Abstract
Exercise has been demonstrated to improve multiple facets of health, including cognitive function. Rodent studies have suggested that exercise has robust effects on the hippocampus, and on tasks that require the hippocampus. However, studies of the effects of exercise in humans often focus on the benefits to cognitive processes that engage areas outside of the hippocampus, such as executive function. Additionally, when exercise’s cognitive benefits are examined, consideration of both males and females, and gonadal hormones, is rarely made. Here we looked at the interaction of gonadal hormones and exercise in terms of the ability of male and female rats to learn to discriminate rewarded from unrewarded arms in a T-maze based on either brightness (white vs. black) or texture (rough vs. smooth), and then to set-shift (a measure of executive function), where this required discrimination based on the opposite dimension. Gonadectomized or intact males and females had access to running wheels for two weeks before being tested. Intact males and females given access to unlocked running wheels performed better at the initial discrimination (Set 1) compared to intact males and females with locked running wheels, but not at the set-shift (Set 2). No advantage of exercise was observed in gonadectomized rats.
Keywords: Set-shifting, voluntary exercis, wheel running, estrogen, testosterone
In addition to the well-known physical benefits of exercising, the cognitive advantages brought by exercise are beginning to become clearer. Rodent studies have demonstrated exercise-related enhancements in learning and brain function, particularly hippocampus. For example, mice with access to a running wheel showed increased cell survival, proliferation and overall neurogenesis in the dentate gyrus (van Praag, Christie, Sejnowski, & Gage, 1999). Many other studies have found that physical activity increased brain-derived neurotrophic factor (BDNF) in the hippocampus (Oliff, Berchtold, Isackson, & Cotman, 1998; Rhodes et al., 2003). These changes on the cellular level, particularly in the hippocampus, have also been shown to be associated with an improvement of performance on tasks such as the radial arm maze and the Morris water maze (Anderson et al., 2000; van Praag et al., 1999). Additionally, Berchtold, Castello, and Cotman (2010) found that mice given 3 weeks of running wheel exercise showed improved performance in a radial arm water maze task that subsisted for up to two weeks after exercise had stopped. BDNF levels were found to be elevated in hippocampus in exercising mice (and positively correlated with their enhanced performance in the maze task), both immediately following exercise and at the one and two week time points after exercise had stopped, demonstrating the lasting effects of voluntary exercise. Establishing the importance of BDNF in mediating these exercise-related improvements in hippocampal function, Vaynman, Ying, and Gomez-Pinilla (2004) showed that blocking BDNF during the one-week period rats were allowed to exercise eliminated the previously observed improvement in performance of the Morris water maze.
While most animal studies to date have focused on exercise’s effects on the hippocampus and behavioral tasks requiring spatial memory, human studies have trended toward examining retention and improvement of executive functions such as working memory, cognitive flexibility, abstract thinking, and planning. Colcombe and Kramer (2003) found evidence that aerobic training may have a protective or enhancing effect for aging populations. In this study, older adults participated in either an aerobic training group or a stretching and toning group (control group) for 6 months. They were tested on a flanker test for cognitive flexibility while undergoing function magnetic resonance imaging (fMRI). Reaction time between presentations of congruent and incongruent arrows was measured, as was patterns of brain activity during responding. Following the exercise intervention, participants were found to have a significant reduction in interference (as indicated by a reduced reaction time ratio between congruent and incongruent presentations), as well as increased activity in brain regions associated with attention (e.g., middle frontal gyrus, superior frontal gyrus, and superior parietal gyrus), and decreased activity in the anterior cingulate cortex, a region associated with response conflict. They were also able to demonstrate that participants who came into the study fit (i.e., no exercise intervention took place) performed more efficiently than their less fit counterparts on the flanker task and had similar areas of increased brain activation to those in the intervention portion of the study, suggesting a potential protective effect of consistent exercise (for a review of human studies, see (Hillman, Erickson, & Kramer, 2008). Despite these findings in humans, there has been little research conducted on the effects of exercise on executive functions, such as cognitive flexibility, in rodents and changes in brain regions outside of the hippocampus. This is an important exclusion, as there are limitations in the extent to which brain mechanisms underlying the beneficial effects of exercise can be studied in humans.
Set-shifting is one measure of cognitive flexibility. Tests of set-shifting ability can be administered to both humans and rodents, serving to bridge between these two research literatures. One common test used to assess the ability of humans to set-shift is the Wisconsin Card Sort Task. In this task, participants must sort cards based on certain stimulus rules such as color, shape, or number. Periodically throughout the test the rules are changed and the participant must inhibit responding to the previous rules and begin responding to the new rules. Individuals with disorders affecting the prefrontal cortex such as schizophrenia and attention-deficit/hyperactivity disorder (ADHD) are impaired at this task in that they have a difficult time suppressing responding to the previously learned rules and continue to make perseverative responses (Green, Satz, Ganzell, & Vaclav, 1992). One way of testing this type of executive cognitive ability in rats analogous to the Wisconsin Card Sort Task involves training the animal to discriminate between rewarded and unrewarded arms in a plus-shaped maze (rotated between trials and always in a T configuration), and then switching the discrimination rule (Chess, Raymond, Gardner-Morse, Stefani, & Green, 2011; Stefani & Moghaddam, 2003). Because the start arm is always in the same location and the position of the response arm is changed between trials, spatial cues cannot be used to solve the task. Moreover, no particular response (e.g., right turn) will always result in a correct choice. Thus, this task is not spatial or response learning and is likely not hippocampus-dependent.
In addition to not relying on the hippocampus, the initial discrimination and the set-shift portion of the task are mediated by different brain substrates. The substrates of the initial discrimination have been less studied, but likely involve the dorsolateral striatum (DLS) (Palencia & Ragozzino, 2005) which has strong connections to the sensorimotor cortex. Lesions of the DLS have been shown to impair the acquisition and performance of stimulus-response associations (Yin, Knowlton, & Balleine, 2004). The set-shift relies on the medial prefrontal cortex (mPFC) and its connections with the dorsomedial striatum (DMS) (Ragozzino, Ragozzino, Mizumori, & Kesner, 2002). The DMS has been demonstrated to be crucial for goal directed behaviors, as lesions of this region impair goal-directed responding, while leaving less flexible S-R responding intact (Yin & Knowlton, 2006).
One purpose of the experiments presented here was to examine the effects of voluntary exercise on the ability of both male and female rats to perform a set-shift task. Studies on the effects of exercise on brain and behavior which include both sexes are still rare, despite the importance of understanding whether the same effects occur in males and females (McCarthy, Arnold, Ball, Blaustein, & DeVries, 2012). We predicted that exercising male and female rats would perform better at the set-shift (i.e., reach learning criterion in fewer trials) than non-exercising controls. A second purpose of the experiments presented here was to examine whether the effects of exercise might be modulated by gonadal hormones. For example, preliminary data from our laboratory suggest that the striatal dopamine (DA) system is importantly involved in our set-shifting task (Eddy, Savrann, Rifken, & Green, 2011) and exercise modulates the striatal DA system (Fisher et al., 2004; Petzinger et al., 2007). In female rats ovariectomy decreases striatal DA, dopamine transporter (DAT) and D1R levels while increasing D2R levels (Becker, 1999). This suggests that exercise might not have the same effects in rats lacking circulating gonadal hormones. On the other hand, the only two studies that we are aware of that have addressed this issue have shown that exercise in female ovariectomized (OVX) rodents produces some of the same effects on the hippocampus as exercise in intact rodents. In one study, exercise in female rats increased BDNF in the hippocampus 3 weeks but not 7 weeks after ovariectomy (Berchtold, Kesslak, Pike, Adlard, & Cotman, 2001). In the other study, exercise in female mice increased cell proliferation in the hippocampus (Jin et al., 2008). To our knowledge, there are no comparable studies in male rodents and no studies of whether exercise-associated effects on behavior are modulated by gonadal hormones.
