Abstract
Background
Recovery from a massive burn is characterized by catabolic and hypermetabolic responses that persist up to 2 years and impair rehabilitation and reintegration. The objective of this study was to determine the effects of long-term treatment with recombinant human growth hormone (rhGH) on growth, hypermetabolism, body composition, bone metabolism, cardiac work, and scarring in a large prospective randomized single-center controlled clinical trial in pediatric patients with massive burns.
Patients and Methods
A total of 205 pediatric patients with massive burns over 40% total body surface area were prospectively enrolled between 1998 and 2007 (clinicaltrials.gov ID NCT00675714). Patients were randomized to receive either placebo (n = 94) or long-term rhGH at 0.05, 0.1, or 0.2 mg/kg/d (n = 101). Changes in weight, body composition, bone metabolism, cardiac output, resting energy expenditure, hormones, and scar development were measured at patient discharge and at 6, 9, 12, 18, and 24 months postburn. Statistical analysis used Tukey t test or ANOVA followed by Bonferroni correction. Significance was accepted at P < 0.05.
Results
RhGH administration markedly improved growth and lean body mass, whereas hypermetabolism was significantly attenuated. Serum growth hormone, insulin-like growth factor-I, and IGFBP-3 was significantly increased, whereas percent body fat content significantly decreased when compared with placebo, P < 0.05. A subset analysis revealed most lean body mass gain in the 0.2 mg/kg group, P < 0.05. Bone mineral content showed an unexpected decrease in the 0.2 mg/kg group, along with a decrease in PTH and increase in osteocalcin levels, P < 0.05.
Resting energy expenditure improved with rhGH administration, most markedly in the 0.1 mg/kg/d rhGH group, P < 0.05. Cardiac output was decreased at 12 and 18 months postburn in the rhGH group. Long-term administration of 0.1 and 0.2 mg/kg/d rhGH significantly improved scarring at 12 months postburn, P < 0.05.
Conclusion
This large prospective clinical trial showed that long-term treatment with rhGH effectively enhances recovery of severely burned pediatric patients.
Introduction
A severe burn is a disastrous injury which results in a hypermetabolic and catabolic state characterized by increases in resting energy expenditure, tachycardia, insulin resistance, a negative muscle protein balance, decrease in bone mass, and growth delay.1,2 Improvements in acute burn care, such as early fluid resuscitation, early burn wound excision and closure, antibiotics, and enteral feeding have significantly decreased mortality after a severe burn in children, thus increasing the number of children entering convalescence after burn.1 The catabolic and hypermetabolic response persists for up to 2 years after burn, which results in a significant delay in recovery and reintegration of pediatric burn victims back into the society.3 Various treatment options have been investigated to attenuate hypermetabolism and catabolism during acute hospitalization as well as after hospital discharge. Recombinant human growth hormone (rhGH) has been proven to be beneficial during the acute phase and during extended treatment after discharge. Growth hormone administration in the acute phase after injury improved protein synthesis, wound healing, and growth.4–8 We have previously shown that low-dose rhGH (0.05 mg/kg/d) therapy from discharge up to 12 months after burn improved height during and after the treatment; however, beneficial effects on lean body mass were only observed during treatment and not after cessation of therapy.9 Furthermore, hypermetabolism, as reflected by an elevated resting energy expenditure and increased cardiac output, was not affected with low-dose rhGH administration.9
In this study, we are summarizing our experience with long term rhGH treatment between 1998 and 2009. During these 12 years, we have treated severely burned children with 3 different doses of rhGH—0.05, 0.1, and 0.2 mg/kg/d—from hospital discharge to 12 months after burn and followed them for additional 12 months to assess continuing therapeutic effects as well as side effects. Results were compared with pediatric burn patients who were randomly assigned to placebo treatment.
