Abstract
Washing cord blood (CB) grafts involves product manipulation and may result in cell loss. We investigated double-unit CB transplantation (CBT) using red blood cell (RBC) depleted units diluted with albumin-dextran in patients with hematologic malignancies. One-hundred thirty-six patients [median 43 years (range 4–71) and 69 kilograms (kg) (range 24–111)] were transplanted with a 4–6/6 HLA-matched graft. Patients ≤ 20 kg were excluded as they only received washed units. Units were diluted a median of 8 fold to a median volume of 200 ml/unit. The median infused TNC doses were 2.7 (larger unit) and 2.0 (smaller unit) × 107/kg respectively, and the median post-thaw recovery was 86%. Units were infused consecutively (median 45 minutes/unit). While only 17 patients (13%) had no infusion reactions, reactions in the remaining 119 patients were almost exclusively mild-moderate (by CTCAE v4 criteria 12 grade 1, 43 grade 2, 63 grade 3) and only 1 patient (< 1%) had a severe (grade 4) reaction. Moreover, most were easily treated. Grade 2–3 hypertension was the most common in 101 (74%) patients. The cumulative incidence of sustained donor-derived neutrophil engraftment was high: 95% in myeloablative and 94% in non-myeloablative CBT recipients. With appropriate supportive care, double-unit CBT with RBC-depleted grafts infused after albumin-dextran dilution is safe with high rates of engraftment in patients > 20 kg.
Introduction
Cord blood (CB) is routinely used as an alternative hematopoietic stem cell source. Traditionally, CB is thawed using albumin-dextran dilution followed by centrifugation and re-suspension i.e. “wash”1. However, the wash of CB grafts involves additional product manipulation, risks potential cell loss and potential contamination, requires increased technologist time, and delays time to infusion2. A faster and easier alternative is albumin-dextran dilution without centrifugation3,4. This approach is controversial, however, as the infusion of non-washed CB has been associated with severe or lethal infusion reactions at some centers [2009 United States National Marrow Donor Program (NMDP) communication and discussed in5]. The majority of non-washed units associated with such serious infusion reactions, however, have been red blood cell (RBC) replete, and the risk of severe infusion reactions appears to be greatly increased if the units are infused as a bedside thaw. This has led the NMDP to recommend that bedside thaw of RBC replete units be avoided.
We have previously demonstrated in 54 double-unit CBT recipients weighing > 20 kilogram (kg) that the use of albumin-dextran dilution without centrifugation is feasible3. However, in that report, 3 patients who received one of 2 units that were RBC-replete and infused after dilution had significant (although non-lethal) infusion reactions. Therefore, since 2009 only RBC depleted units are considered for the “no wash” approach at Memorial Sloan-Kettering Cancer Center (MSKCC). We now report the safety (infusion reactions) and efficacy (engraftment) of this approach in 136 patients transplanted with RBC-depleted double-unit CB grafts thawed with albumin-dextran dilution with a focus on the nature, severity, and management of infusion reactions.
Materials and Methods
Patients and CB Grafts
This is a retrospective analysis of 136 consecutive double-unit CBT recipients transplanted between February 2006 and November 2012 at MSKCC for the treatment of hematological malignancies who fulfilled eligibility criteria. Eligible patients were at least 20 kilograms in weight and received two RBC depleted CB units as their first allograft for the treatment of acute leukemia in morphologic remission or aplasia, myelodysplasia or lymphoma. Double-unit grafts were used to augment engraftment6–8 and potentially protect against relapse9–13. Twenty kilograms was set as the lower limit of recipient weight for administration of non-washed CB given the higher risk of dimethyl sulfoxide (DMSO) toxicity in smaller patients14. Furthermore, small pediatric patients may not tolerate large infusion volumes, and the potential cell loss with a wash has little impact on the infused total nucleated cell (TNC) dose in this group. During the time period of the study, 20 patients received double-unit CBT but were excluded from this analysis. In 14 small children both units were washed, in 4 patients transplanted early in the study period one of the units of the graft was RBC replete but was not washed, and in the remaining 2 patients one unit of the graft was washed due to being RBC replete.