Methods
Subjects
Male Wistar rats (n= 36) and female Wistar rats (n= 32) obtained from Harlan Sprague Dawley were used. Sixteen of the female rats arrived OVX, and 16 of the male rats arrived castrated. Staples were removed from rats 10 days following surgery. Rats were between 59 and 63 days old when they arrived in the colony, and housed individually. All rats were given at least 5 days of acclimation in the colony following their arrival, during which time they had ad lib access to food and water. Prior to training baseline weights were obtained and rats were then food deprived to 85% or 80% (males and females, respectively) of their free feeding weight. The University of Vermont Institutional Animal Care and Use Committee approved all procedures.
Voluntary Exercise
Rats assigned to the exercise group were given unlocked running wheels following colony acclimation. Non-exercise animals were given identical wheels that were locked in place to control for environmental enrichment effects. Rats had 24 hour ad lib access to running wheels (except during maze habituation procedures) for approximately two weeks prior to testing. The running wheels (Med Associates Inc., St Albans, VT) were 36 cm in diameter and had an automatic counter attached that recorded every quarter revolution. Wheel counts were taken at approximately the same time each day.
Apparatus
Set-shift apparatus and procedures are modifications of those previously described by Stefani et al., (2003). The cross-maze was on a table located in a quiet, nondescript room with minimal overhead lighting (illumination at the top of the center square of the maze = 48 lux; illumination at the floor of the center square = 26 lux), was constructed of painted polycarbonate, and consisted of a square central platform (each side measured 14.0 cm), to which four arms were attached. Arms were 14.0 cm wide, 40.6 cm long, and 20.3 cm high. A food well was located 2.5 cm from the end of each arm, and measured 1.9 cm in diameter and 0.63 cm deep. The food pellets could not be detected visually from the arm entrance. The four arms varied along two dimensions: brightness and texture. Two of the arms were painted black, while two of the arms were painted white. Of the four arms, two had a smooth texture, while two of the arms had a rough texture that was achieved by mixing a small amount of sand into the paint. Thus, the arms were black/smooth, black/rough, white/smooth, and white/rough. The central platform was painted grey. A polycarbonate insert (also painted grey) could be positioned between the central platform and any one of the arm entrances to create a T configuration with the remaining open arms and the central platform. The rats were held in a gray polycarbonate holding chamber (35.6 cm × 35.6 cm × 35.6 cm) containing animal bedding during the inter-trial intervals (ITIs).
Habituation
Approximately 5–7 days following arrival in the colony, a daily handling regimen commenced. During the first three days of this handling regimen, each rat was handled for approximately 5 minutes and then weighed. On the third day of handling and weighing, food restriction began. On subsequent days rats were handled, weighed, and then fed an amount of food that would maintain the rats at 80% (females) or 85% (males) of their baseline weight. Once the baseline weight was achieved, habituation training began. The first phase of habituation involved “open-arm” habituation. This phase was designed to acquaint the rats with the maze and shape the rat so that they reliably ate from all of the food wells. On the first day of open-arm habituation, 45-mg pellets (Purina TestDiet) were scattered throughout all arms of the maze, the central platform, and in each food well. Each rat was allotted 10 minutes to freely explore the entire maze and eat the pellets. At the end of the 10 minutes, rats were removed and placed in the holding chamber for two minutes. The rats were then placed back into their home cages where they received approximately 1 gram of the 45-mg pellets used in the maze, in addition to their daily food rationing. This helped acquaint the rats with the taste of the new pellets and encouraged them to eat during subsequent days in the maze. On the second day of “open-arm” habituation, each food well was baited with several pellets, and pellets were scattered only in the half of each arm closest to the food well. Rats were allowed to explore the maze for five minutes or until all pellets were consumed. Rats were again placed in the holding chamber for two minutes at the conclusion of their session. On the third day of habituation, four pellets were placed in each of the four food wells. Rats were again allowed to explore the maze until five minutes had elapsed or all pellets had been consumed. On the fourth day of open-maze habituation, a single pellet was placed in each food well. Rats were given two minutes to consume all of the food pellets, and were placed in the holding chamber for two minutes at the conclusion of the session. If rats did not meet the criterion of consuming all four food pellets within two minutes, they were given an additional session of open-arm habituation.
The next phase of habituation was “blocked arm habituation” and consisted of eight trials per day over two consecutive days. The purpose of this habituation phase was to acquaint the animal with the T configuration of the maze, as well as being repeatedly moved from the maze to the holding chamber in quick succession. The polycarbonate insert was placed between the central platform and one of the arms. The start arm (the stem of the T) was randomly selected and differed for each rat. The other two arms that remained accessible from the central platform were baited with a single pellet each. Rats were placed in the distal end of the start arm, allowed to choose one of the arms and consume the food reward located in the food well of the chosen arm. Rats were then removed from the maze and placed in the holding chamber for a 15-s ITI. During the ITI, the maze was rotated so that the rat would begin the next trial from a different start arm. The start arm was altered on each trial to discourage the development of habit- or place-based strategies. On the second trial, the arm opposite of the chosen arm on the previous trial was baited. The rat was again placed in the start arm, allowed to choose one of the arms, obtain a reward if the correct arm was chosen, and was removed to the holding chamber for the 15-s ITI. This procedure was repeated for 6 more trials. The arms were baited randomly, such that the probability of being rewarded for any particular arm choice (e.g. black arms, smooth arms, left arms) was equal. The goal was to have the rat make an arm choice in order to receive a food pellet. At this stage, it was not desirable for the rats to make associations between particular responses and reinforcement. If rats obtained a reinforcer on three consecutive trials, the food pellet was omitted from the subsequent trial (e.g. neither of the arms was baited). A second day of blocked-arm habituation was conducted. Time to complete the 8 habituation trials was recorded on both days.
Set-Shift Procedure
The set-shifting procedure was comprised of two daily sessions, Set 1 and Set 2. Prior to the set-shifting portion of the experiment, rats were randomly assigned to a stimulus-reward contingency (e.g., stimulus dimension: Texture; rewarded stimulus attribute: Smooth). During Set 1, the rat was first placed in the holding chamber for two minutes. Next, the rat was placed in the start arm (which was changed for each trial), and was allowed to make an arm choice. If the rat chose the rewarded stimulus attribute (correct arm), the rat would find a pellet at the end of the arm. If the rat chose a non-rewarded stimulus attribute (incorrect arm), the rat would not find a pellet. The rat was then removed to the holding chamber for the 15-s ITI, the maze rotated and re-baited, and the rat would receive further trials until a criterion of eight consecutive correct choices was reached.
For Set 2, the rewarded stimulus dimension was shifted. For example, a rat that was previously rewarded for choosing smooth arms might now be rewarded for choosing white arms. The shift in stimulus-reward contingency always occurred across stimulus dimensions. In other words, if texture (smooth or rough) was rewarded during Set 1, brightness (black or white) would be rewarded during Set 2. Unlike during Set 1, all rats were trained for at least 80 trials, regardless of how many trials were required to reach the criterion of eight consecutive correct arm choices. Time to reach criterion was recorded for both sets.