Patients and Methods
Study Population
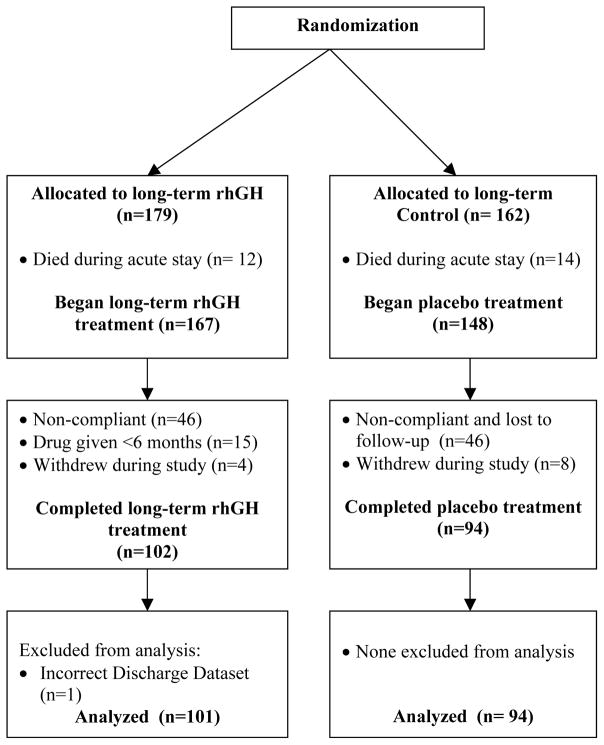
Severely burned children were enrolled at a single institution between 1998 and 2007 using a randomization schedule to test the efficacy of 3 different doses of rhGH, administered after hospital discharge to 12 months after burn in a dose-finding design (Fig. 1). Burn patients were studied for an additional 12 months after treatment was discontinued. Patients were enrolled in a double-blinded, randomized fashion. Inclusion criteria were: age ≤ 19 years, total body surface area burns of over 40%, and absence of cardiovascular disease. The study was approved by the Institutional Review Board at the University of Texas Medical Branch. Informed written consent was obtained from each patient’s guardian with the assent of the child prior to enrollment. Patients received 0.05 mg/kg, 0.1 mg/kg or 0.2 mg/kg rhGH (Lilly, Indianapolis, IN) or placebo subcutaneously daily from hospital discharge to 1 year after burn. Four patients in the 0.2 mg/kg rhGH group received the drug up to 24 months post burn; we did not include these patients in the 18- and 24-month follow-up measurements (see Fig. 1). Guardians and patients were instructed and supervised in the proper use of the drug and compliance was checked by questionnaires and interviews with study nurses and physicians during the follow-up appointments.10 Dietary needs for the entire time of the study were calculated as between 1.2 and 1.4 times the resting energy expenditure11 and were adjusted as needed during follow-up visits. Patients and their parents were monitored and counseled by dietary nurses and required to keep a nutrition log. Patients were studied at discharge (3–12 weeks after burn), and 6, 12, 18, and 24 months after injury. At the time of hospital admission and follow-up, patients were examined by physicians including a pediatric endocrinologist and reviewed by a safety committee to screen for compliance and adverse side effects such as hypercalcemia, glucose intolerance, acromegaly, pseudotumor cerebri, and gynecomastia.
Figure 1.
Randomization chart.
Study Flowchart
Weights and Heights
Height and weight measurements were obtained at discharge and all follow-up visits. Body weight was measured using a standard calibrated scale. Height was measured with a standardized scale and height percentiles were determined by growth charts obtained from the National Center for Health Statistics.12 Weight measurements are expressed as percent change to patient discharge values. Height percentiles are expressed as means at discharge and follow-up time points.
Body Composition
Lean body mass (LBM), bone mineral content (BMC), and percent total body fat content were measured by dual-energy x-ray absorptiometry. A Hologic model QDR-4500A absorptiometer (Hologic Inc., Waltham, MA) was used for these studies. To minimize systematic deviations, the system was calibrated daily against a spinal phantom in the anteroposterior, lateral, and single beam modes. Individual pixels were calibrated against a tissue bar phantom to determine whether the pixel was reading bone, fat, lean tissue, or air. All studies were performed after feedings and intravenous fluids were discontinued. Results are expressed as percent change to patient discharge values.