The 136 recipients of grafts prepared with albumin-dextran dilution received myeloablative or non-myeloablative conditioning according to patient age, extent of prior therapy, co-morbidities, and diagnosis. All patients received a calcineurin-inhibitor and mycophenolate mofetil immunosuppression and post-transplant granulocyte colony stimulating factor. Units were selected according to their TNC dose, human leukocyte antigen (HLA)-A, -B antigen, -DRB1 allele match, and bank of origin15. All patients or their guardians signed informed consent prior to transplantation. Fifty of the 136 patients in this study have previously been reported in 20093.
Preparation of CB Units for Infusion
Units were stored at ≤ −180°C upon arrival at MSKCC. Each unit was thawed separately as previously described3. In brief, units were thawed in a 37°C waterbath and transferred to a bio-safety cabinet. A stock diluent solution with a 5:1 ratio of 10% dextran 40 (molecular weight 40,000, Hospira, IL, USA) and 25% human serum albumin (CSL Behring, IL, USA) was prepared at room temperature and aliquoted into 300 or 600 ml transfer packs for units in single compartment bags or MedSep double compartment units, respectively. All units were diluted ≥ 6 fold with the standard dilution being 8-fold for a final volume of at least 200 ml/unit. Diluent approximately equal to the cryopreserved volume of the unit was slowly transferred to the cryo-bag. Once a 1:1 dilution was achieved the tubing was clamped. After thorough mixing the product was allowed to equilibrate for 1–2 minutes. It was then drained back into the transfer pack or cell wash infusion bag to achieve the final dilution. Units frozen in 2 bags were combined after albumin reconstitution before samples were taken.
CB Unit Assessment
A small aliquot was removed from the albumin-dextran diluted units for RBC count, TNC count, ABO blood group, culture, and assays of unit quality. Unit quality was evaluated by flow cytometric assay of CD34+ cell viability using 7-amino-actinomycin D16, and colony-forming unit (CFU) assays. CFU assays were performed using a total of 1 × 105 cells plated in duplicate and colony growth was evaluated by light microscopy at 14 days. The RBC volume (ml/kg) was calculated as: MCV (110 for CB) × post-thaw RBC content (per L) × final product volume (ml)/kg of patient weight.
Management of CB Infusion and Infusion Reactions
All patients received pre-medications, mostly with oral acetaminophen, intravenous diphenhydramine (or hydroxyzine), and intravenous lorazepam. In addition, patients received hydration approximately twice maintenance 4–6 hours pre-transplant and 12–24 hours post-transplant. Experienced transplant nurses or physicians were present during infusion of both units and patients were closely monitored. Vital signs were monitored before the start of the infusion of each unit, every 15 minutes during infusion, at the completion of infusion, and then every 15 minutes × 2, every 30 minutes × 2, and every 60 minutes × 4, and subsequently every 4 hours. Each unit was administered to completion within 4 hours of thaw. Emergency medications including intravenous lorazepam, hydralazine, furosemide, hydrocortisone and oxygen were at the bedside for immediate administration if needed.
Definitions
Infusion reactions were graded according to the Common Terminology Criteria for Adverse Events version 4 (CTCAE v4.0). A serious infusion reaction was defined as any life-threatening (grade 4) or lethal (grade 5) event related to CB infusion such as anaphylaxis, acute renal failure, seizure, transfer to intensive care unit, or death within 48 hours of CB infusion. Grade 1 hypertension was systolic blood pressure (BP) 120–139 mm Hg or diastolic 80–89 mm Hg, grade 2 was systolic 140–159 mm Hg or diastolic 90–99 mm Hg or symptomatic increase by > 20 mm Hg, grade 3 was systolic ≥ 160 mm Hg or diastolic ≥ 100 mm Hg, and grade 4 was life-threatening consequences (e.g. neurologic deficit or hypertensive crisis). Grade 1 nausea/vomiting was nausea requiring intra-venous medications or 1–2 episodes of vomiting separated by 5 minutes in 24 hours. Bradycardia (heart rate < 60 beats/minute) or tachycardia (> 100 beats/minute) had grade 1 severity if asymptomatic and intervention was not indicated whereas grade 2 was symptomatic and medical intervention was required. Grade 2 hypoxia was decreased oxygen saturation < 88% or intermittent supplemental oxygen. In addition, for the purposes of this analysis, we defined renal insufficiency as a 1.5 fold increase in serum creatinine over the day zero baseline and greater than the upper limit of normal for age.