Results
Male Rats
Wheel Running
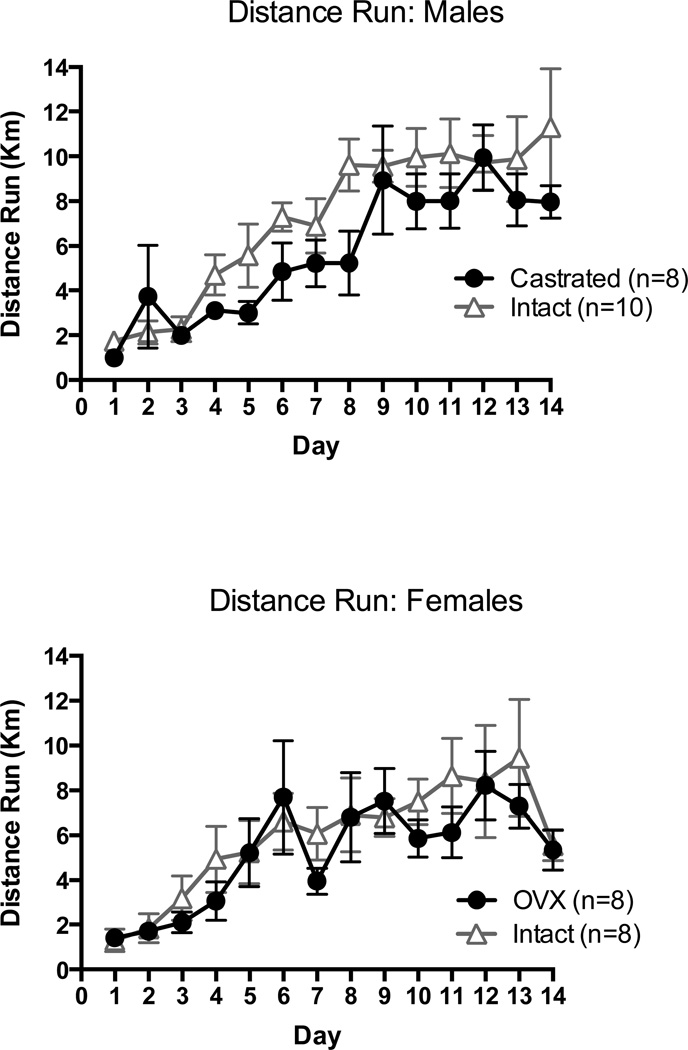
A 2 (intact vs. castrated) X 14 (day of wheel access) repeated-measures ANOVA showed that both intact and castrated male rats increased their running across the 14 day period of wheel access, F(13, 208) = 58.09, p < 0.01. There was no group effect or group by day interaction, indicating that both groups ran equal distances (Figure 1, left panel).
Figure 1.
Distance run (kilometers) per day by intact vs. gonadectomized rats.
Intact Males
Set 1: Trials to Criterion
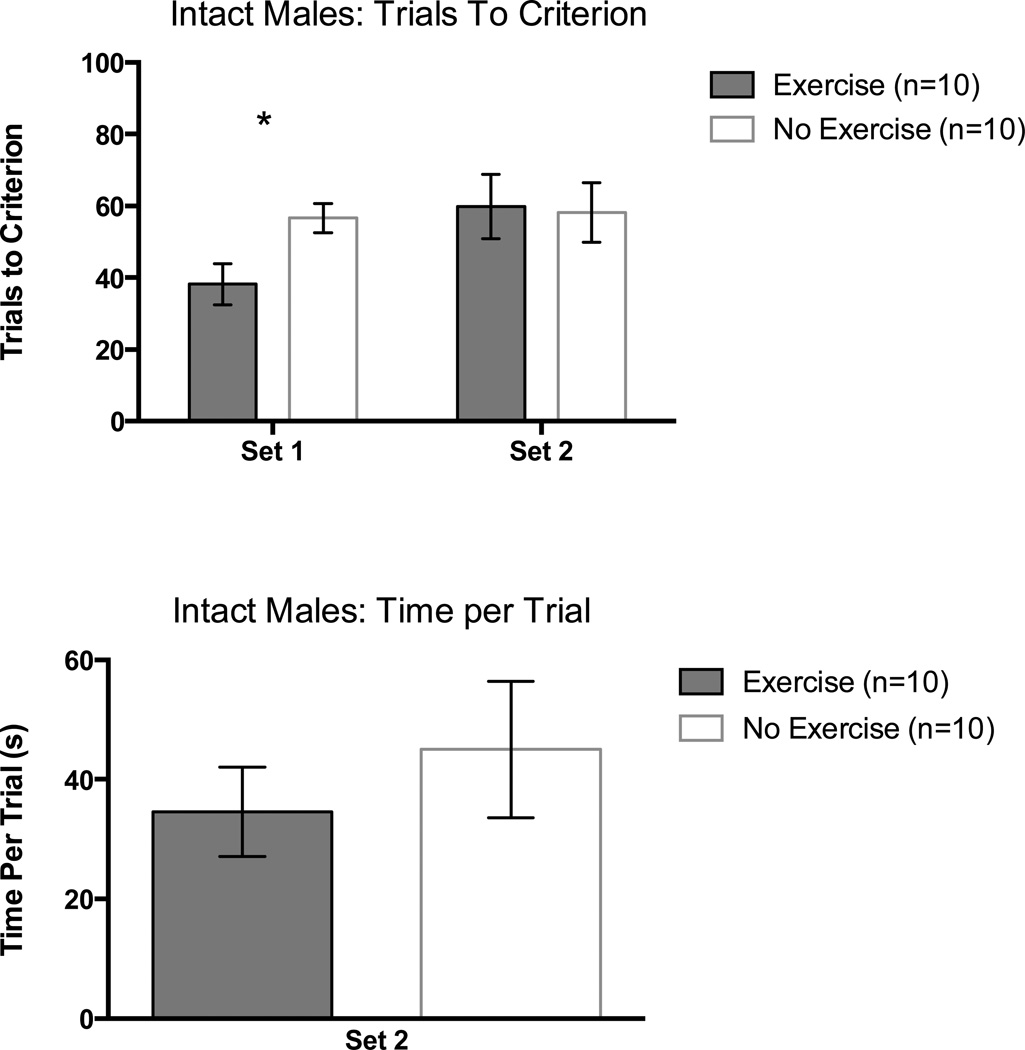
Exercising intact male rats learned the initial discrimination in fewer trials than non-exercising intact males. A one-way ANOVA on trials to criterion in Set 1 supported this observation, F(1,18) = 6.90, p < 0.05 (Figure 2, top panel).
Figure 2.
Trials to a criterion of 8 consecutive correct arm choices as a function of set in intact males (top panel) and time per trial (seconds) in Set 2 (bottom panel). Error bars are SEM. *= p < 0.05.
Set 2: Trials to Criterion
Exercising and non-exercising intact rats performed equivalently on the set-shift portion of the task as confirmed by a one-way ANOVA on trials to criterion in Set 2, F(1,18) = 0.02, p = 0.89 (Figure 2, top panel).
Set 2: Time per Trial
The time per trial measure for Set 2 was used to assess motivation and motor abilities. There were no significant differences between groups. This was confirmed with a one-way ANOVA, p > 0.05 (Figure 2, bottom panel).