Cardiac Function
Cardiac function measurements included heart rate, stroke volume, and cardiac output (CO). All ultrasound measurements were made with the HP SONOS 100 CF echocardiogram (Hewlett Packard Imaging System, Andover, MA,) with a 3.5 MHz transducer. Recordings were performed with the subjects in a supine position and breathing freely. M-mode tracings were obtained at the level of the tips of the mitral leaflets in the parasternal long axis position and measurements were performed according to the American Society of Echocardiography recommendations.13 Left ventricular end-systolic and end-diastolic volumes were used to calculate stroke volume and CO. Three measurements were performed and averaged for data analysis. Results are expressed as means at discharge and follow-up time points.
Indirect Calorimetry
Resting energy expenditure (REE) was measured using a Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA). Composition of inspired and expired gases were sampled and analyzed at 60-second intervals. Values obtained during a 5-minute steady state were accepted. The average REE was calculated from steady state measurements. For statistical comparisons, energy expenditure was expressed as REE percent predicted, a quotient of REE and the basal metabolic rate, predicted by the Harris-Benedict equation.14,15 Values are expressed as percent change to discharge values.
Hormone Panel
Serum growth hormone (GH), insulin-like growth factor-I (IGF-1), parathormone (PTH), and osteocalcin were measured using enzyme linked immunosorbent assays from Diagnostic Systems Laboratory (Webster, TX).16 Results are expressed as means at discharge and follow-up time points.
Serum Proteins, Calcium, and Glucose
Total calcium, glucose, cholesterol, triglycerides, and total protein were analyzed using the VITROS colorimetric system, according to the protocols provided by the manufacturer (Ortho-Clinical Diagnostics, Rochester, NY). Transferrin and free fatty acids were determined using the Behring nephelometer-100 m (Behring, Deerfield, IL).
Cosmetic Outcome
In a subgroup of patients (0.1 and 0.2 mg/kg/d rhGH and control patients), burn scars were assessed by 5 blinded volunteer clinicians using a modified Seattle scar score.17 Briefly, this scoring system assesses 4 characteristics of the scars: scar surface, thickness, border height, and color differences; each parameter is scored from 0 to 4, 0 being the normal value and 4 the most severe, for a total possible score from 0 to 16. The evaluators were blinded to the identity, treatment group, and study time-point of the patients whose photographs were assessed. Evaluators were not involved in the treatment of these patients. Each observer analyzed photographs of representative scars at 6, 12 months and 18 to 24 months postinjury.
Exercise
A subgroup of patients was part of a 12-week exercise program that took place between 3 and 9 months post burn. The program was administered 3 times a week with patients remaining as resident out-patients with their families and included basic resistance exercises and aerobic conditioning exercises on a treadmill or cycle ergometer. Strength assessment was performed according to manufacturer instructions, using the Biodex System 3 dynamometer (Biodex Medical Systems, Shirley, NY). Isokinetic testing of knee extensor strength of the patient’s dominant leg was performed at an angular velocity of 150°/s. After the submaximal warm-up repetitions, 10 maximal voluntary muscle contractions (full extension and flexion) were performed consecutively. The test was repeated following 3 minutes of rest to minimize the effects of fatigue. Peak torque values were calculated with the Biodex software system. The highest peak torque measurement attained from the 2 trials was selected.
Statistical Analysis
Data are presented as means ± SEM in graphs and means ± SD in tables. Statistical analysis used a Tukey t test or a 1-way ANOVA, followed by Bonferroni multiple comparison tests, when appropriate. For scar scores, the Holm-Sidak post hoc test was used and significance was accepted at P < 0.05. Statistical software (SigmaStat and SigmaPlot, SPSS, Chicago, IL) was used for analyses.
Results
Demographics
There was no significant difference between groups with regard to age, gender, ethnicity, and burn size (Table 1).
Table 1.