As neonatal blood does not contain isohemaglutinins17, only major ABO incompatibilities between patients and CB units were considered for possible association with infusion reactions. The following donor/recipient pairings defined major ABO incompatibility: “AB” CB unit infused into a “A”, “B” or “O” recipient; “A” CB unit infused into a “B” or “O” recipient; and a “B” CB unit infused into a “A” or “O” recipient.
Assessment of Donor Engraftment
Time to neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count ≥ 0.5 × 109/l. Time to platelet recovery was defined as the first of 3 consecutive days at ≥ 20 × 109/l and at least 7 days without platelet transfusion support. Sustained engraftment was defined as sustained donor-derived neutrophil recovery with donor chimerism of at least 90% (both units combined). The probabilities of neutrophil and platelet engraftment were computed using the cumulative incidence function with early death being the competing risk.
Results
Patients and CB Grafts
Patient characteristics are summarized in Table 1. The 136 consecutive patients (median age 43 years and weight 69 kg) were transplanted for high or standard-risk hematological malignancies. Eighteen patients were children (aged 4–16 years). Conditioning was myeloablative (n = 102) or non-myeloablative (n = 34) conditioning. The 272 units were obtained from domestic (n = 198, 73%) or international (n = 74, 27%) banks. The units were 6/6 (n = 14, 5%), 5/6 (n = 134, 49%), and 4/6 (n = 124, 46%) HLA-matched to the patient.
Table 1.
Patient characteristics (n = 136)
| Patient Characteristic | Value |
|---|---|
|
| |
| Median (range) age (years) | 43 (4–71) |
|
| |
| Median (range) weight (kg) | 69 (24–111) |
|
| |
| N (%) diagnosis | |
| Acute leukemia | 73 (54%) |
| MDS/Myeloproliferative disease | 9 (7%) |
| NHL/HD/CLL | 54 (40%) |
|
| |
| N (%) disease risk | |
| Standard | 18 (13%) |
| High | 118 (87%) |
|
| |
| Conditioning intensity | |
| N (%) high dose | |
| Chemo + TBI 1320–1375 cGy | 38 (28%) |
| Clo100–150/Mel140/Thio10 | 7 (5%) |
| N (%) reduced intensity | |
| Flu150/Cy50/Thio10/TBI 400 cGy | 48 (35%) |
| Mel140/Flu150 | 8 (6%) |
| Flu/TBI/Decitabine/6Thioguanine | 1 (1%) |
| N (%) non-myeloablative | |
| Flu150/Cy50/TBI200 cGy | 34 (25%) |
N indicates number; kg, kilogram; MDS, myelodysplasia; NHL, Non-Hodgkin lymphoma; HD, Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; Chemo, chemotherapy; TBI, total body irradiation; Clo, clofarabine; Mel, melphalan; Thio, thiotepa; Flu, fludarabine; Cy, cyclophosphamide.
CB units were diluted to a median of 8 fold (Table 2). All patients received diluted grafts except for one who developed a CTCAEv4.0 grade IV anaphylaxis at initiation of infusion of the first unit. This patient’s infusion was aborted, and the graft was washed. The median infused TNC doses × 107/kg of the remaining 135 diluted CB grafts were 2.7 (larger unit) and 2.0 (smaller unit), respectively, with a median TNC recovery of 86%.
Table 2.