Set 2: Performance Across Trial Blocks
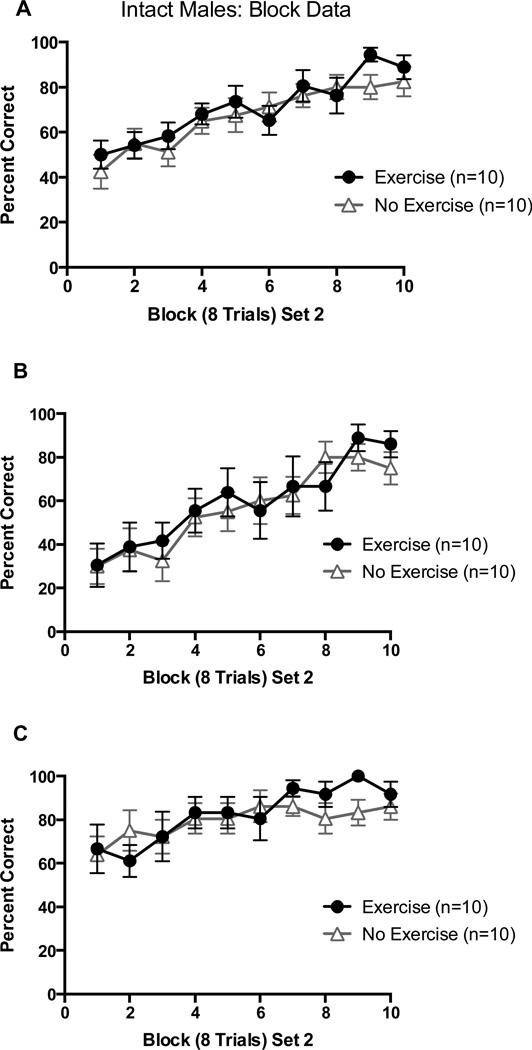
All four groups began at approximately chance levels (~50%) of overall choices in block 1 of Set 2 and learned the new set across trial blocks (Figure 3A). There was no performance difference across blocks between groups. This was confirmed by a 2 (Exercise vs. No Exercise) X 10 (8-trial block) repeated-measures ANOVA on percentage of correct choices which revealed only a significant main effect of trial block, F(9,144) = 14.09, p < 0.01.
Figure 3.
(A) Percentage of correct arm choices in Set 2 as a function of block of 8 trials in intact males; (B) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is an incorrect arm choice in Set 2; (C) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is a correct arm choice in Set 1. Error bars are SEM.
Block data were also analyzed based on percentage of perseverative arm choices (choosing the arm that was reinforced in Set 1 but not in Set 2) and percentage of reinforcement arm choices (choosing the arm that was reinforced in both Set 1 and Set 2). Both groups made perseverative errors in the early blocks of Set 2 and made fewer perseverative choices across trial blocks. When confirmed with a 2 (Exercise vs. No Exercise) X 10 (8-trial Block) repeated measures ANOVA on percentage of perseverative choices, it was shown that only the block main effect was significant, F(9, 144) = 10.90, p < 0.01 (Figure 3B). There was also a significant block main effect for reinforcement arms, F(9,144) = 4.24, p < 0.05, indicating that both groups continued to choose the previously reinforced arm in early trials blocks in Set 2, and showed a slight increase in later trial blocks (Figure 3C).
Habituation Data
Habituation data from the day before Set 1 were also compared to check for any motivational differences between the exercising and non-exercising rats. During this session, rats were run through 8 trials with one arm randomly baited on each trial. Time to complete the 8 trials was recorded. An independent samples t-test revealed no difference in time to complete 8 trials between exercising (M = 5.18, SD = 1.77) and non-exercising (M = 5.33, SD = 1.56) rats; t(18)= 0.18, p > 0.05.
Castrated Male Rats
Set 1: Trials to Criterion
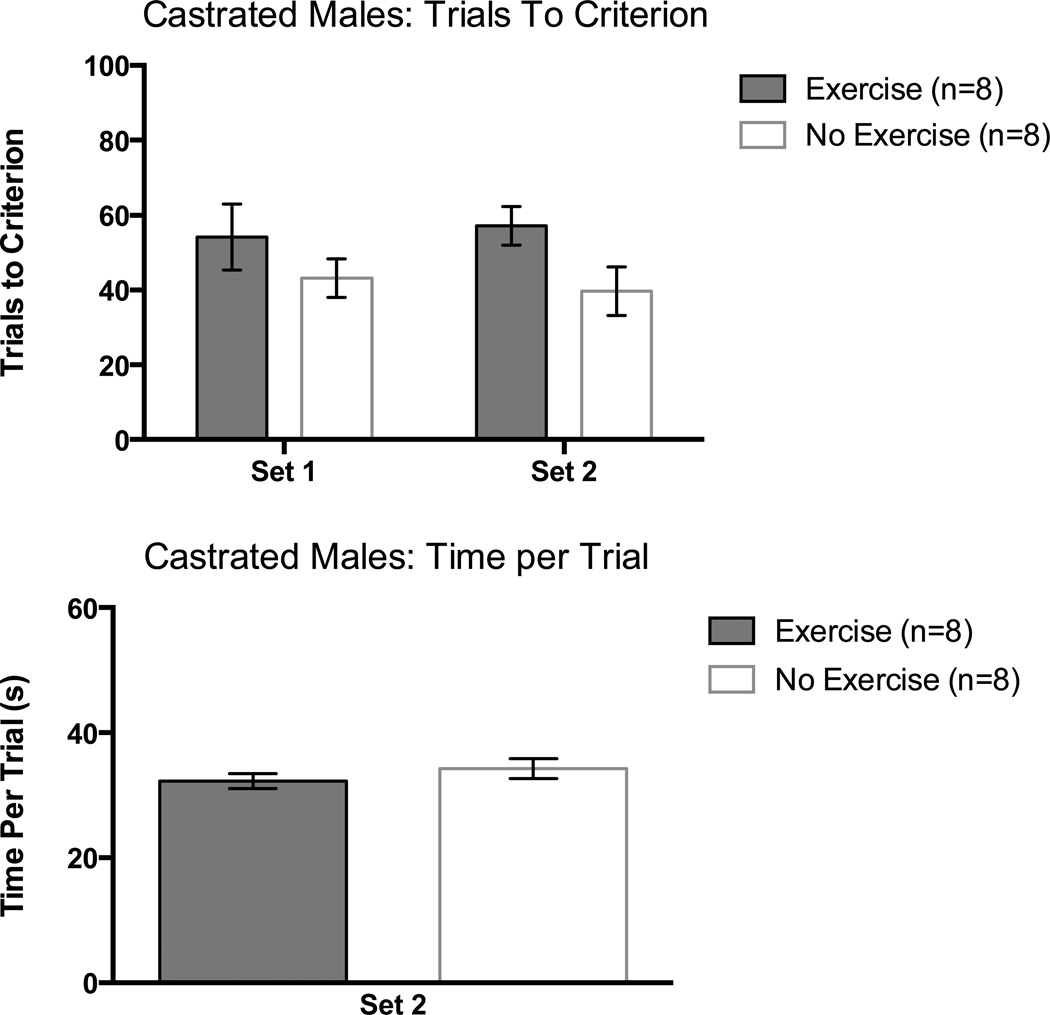
Unlike intact males, there was no significant difference between exercisers and non-exercisers in trials to criterion in Set 1 for castrated rats, F(1,14) = 1.15, p > 0.05 (Figure 4, top panel).
Figure 4.
Trials to a criterion of 8 consecutive correct arm choices as a function of set in castrated males (top panel) and time per trial (seconds) in Set 2 (bottom panel). Error bars are SEM.
Set 2: Trials to Criterion
Similar to intact males, there was no difference in performance on Set 2 between castrated exercisers and castrated non-exercisers, F(1,14) = 4.47, p > 0.05 (Figure 4, top panel).
Set 2: Time per Trial
The time per trial measure for Set 2 was used to assess motivation and motor abilities. There were no significant differences between groups. This was confirmed with a one-way ANOVA, p > 0.05 (Figure 4, bottom panel).