Study Demographics
| Treatment Groups | 0.05 mg/kg rhGH | 0.1 mg/kg rhGH | 0.2 mg/kg rhGH | Placebo | P |
|---|---|---|---|---|---|
| No. patients per group (n) | 37 | 41 | 23 | 94 | |
| No. patients, totals (n) | All rhGH patients: 101 | 94 | |||
| Age (yrs, mean ± SD) | 9±5 | 9±4 | 10±5 | 9±5 | ns |
| Gender | |||||
| Male (n) | 21 | 30 | 17 | 61 | ns |
| Female (n) | 16 | 11 | 6 | 33 | ns |
| Burn to admission (d, median [25%/75%]) | 3 (2/7) | 3 (1/6) | 3 (2/6) | 3 (2/5) | ns |
| Inhalation injury (%) | 20 | 14 | 18 | 17 | ns |
| Total burn size (%TBSA, mean ± SD) | 61±16 | 60±15 | 67±16 | 60±14 | ns |
| Full thickness burn size (%TBSA, mean ± SD) | 49±24 | 47±22 | 55±15 | 48±22 | ns |
| Burn type | |||||
| Flame (%) | 77 | 82 | 80 | 78 | ns |
| Scald (%) | 10 | 9 | 11 | 9 | ns |
| Other (%) | 13 | 9 | 9 | 13 | ns |
| Length of stay (d, mean ± SD) | 37±31 | 32±24 | 48±38 | 32±24 | ns |
Heights and Weights
Growth was significantly improved in the rhGH group, as compared with controls, starting at 9 months post burn. Mean height percentiles were reaching normal levels (50th percentile) at 