Characteristics of 135 CB grafts after albumin-dextran reconstitution (n = 270 units)*
| Unit Characteristics | Value |
|---|---|
|
| |
| Median (range) volume (ml) | |
| Pre-thaw | 25 (21–80) |
| Post-thaw | 200 (200–600) |
| Fold dilution | 8 (6–12) |
|
| |
| Median (range) TNC/kg × 107/kg | |
| Pre-thaw (n = 270) | 2.7 (1.1–7.5) |
| Post-thaw (n = 270) | 2.3 (0.9–6.9) |
|
| |
| Percent (range) TNC recovery | 86% (50–125) |
|
| |
| Median (range) inf. TNC × 107/kg | |
| Larger unit (n = 135) | 2.7 (1.4–6.9) |
| Smaller unit (n = 135) | 2.0 (0.9–4.5) |
|
| |
| Median (range) inf. CD34+ cell × 105/kg | |
| Larger unit (n = 135) | 1.3 (0.3–4.5) |
| Smaller unit (n = 135) | 0.7 (0.1–2.1) |
|
| |
| Median inf. CFU × 104/kg (range) | |
| Larger unit (n = 135) | 3.7 (0.4–18.4) |
| Smaller unit (n = 135) | 1.8 (0.01–7.2) |
|
| |
| Median (range) CD34+ cell viability** | 93% (34–99) |
One graft is excluded as it was washed in the patient with a grade 4 reaction.
Reflects post-thaw measurement.
N indicates number; TNC, total nucleated cell; kg, kilogram; inf, infused; CFU, colony-forming unit.
Administration of Diluted CB Products
Each unit was administered within approximately 60–90 minutes of dilution. The median time between pre-medication and initiation of the first unit was 48 minutes (range 5–100 minutes). CB units were infused consecutively shortly after thaw. The median interval between infusion of the first and second unit was 15 minutes (range 3–133 minutes). The median time to infuse each unit was 45 minutes (range 15–285 minutes), and the median time to infuse the entire diluted graft was 115 minutes (range 42–438).
Infusion Reactions
The frequency, nature, and severity of infusion reactions by CTCAE v4.0 criteria are shown in Tables 3A and 3B. There were no grade 5 (lethal) infusion reactions. While only 17 patients (13%) had no reactions, most reactions were mild-moderate and easily treated. By CTCAE v4.0 criteria, 12 of a total of 136 (9%) patients had grade 1 reactions, 43 (32%) grade 2, 63 (46%) grade 3, and only 1 (<1%) patient had a grade 4 reaction.
Table 3A.
Infusion reactions by type and CTCAE v4 grade (157 reactions in 119 patients).
| Grade | HTN | N ± V | Cardiopulmonary* | Allergic** | Other*** | Total |
|---|---|---|---|---|---|---|
| 1 | 8 | 22 | 11 | - | 3 | 44 |
| 2 | 38 | - | 8 | - | 2 | 48 |
| 3 | 63 | - | - | 1 | - | 64 |
| 4 | - | - | - | 1 | - | 1 |
| 5 | - | - | - | - | - | - |
| Total | 109 | 22 | 19 | 2 | 5 | 157 |
Cardio-pulmonary reactions included: bradycardia, tachycardia, chest pain, dyspnea, wheezing, hypoxia, or cough.
Allergic reactions are angioedema or anaphylaxis.
Other reactions include fever, chills, shoulder or abdominal pain.
HTN indicates hypertension; N ± V, nausea ± vomiting.
The most common infusion reaction was hypertension. Eight patients had grade 1, 38 had grade 2, and 63 had grade 3 hypertension (systolic ≥ 160 mm Hg or diastolic ≥ 100 mm Hg). Thirty patients did not require treatment due to prompt spontaneous resolution of hypertension whereas 79 patients (58% of patients overall) were treated with hydralazine, furosemide, or both. For the patients with the most severe hypertension (grade 3), the onset was during the first unit in 37 patients, and during the second unit in 26. In this grade 3 group, the median peak systolic BP overall was 161 mm Hg (range 132–213) and the median peak diastolic was 101 mm Hg (range 83–140).