Set 2: Performance Across Trial Blocks
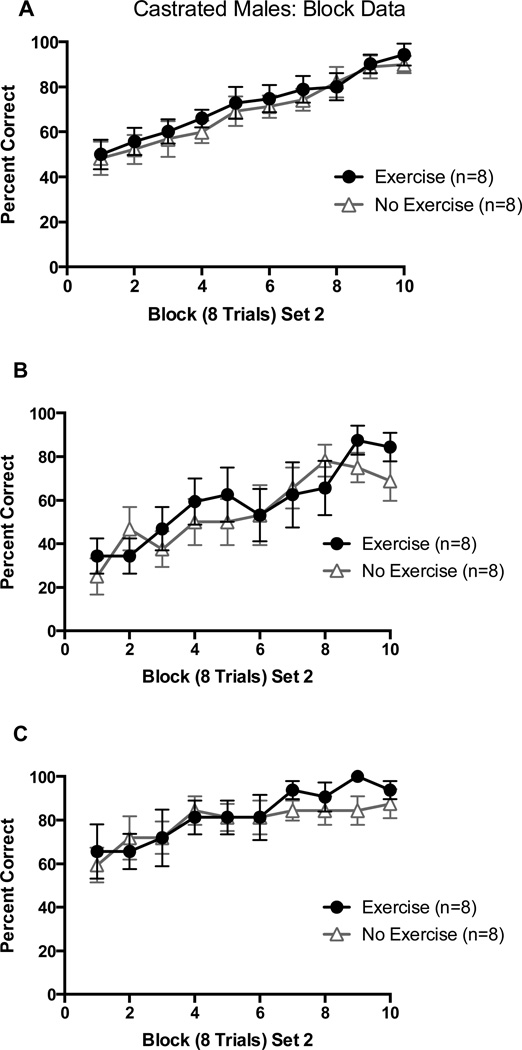
Both groups began at approximately chance levels (~50%) of overall choices in block 1 of Set 2 and learned the new set across trial blocks. There was no performance difference across blocks between groups. This was confirmed by a 2 (Exercise vs. No Exercise) X 10 (8-trial block) repeated-measures ANOVA on percentage of correct choices which revealed only a significant main effect of trial block, F(7,144) = 13.83, p < 0.01 (Figure 5A).
Figure 5.
(A) Percentage of correct arm choices in Set 2 as a function of block of 8 trials in castrated males; (B) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is an incorrect arm choice in Set 2; (C) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is a correct arm choice in Set 1. Error bars are SEM.
Block data were also analyzed based on percentage of perseverative arm choices (choosing the arm that was reinforced in Set 1 but not in Set 2) and percentage of reinforcement arm choices (choosing the arm that was reinforced in both Set 1 and Set 2). Both groups made perseverative errors in the early blocks of Set 2 and made fewer perseverative choices across trial blocks. When confirmed with a 2 (Exercise/No Exercise) X 10 (8-trial Block) repeated measures ANOVA on percentage of perseverative choices, it was shown that only the block main effect was significant, F(7, 144) = 9.90, p < 0.01 (Figure 5B). There was also a significant block main effect for reinforcement arms, F(7,144) = 4.16, p < 0.05, indicating that both groups continued to choose the previously reinforced arm in early trials blocks in Set 2, with a slight increase in later trial blocks (Figure 5C).
Habituation Data
Habituation data from the day before Set 1 were also compared to check for any motivational differences between the exercising and non-exercising rats. An independent samples t-test revealed no difference in time to complete 8 trials between exercising (M = 2.65, SD = 0.48) and non-exercising (M = 3.05, SD = 0.39) rats; t(14)= 0.20, p > 0.05.
Female Rats
Wheel Running
A 2 (intact vs. OVX) X 14 (day of wheel access) repeated-measures ANOVA showed that both intact and OVX female rats increased their running across the 14 day period of wheel access, F(13,182) = 10.55, p < 0.01. There was no group effect or group by day interaction, indicating that both groups ran equal distances (Figure 1, right panel). There has been some indication that OVX rats may run less than intact females (Rodier, 1971), though unpublished data suggest that food deprivation eliminates this difference (D. J. Bucci, personal communication, October 18, 2012).
Intact Female Rats
Set 1: Trials to Criterion
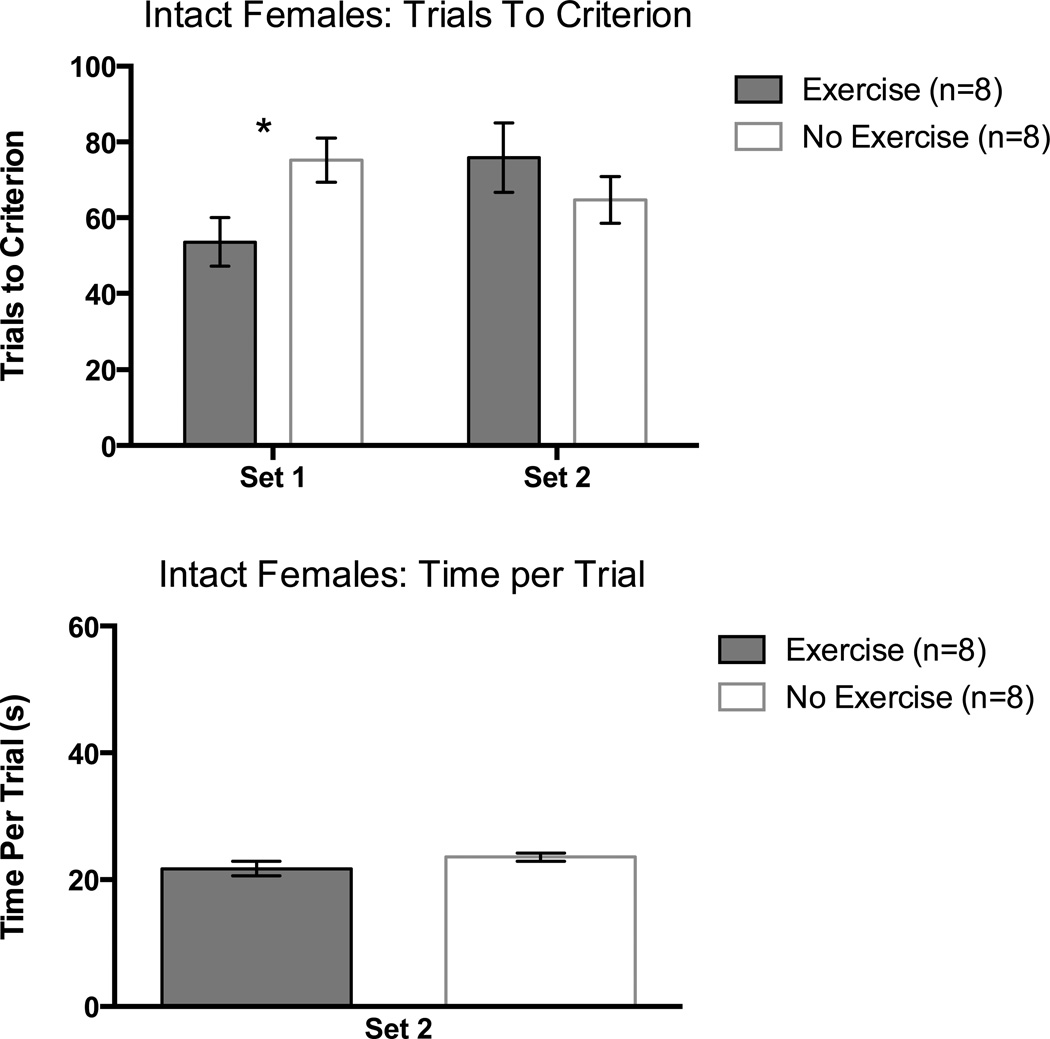
Exercising intact female rats learned the initial discrimination in fewer trials than non-exercising intact females. A one-way ANOVA on trials to criterion in Set 1 supported this observation, F(1,14) = 6.18, p < 0.05.
Set 2: Trials to Criterion
Exercising and non-exercising intact female rats performed equivalently on the set-shift portion of the task as confirmed by a one-way ANOVA on trials to criterion in Set 2, F(1,14) = 1.01, p = 0.33 (Figure 6, top panel).
Figure 6.