12, 18, and 24 months postburn in the rhGH group, whereas control patients showed a prolonged growth delay with an average height at the level of the 33rd percentile throughout the entire study (Fig. 2A). Patients receiving 0.1 mg/kg/d rhGH showed the most sustained growth improvement, whereas those receiving 0.2 mg/kg/d rhGH did not improve height at any time point (Fig. 2B). No differences in total body weight were observed between the individual rhGH groups and placebo patients (Fig. 2D). A significant weight gain between the entire rhGH group and controls was observed at 12 months post burn (Fig. 2C).
Figure 2.
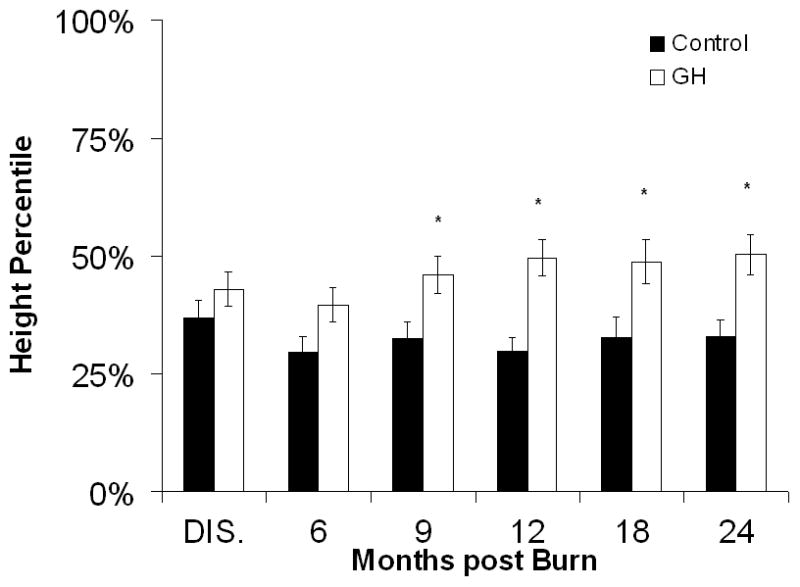
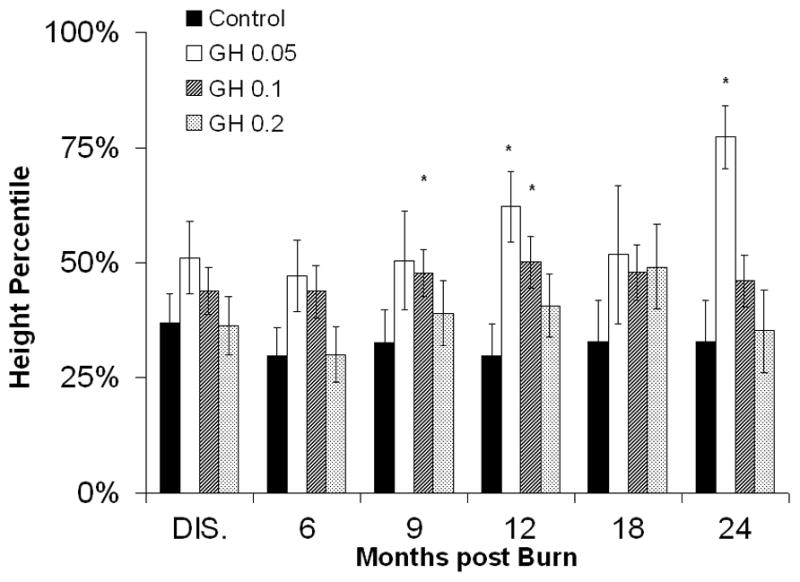
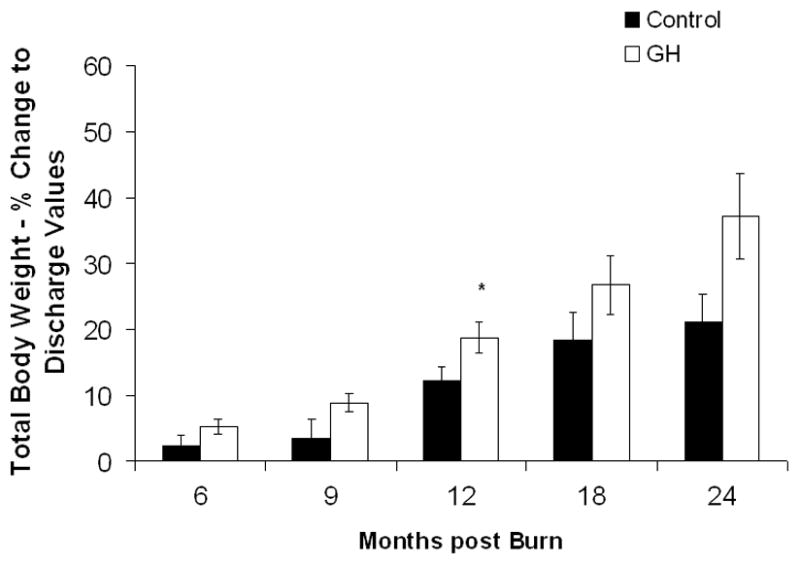
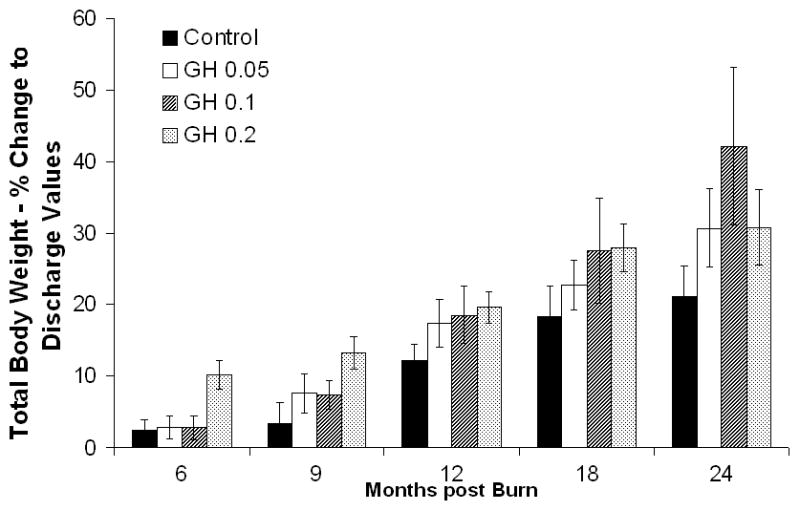
Heights and Weights. A and B, Height percentiles measured at patient discharge (Dis.), 6, 9, 12, 18, and 24 months post burn. C and D, Total Body Weights. Data are shown as percent change from hospital discharge to 24 months after burn. *Significant difference between rhGH group(s) and placebo, P < 0.05.