The second most common reaction was nausea and/or vomiting which affected 22 (14%) patients; all were of grade 1 severity and were managed by anti-emetics (usually lorazepam). The next most common reaction was cardio-pulmonary symptoms which occurred in 19 patients. These included bradycardia, tachycardia, dyspnea, wheezing, hypoxia, cough, or chest pain/tightness. Six patients had bradycardia ranging from 42–56 beats per minute. All were asymptomatic and resolved without intervention. Two patients had tachycardia at 119 and 124 beats/minute; both resolved without intervention. Six patients had transient hypoxia with a minimum oxygen saturation ranging from 88–93%. All were managed with oxygen by nasal cannulae, and 3 patients received nebulized bronchodilator. Overall, cardio-pulmonary symptoms developed during the first unit in 7 patients and with the second unit in 12.
Severe reactions were rare. One patient had grade 3 angioedema along with grade 3 hypertension, grade 1 bradycardia (lowest heart rate 53), and grade 1 nausea. The angioedema involved hoarse voice, facial and lip swelling and developed approximately 10 minutes into the first unit. Symptoms were managed with corticosteroids, diphenhydramine, oxygen via nasal cannula, and slowing the infusion. A second patient developed a grade 4 anaphylaxis-type reaction with severe bronchospasm, hypoxia (oxygen saturation 89%) approximately 10 mls into the first unit, and grade 1 hypertension (132/82 mmHg). This unit was ABO compatible with the patient. The infusion was aborted while corticosteroids, epinephrine, diphenhydramine, inhaled levalbuterol, oxygen via nasal cannula, and lorazepam were administered. The patient was transferred to the intensive care unit for observation and a washed graft was administered uneventfully. The patient did not require pressors or intubation, had complete recovery within < 24 hours, and engrafted successfully. This patient later had similar reactions to platelets. Other infrequent reactions included fever (observed), chills (treated with meperidine), and shoulder or abdominal pain (treated with slowing of the infusion and/or narcotics).
Seventy-six grafts (55%) administered after dilution were ABO incompatible with the recipients (26 patients received grafts in which both units had major ABO incompatibility and 50 received grafts in which one of the 2 units had major incompatibility). However, there was no relationship between major ABO incompatibility and incidence of grade 1–4 infusion reactions. Overall, in 70/76 (92%) recipients of grafts with major ABO incompatibility there were a total of 97 reactions (including 67 HTN, 13 cardio-pulmonary and 1 allergic). This compared to 60 reactions in a total of 49/60 (82%) recipients of ABO compatible grafts (including 42 HTN, 6 cardiopulmonary and 1 allergic). Furthermore, after albumin-dextran reconstitution, the median dose of RBC/unit was 0.08 ml/kg (range 0.003–0.56). In the 16 pediatric patients with weight 20–40 kg the median dose of RBC/kg was 0.16 ml/kg (range 0.08–0.5). No patient had macroscopic hematuria or renal impairment.