Trials to a criterion of 8 consecutive correct arm choices as a function of set in intact female rats (top panel) and time per trial (seconds) in Set 2 (bottom panel). Error bars are SEM. *= p<.05.
Set 2: Time per Trial
The time per trial measure for Set 2 was used to assess motivation and motor abilities. There were no significant differences between groups. This was confirmed with a one-way ANOVA, p > 0.05 (Figure 6, bottom panel).
Set 2: Performance Across Trial Blocks
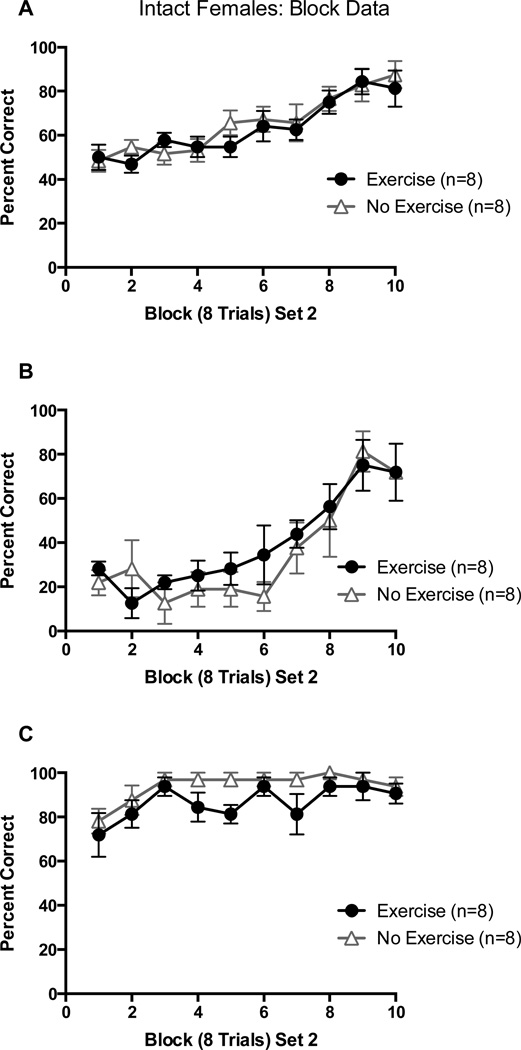
Both groups began at approximately chance levels (~50%) of overall choices in block 1 of Set 2 and learned the new set across trial blocks. There was no performance difference across blocks between groups. This was confirmed by a 2 (Exercise vs. No Exercise) X 10 (8-trial block) repeated-measures ANOVA on percentage of correct choices which revealed only a significant main effect of trial block, F(9, 126) = 15.26, p < 0.01 (Figure 7A).
Figure 7.
(A) Percentage of correct arm choices in Set 2 as a function of block of 8 trials in intact females; (B) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is an incorrect arm choice in Set 2; (C) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is a correct arm choice in Set 1. Error bars are SEM.
All four groups made a high percentage of perseverative errors in block 1 of Set 2 and made fewer perseverative choices across trial blocks. When confirmed with a 2 (Exercise vs. No Exercise) X 10 (8-trial Block) repeated measures ANOVA on percentage of perseverative choices, it was shown that only the block main effect was significant, F(9, 126) = 12.51, p < 0.01 (Figure 7B). Both groups also continued to choose the arm that was reinforced in Set 1 during Set 2. This was confirmed by a 2 (Exercise vs. No Exercise) X 10 (8-trial Block) repeated-measures ANOVA on percentage of reinforcement choices which revealed a significant block main effect, F(9,126) = 3.37, p < 0.01 (Figure 7C).
Habituation Data
An independent samples t-test revealed no difference in time to complete 8 trials between exercising (M = 3.65, SD = 0.81) and non-exercising (M = 4.16, SD = 0.75) rats; t(14)= −1.31, p > 0.05.
Ovariectomized Females
Set 1: Trials to Criterion
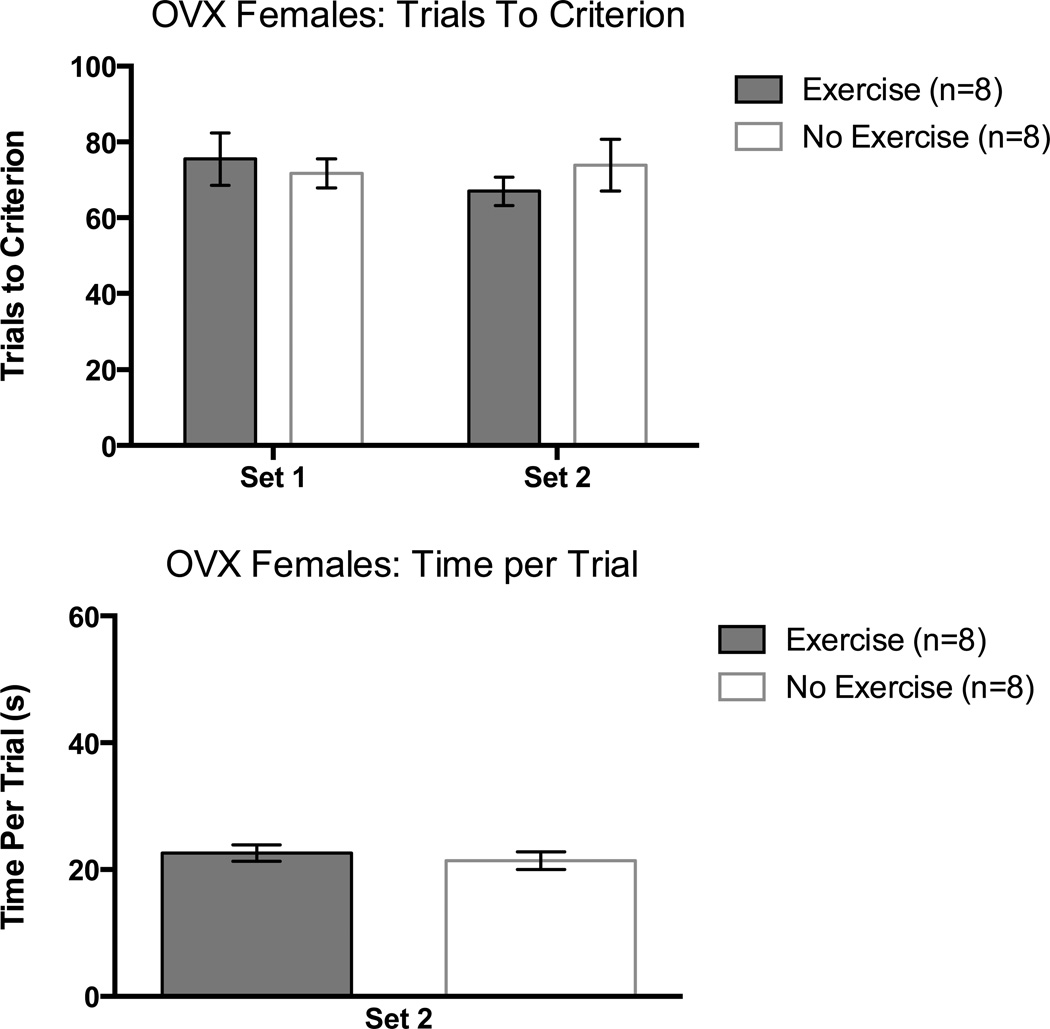
Unlike exercising intact female rats, OVX exercisers did not learn the initial discrimination in fewer trials than non-exercising OVX females. A one-way ANOVA on trials to criterion in Set 1 supported this observation, F(1,14) = 0.22, p > 0.05.
