov;35(8):752–759. doi: 10.1055/s-0029-1245107. [DOI] [PubMed] [Google Scholar]
- 21.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999 Feb 1;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]