Sustained Donor Engraftment
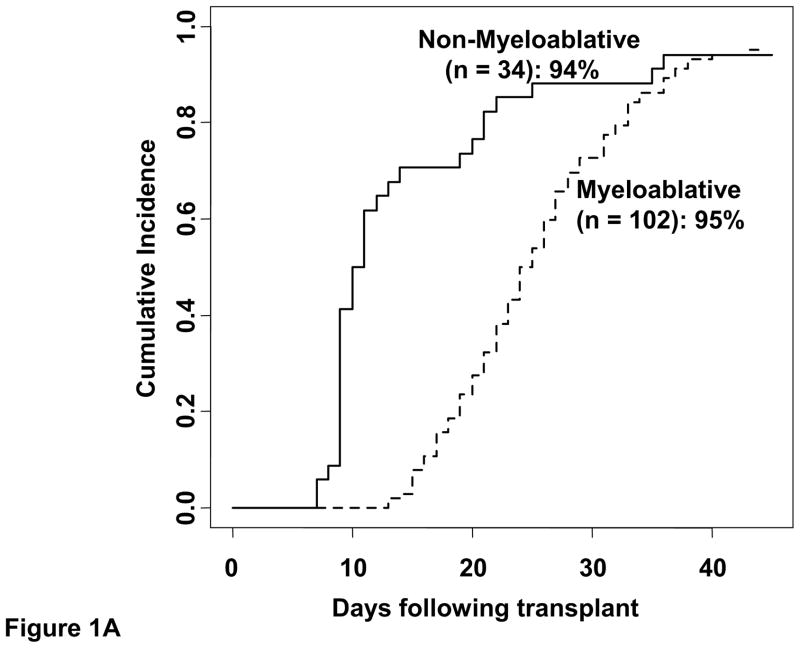
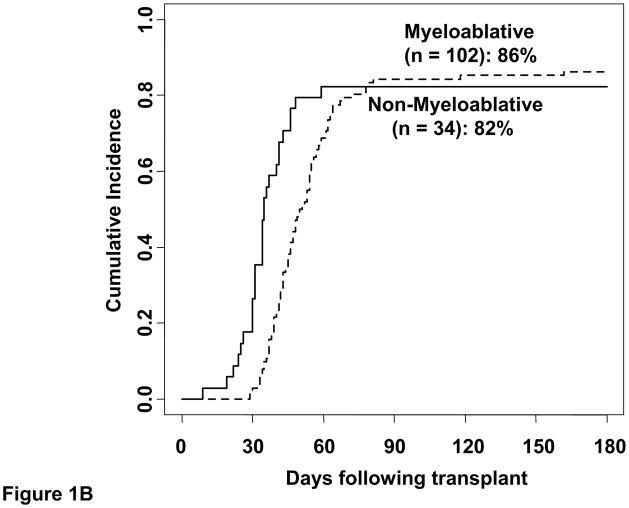
Five of the 136 patients (3 recipients of myeloablative and 2 of non-myeloablative conditioning) had graft failure. The cumulative incidence of sustained donor-derived neutrophil engraftment by day 45 was 95% (95%CI:88–98) in myeloablative CBT recipients (median recovery 24 days, range 13–43) and 94% (95%CI:74–99) in non-myeloablative CBT recipients (median 10 days, range 7–36) (Figure 1A). The cumulative incidence of day 180 platelet recovery was 86% (95%CI:77–92) (median 47 days, range 29–162) and 82% (95%CI:64–92) (median 34 days, range 9–59) after myeloablative and non-myeloablative conditioning, respectively (Figure 1B).
Figure 1. The cumulative incidence of sustained donor neutrophil engraftment (1A) and platelet engraftment (1B) after double-unit CBT performed with albumin-dextran-dilution according to conditioning regimen intensity.
For non-myeloablative recipients, while early count recovery may have been autologous, only those with sustained donor-derived count recovery are counted as having engrafted.
Discussion
Preparation of CB units for infusion by thaw of CB followed by washing and removal of the supernatant was originally developed for children and has been used for larger children and adults by many centers as a matter of tradition. However, given that autologous products with higher DMSO doses are routinely administered without wash, it stands to reason that infusion of unwashed CB should be investigated. In CBT such an approach is even more desirable as avoidance of centrifugation results in considerably less product manipulation and could translate to lower cell loss. Further additional benefits of a no wash approach are less technologist time, lower contamination rate, and a shorter time between thaw and infusion. Such advantages can be achieved with albumin-dextran dilution of the graft. With such an approach thaw and dilution occurs in the safety of the laboratory. Additionally, given the graft is diluted, infusion can be slowed or even temporarily held in the event of a reaction without risk of significant viability compromise. This is in contrast to a bedside thaw in which infusion of a concentrated product has to be completed within a short time interval.