Set 2: Trials to Criterion
Like intact female rats, exercising and non-exercising OVX female rats performed equivalently on the set-shift portion of the task as confirmed by a one-way ANOVA on trials to criterion in Set 2, F(1,14) = 4.47, p > 0.05 (Figure 8, top panel).
Figure 8.
Trials to a criterion of 8 consecutive correct arm choices as a function of set in OVX female rats (top panel) and time per trial (seconds) in Set 2 (bottom panel). Error bars are SEM.
Set 2: Time per Trial
There were no significant differences between groups. This was confirmed with a one-way ANOVA, p > 0.05 (Figure 8, bottom panel).
Set 2: Performance Across Trial Blocks
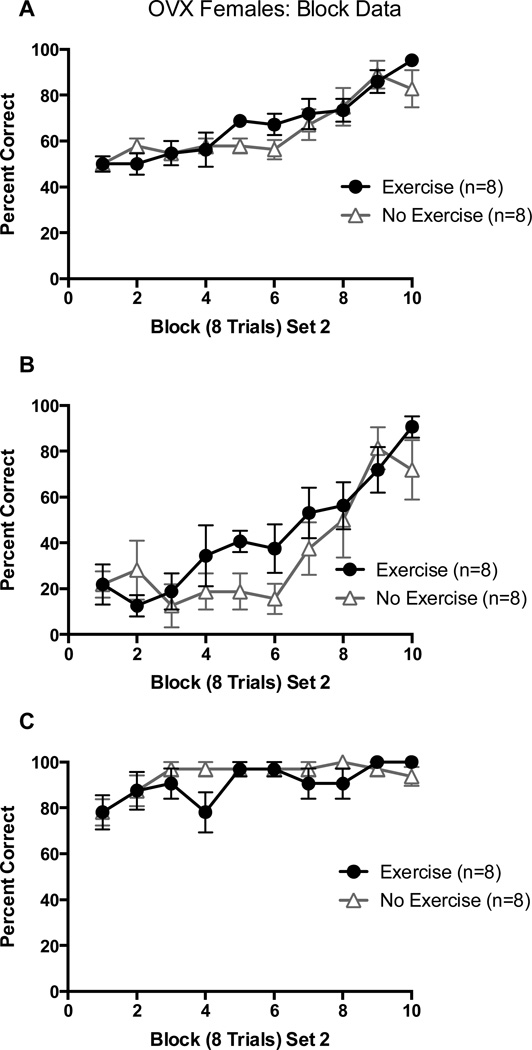
Both groups began at approximately chance levels (~50%) of overall choices in block 1 of Set 2 and learned the new set across trial blocks. There was no performance difference across blocks between groups. This was confirmed by a 2 (Exercise vs. No Exercise) X 10 (8-trial block) repeated-measures ANOVA on percentage of correct choices which revealed only a significant main effect of trial block, F(9, 126) = 16.24, p < 0.01 (Figure 9A).
Figure 9.
(A) Percentage of correct arm choices in Set 2 as a function of block of 8 trials in OVX females; (B) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is an incorrect arm choice in Set 2; (C) Percentage of correct arm choices in Set 2 from arm starts where a correct arm choice in Set 1 is a correct arm choice in Set 1. Error bars are SEM.
All four groups made a high percentage of perseverative errors in block 1 of Set 2 and made fewer perseverative choices across trial blocks. When confirmed with a 2 (Exercise vs. No Exercise) X 10 (8-trial Block) repeated measures ANOVA on percentage of perseverative choices, it was shown that only the block main effect was significant, F(9, 126) = 13.10, p < 0.01 (Figure 9B). Both groups also continued to choose the arm that was reinforced in Set 1 during Set 2. This was confirmed by a 2 (Exercise vs. No Exercise) X 10 (8-trial Block) repeated-measures ANOVA on percentage of reinforcement choices which revealed a significant block main effect, F(9,126) = 4.72, p < 0.01 (Figure 9C).
Habituation Data
An independent samples t-test revealed no difference in time to complete 8 trials between exercising (M = 3.55, SD = 0.62) and non-exercising (M = 4.56, SD = 0.97) rats; t(14)= −1.49, p > 0.05.
Males vs. Females
A final set of comparisons was made between non-exercising males and females for examination of sex differences in performance. These comparisons should be treated cautiously, as males and females were trained and tested in different experiments.
Set 1: Trials to Criterion
Non-exercising intact male rats attained criterion more quickly than non-exercising intact female rats. The same was true of non-exercising castrated male rats compared to non-exercising OVX female rats. One-way ANOVAs on trials to criterion in Set 1 supported these observations, F(1,16) = 7.25, p < 0.02 and F (1,14) = 19.51, p < 0.01.
Set 2: Trials to Criterion
There was no difference in trials to criterion between non-exercising intact male rats and non-exercising intact female rats (p > 0.05). In contrast, non-exercising castrated male rats attained criterion more quickly than non-exercising OVX female rats. A one-way ANOVA on trials to criterion in Set 1 supported this observation, F(1,14) = 13.19, p < 0.01.
Set 2: Performance Across Trial Blocks
Females (intact or OVX) showed a greater propensity to behave in Set 2 as if they were still in Set 1. This was supported by two findings. First, females showed greater perseveration in Set 2 compared to males; percentage of correct choices when the choice involved an arm that had been reinforced in Set 1 but was not reinforced in Set 2 was significantly lower in intact females compared to intact males, F(1,16) = 8.35, p = 0.01, and in OVX females compared to castrated males, F(1,14) = 6.96, p = 0.02. Second, females showed greater continued choice of arms in Set 2 that had also been reinforced in Set 1; percentage of correct choices when the choice involved an arm that had been reinforced in Set 1 and was still reinforced in Set 2 was significantly higher in intact females compared to intact males, F(1,16) = 9.15, p < 0.01, and in OVX females compared to castrated males, F(1,14) = 11.88, p < 0.01.
Discussion
There are two major gaps in the exercise literature amongst human and rodent studies; while human studies tend to focus on exercise-related improvements in executive function, rodent studies typically focus on hippocampus-dependent spatial tasks such as the Morris water maze or radial arm maze. Moreover, human studies more commonly compare males and females, while animal studies typically use one sex (most often males) or the other. These experiments looked at the effect of two weeks of voluntary wheel running on male and female rats, with or without gonadal hormones, and their ability to perform an executive function task.
Both male and female rats were predicted to perform better on the “shift” portion of the task following exercise, due to previous findings in humans suggesting exercise can improve PFC function. However, it was found that exercise only positively affected their ability to perform the initial discrimination. Both exercising intact male and female rats were able to reach criterion in fewer trials than non-exercising controls during the initial (Set 1) discrimination, while there was no difference in trials to criterion between groups in the set-shift portion (Set 2). Based on comparable time per trial and times to complete habituation sessions, differences in motor abilities and motivation between exercisers and non-exercisers do not appear to explain observed differences in the initial discrimination.
Previous research implicates the striatum as a substrate involved in the initial portion of the set-shift task. (Palencia & Ragozzino, 2005) demonstrated the importance of the DLS in the initial discrimination of a response learning task. In their study, prior to the initial acquisition of a T-maze task rats were given an infusion of AP-5, an NMDA antagonist, into the DLS. In their task, rats were trained that a particular response, for example a right turn, would result in a reward. The following test session required reversal learning. Infusion of AP-5 prior to testing resulted in poorer learning of the initial discrimination, but did not affect reversal learning. Preliminary data from our laboratory suggest that the exercise-associated improvement in the initial discrimination (Set 1) relies on the DA receptors in the DLS (Eddy et al., 2011). In this set of experiments, rats had bilateral cannulae implanted in the DLS. Prior to Set 1, rats received an infusion of SCH23390 (D1 antagonist) or eticlopride (D2 antagonist) into DLS. Non-exercising rats receiving the D1 antagonist showed an improvement in discrimination ability, performing at levels similar to exercising rats while there was no effect on exercisers. In contrast, the D2 antagonist did not affect non-exercising rats, but appeared to diminish the exercise-related improvement in performance. We, as well as others (e.g. Fisher et al. (2004) have also shown a decrease in DAT in the striatum following exercise, suggesting that the DLS DA system may be importantly involved in the beneficial effects of exercise on Set 1 of our task.