Our analysis in 136 patients demonstrates that with close medical staff supervision, adequate hydration, appropriate pre-medication, and prompt management of infusion reactions, a tolerable infusion reaction profile in double-unit CBT recipients transplanted with RBC depleted units can be achieved. Mild and moderate reactions were common but easily treated. There were two severe reactions, one grade III angio-edema and one grade IV anaphylaxis. From a practical standpoint it is important to note that both of these reactions occurred within the first 10 minutes of the infusion. This mandates medical staff be at the bedside during this time as is the practice with autologous hematopoietic stem cell products or platelets, and that emergency medications and oxygen be available near the bedside for immediate administration as needed. Moreover, the patient with severe bronchospasm subsequently had similar reactions to platelets. Therefore, it is reasonable to recommend that CBT with dilution not be performed for any patient with a history of severe reactions to blood products, or at least not without aggressive pre-medications and intense monitoring. In addition, as hypertension is the most common infusion reaction, elevated blood pressure existing prior to CB infusion should be treated and controlled before transplant is initiated, and given fluid overload could contribute to hypertension the fluid balance should be maintained with intra-venous furosemide as needed.
In addition to the tolerable infusion reaction profile, a high rate of sustained donor neutrophil and platelet engraftment was observed in this study. It is tempting to hypothesize that this could be related, at least in part, to reduced manipulation resulting in improved product quality. While infusion reactions are common, the high rates of engraftment combined with the fact that most reactions are easily managed justifies this approach. For these reasons, we now exclusively use albumin-dextran dilution for thaw and infusion of RBC depleted CB units in recipients > 20 kg. In the decision to adopt an albumin-dextran diluted thaw, it is important that transplant centers ensure units are RBC depleted. Additionally, our results are likely dependent on adequate dilution, slow infusion, and especially on prompt infusion reaction management. It is likely that a lower incidence of reactions could be achieved with intensifying the pre-medications such as increasing the dose of diphenhydramine and/or adding intravenous corticosteroids such as hydrocortisone and this is now being investigated at MSKCC since the infusion reactions in this report have been analyzed.
We do not have any experience with this approach in patients under 20 kg. Moreover, dilution CBT without wash is not recommended for transplantation of RBC-replete units, or for patients with prior severe reactions to blood products. As life threatening or lethal infusion reactions have been reported with units that contain high numbers of RBC, transplant centers need to be vigilant of the processing methodology at the time of unit selection. We recommend that if RBC containing units are selected, such units are washed and the transplant center develops a standard operating procedure for the centrifugation and reconstitution of these products to ensure adequate cell recovery. However, the RBC dose in diluted RBC depleted products appears to be acceptable. Despite the lack of wash the dose of RBC in this study was well below the dose of 1.5 ml/kg that has been associated with reactions with autologous bone marrow infusions18.
Finally, it is our recommendation that in the setting of double-unit CBT, the second unit should not be thawed until the patient is confirmed to be stable during the infusion of the first unit. At our center, as any serious reactions have occurred during the first 10 minutes of infusion, the medical staff now communicates with the laboratory approximately 15 minutes into the infusion of the first unit to confirm the appropriateness of commencing the thaw of the second unit. These procedural interventions permit infusion of diluted but unwashed products with a high degree of safety.
Table 3B.
Details of infusion reactions (grade 1–4) listed by maximal grade*.
| Infusion Reactions by Maximum Grade (CTCAE v4 criteria) | N |
|---|---|
|
| |
| Grade 1 (n = 12 of total 136, 9%) | |
|
| |
| HTN | 4 |
|
| |
| HTN, N ± V | 1 |
| HTN, bradycardia, N ± V | 1 |
|
| |
| Bradycardia | 1 |
| Bradycardia, N ± V, abdominal pain | 1 |
| N ± V | 3 |
| Coughing, shoulder pain | 1 |
|
| |
| Grade 2 (n = 43 of total 136, 32%) | |
|
| |
| HTN | 32 |
|
| |
| HTN + grade 1 bradycardia | 1 |
| HTN + grade 1 N ± V | 5 |
|
| |
| Wheezing | 2 |
| Hypoxia + grade 1 N ± V | 2 |
| Hypoxia + grade 1 HTN | 1 |
|
| |
| Grade 3 (n = 63 of total 136, 46%) | |
|
| |
| HTN | 46 |
|
| |
| HTN + grade 1 N ± V | 6 |
| HTN + grade 1 N ± V, chest tightness, dyspnea**, tachycardia | 1 |
| HTN + grade 1 N ± V, chest pain | 1 |
| HTN + grade 2 hypoxia | 2 |
| HTN + grade 1 dyspnea** | 1 |
| HTN + grade 1 tachycardia | 1 |
| HTN + grade 2 chills, grade 1 chest pain, | 1 |
| HTN + grade 2 hypoxia, grade 1 bradycardia, chest pain | 1 |
| HTN + grade 3 angioedema, grade 1 bradycardia, N ± V | 1 |
| HTN + grade 2 chills, grade 1 fever | 1 |
| HTN + grade 1 chills | 1 |
|
| |
| Grade 4 (n = 1 of total 136, <1%) | |
|
| |
| Anaphylaxis + grade 1 HTN | 1 |
N ± V indicates nausea ± vomiting; HTN, hypertension; sat, saturation.
Of 136 patients, 119 had grade 1–4 reactions whereas 17 patients had no reactions.
Normal oxygen saturation.
Acknowledgments
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research, the Memorial Sloan-Kettering Cancer Center Society, the Translational and Integrative Medicine Research Grant, and P01 CA23766 from the National Cancer Institute, National Institutes of Health.
Footnotes
Author Contributions
P.B.D. interpreted the data and wrote the manuscript. D.P. wrote the manuscript, S.D analyzed the statistics and wrote the manuscript, K.E., M.L and A.M.G. collected the data, J.T., R.M., S.G, N.A.K and A.S wrote the manuscript. J.N.B. designed the study, interpreted the data, and wrote the manuscript.
Disclosure of Conflicts of Interest
The authors have no relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laroche V, McKenna DH, Moroff G, Schierman T, Kadidlo D, McCullough J. Cell loss and recovery in umbilical cord blood processing: a comparison of postthaw and postwash samples. Transfusion. 2005;45:1909–1916. doi: 10.1111/j.1537-2995.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 3.Barker JN, Abboud M, Rice RD, et al. A “no-wash” albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15:1596–1602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regan DM, Wofford JD, Wall DA. Comparison of cord blood thawing methods on cell recovery, potency, and infusion. Transfusion. 2010;50:2670–2675. doi: 10.1111/j.1537-2995.2010.02803.x. [DOI] [PubMed] [Google Scholar]
- 5.Barker JN, Scaradavou A. Response: the controversy of red cell replete cord blood units. Blood. 2011;118:480. [Google Scholar]
- 6.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 7.Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117:3277–3285. doi: 10.1182/blood-2010-08-300491. quiz 3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 11.Brunstein C, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after non-myeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47:924–933. doi: 10.1038/bmt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labopin M, Ruggeri A, Gorin NC, et al. Cost-effectiveness and clinical outcomes of double versus single cord bloodtransplantation in adults with acute leukemia in France. Haematologica. 2013 doi: 10.3324/haematol.2013.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perseghin P, Balduzzi A, Bonanomi S, et al. Infusion-related side-effects in children undergoing autologous hematopoietic stem cell transplantation for acute leukemia. Bone Marrow Transplant. 2000;26:116–118. doi: 10.1038/sj.bmt.1702462. [DOI] [PubMed] [Google Scholar]
- 15.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117:2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scaradavou A, Smith KM, Hawke R, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16:500–508. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snell M, Chau C, Hendrix D, et al. Lack of isohemagglutinin production following minor ABO incompatible unrelated HLA mismatched umbilical cord blood transplantation. Bone Marrow Transplant. 2006;38:135–140. doi: 10.1038/sj.bmt.1705409. [DOI] [PubMed] [Google Scholar]
- 18.Alessandrino P, Bernasconi P, Caldera D, et al. Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant. 1999;23:533–537. doi: 10.1038/sj.bmt.1701609. [DOI] [PubMed] [Google Scholar]