Exercise has been suggested to increase DA release and up-regulate D2 receptors in the striatum (Dishman et al., 2006; MacRae, Spirduso, Cartee, Farrar, & Wilcox, 1987; Teixeira et al., 2011). Gorton et al. (2010) found that in mice treated with MPTP, a neurotoxin that induces Parkinson’s Disease-like symptoms, 6 weeks of treadmill running produced an increase in D2 receptor protein, as well as D2/D3 ligand binding in the striatum. Foley and Fleshner (2008) also showed that 6 weeks of voluntary wheel running resulted in an increase in D2 receptor mRNA in the caudate-putamen. DAT has also been shown to be down-regulated following exercise, which (in the short term, at least) results in an increase of synaptic DA (Fisher et al., 2004; Petzinger et al., 2007).Fisher et al. (2004) found a reduction in DAT expression in the striatum of mice that exercised for 30 days on a treadmill and were treated with MPTP. Decreases in DAT may slightly bias the DA system towards activation of higher affinity D2 receptors and away from activation of lower affinity D1 receptors (cf. Lovinger (2010). The up-regulation of D2 receptors may be involved in the shifting of dependence from D1 receptors to D2 receptors when well-learned behaviors become less dependent on external reinforcement, or habitual (Canales, 2005). If exercising rats have more D2 receptors, they may shift from D1 to D2 receptor-dependent behavior more quickly, making performing well-learned behaviors more efficient.
Our results also showed that a lack of gonadal hormones eliminated the exercise-associated benefit in discrimination learning. Thus, only rats with circulating gonadal hormones showed an improvement in DLS-dependent discrimination learning, indicating a possible interaction between gonadal hormones and exercise that initiated this enhancement. This possibility is supported by several findings. Erickson et al. (2007) examined the effect of hormone treatment and exercise on postmenopausal women. It was found that hormone treatment in the first ten years following menopause generally ameliorated the normal decline in executive function. They also found that exercise during the ten-year span further increased executive function. Exercise and estrogen appear to act together to improve cognitive function, and while this interaction has been less explored in the rodent literature, there is some suggestion that it may underlie changes following exercise in female rodents.
Several studies have found that OVX resulted in decreased levels of DAT in the striatum (Bosse, Rivest, & Di Paolo, 1997; Chavez et al., 2010; Yu et al., 2009) which can be reversed (at least to some degree) via estrogen treatment. These data, along with studies suggesting an interaction between estrogen and exercise, implicate the striatal DA system as a potential substrate responsible for the effects of exercise seen in intact, cycling females and not OVX females in this experiment. The absence of an enhancement in Set 1 performance in castrated males may be due to similar mechanisms, as male rats without testosterone have been shown to have lower DA concentrations (although this was measured in the PFC) compared to males with circulating hormones (Aubele & Kritzer, 2011). These studies indicate that this is an important avenue of study, as the effects of gonadal steroids like estrogen and testosterone in combination with exercise have been largely unstudied systematically in rodents, and in brain substrates outside of the hippocampus. Future experiments will examine whether replacing gonadal hormones is sufficient to restore exercise-associated benefits in discrimination learning.
These studies yielded several novel data. First, exercising female and male rats learned a reinforced vs. non-reinforced arm discrimination, a DLS-dependent task, in fewer trials than non-exercising rats. This exercise-related enhancement was not present in the DMS/mPFC-dependent extra-dimensional set-shift portion of the task. Second, gonadectomy was sufficient to eliminate the improvement in performance of the DLS-dependent discrimination. The findings presented here suggest that voluntary exercise can improve learning of a DLS-dependent discrimination task, but only when gonadal hormones are present.
Acknowledgements
The authors wish to acknowledge Katherine Stansfield, Samantha Luce, and Jessica Savrann for their help in data collection. Funding for this work was provided by NIMH R01MH082893 and by a University of Vermont Undergraduate Research Award.
References
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiology & Behavior. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Gonadectomy and hormone replacement affects in vivo basal extracellular dopamine levels in the prefrontal cortex but not motor cortex of adult male rats. Cereb Cortex. 2011;21(1):222–232. doi: 10.1093/cercor/bhq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology Biochemistry and Behavior. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167(3):588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14(12):1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Bosse R, Rivest R, Di Paolo T. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Brain Res Mol Brain Res. 1997;46(1–2):343–346. doi: 10.1016/s0169-328x(97)00082-x. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005;83(2):93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–59. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Chess AC, Raymond BE, Gardner-Morse IG, Stefani MR, Green JT. Set shifting in a rodent model of attention-deficit/hyperactivity disorder. Behav Neurosci. 2011;125(3):372–382. doi: 10.1037/a0023571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Eddy M, Savrann J, Rifken K, Green J. Program No. 99. 10. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. The effects of voluntary exercise on discrimination and set-shifting in male Wistar rats. [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28(2):179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10(2):67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- Gorton LM, Vuckovic MG, Vertelkina N, Petzinger GM, Jakowec MW, Wood RI. Exercise effects on motor and affective behavior and catecholamine neurochemistry in the MPTP-lesioned mouse. Behav Brain Res. 2010;213(2):253–262. doi: 10.1016/j.bbr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Satz P, Ganzell S, Vaclav JF. Wisconsin Card Sorting Test performance in schizophrenia: remediation of a stubborn deficit. Am J Psychiatry. 1992;149(1):62–67. doi: 10.1176/ajp.149.1.62. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Jin J, Jing H, Choi G, Oh MS, Ryu JH, Jeong J-W, Park C. Voluntary exercise increases the new cell formation in the hippocampus of ovariectomized mice. Neuroscience Letters. 2008;439:260–263. doi: 10.1016/j.neulet.2008.04.103. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58(7):951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae PG, Spirduso WW, Cartee GD, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neurosci Lett. 1987;79(1–2):138–144. doi: 10.1016/0304-3940(87)90686-0. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, DeVries GJ. Sex differences in the brain: The not so inconvient truth. Journal of Neuroscience. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61(1–2):147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The contribution of NMDA receptors in the dorsolateral striatum to egocentric response learning. Behav Neurosci. 2005;119(4):953–960. doi: 10.1037/0735-7044.119.4.953. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27(20):5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116(1):105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, T. Garland J, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behavioral Neuroscience. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rodier WI., 3rd Progesterone-estrogen interactions in the control of activity-wheel running in the female rat. J Comp Physiol Psychol. 1971;74(3):365–373. doi: 10.1037/h0030568. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Distinct contributions of glutamate receptor subtypes to cognitive set-shifting abilities in the rat. Ann N Y Acad Sci. 2003;1003:464–467. doi: 10.1196/annals.1300.064. [DOI] [PubMed] [Google Scholar]
- Teixeira A, Muller LG, Reckziegel P, Boufleur N, Pase CS, Villarinho JG, Burger ME. Beneficial effects of an innovative exercise model on motor and oxidative disorders induced by haloperidol in rats. Neuropharmacology. 2011;60(2–3):432–438. doi: 10.1016/j.neuropharm.2010.10.017. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yu PL, Wu CI, Lee TS, Pan WH, Wang PS, Wang SW. Attenuation of estradiol on the reduction of striatal dopamine by amphetamine in ovariectomized rats. J Cell Biochem. 2009;108(6):1318–1324. doi: 10.1002/jcb.22361. [DOI] [PubMed] [Google Scholar]