Body Composition
Lean body mass in the rhGH group was increased when compared with placebo up to 12 months postburn. (Fig. 3A). Individually, 0.2 mg/kg/d showed the highest increase in LBM, reaching significant levels at 6, 9, 12, and 24 months postburn, whereas 0.1 mg/kg/d rhGH, had a significant effect at 12 months after the end of therapy (Fig. 3B). Bone mineral content values showed partly opposing patterns: while 0.05 mg/kg/d significantly increased BMC at 12 to 24 month postburn, higher doses did not affect BMC or even decreased it when compared with placebo (Fig. 3D). The entire rhGH group showed no differences when compared with placebo (Fig. 3C). A similar opposing pattern was evident in percent body fat measurements. Controls versus rhGH showed a significantly lower percentage of body fat in the rhGH group during the entire time of drug administration (Fig. 3E). Individually, 0.1 and 0.2 mg/kg/d rhGH showed a significant decrease at 9 to 12 and 12 months, respectively, while patients in the 0.05 mg/kg/d rhGH group had increased body fat as compared with controls after the cessation of therapy (Figs. 3E, F).
Figure 3.
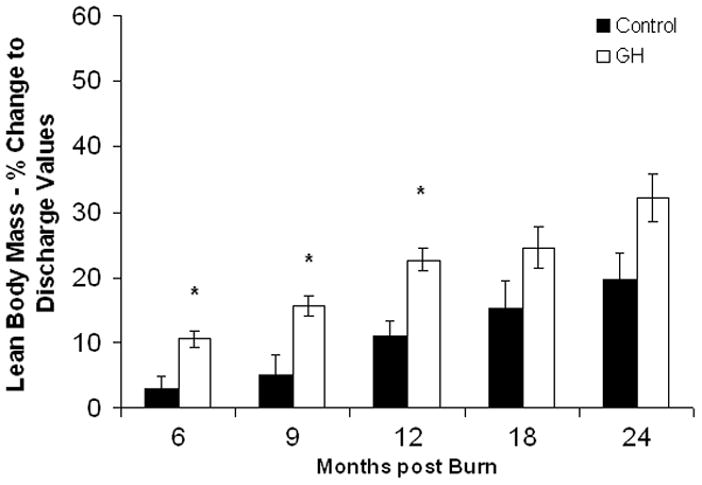
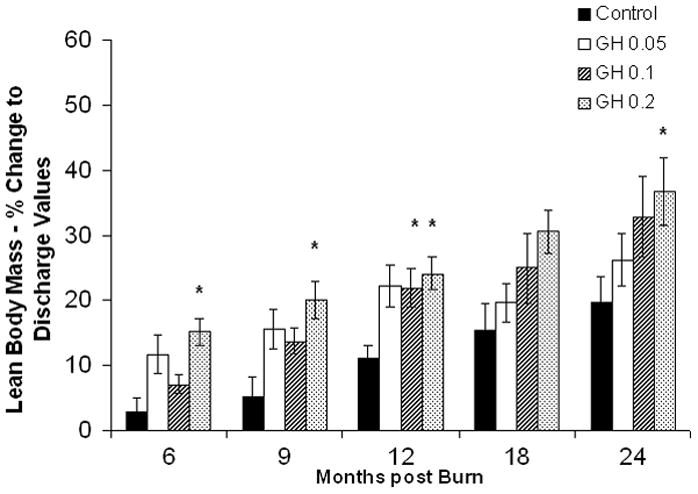
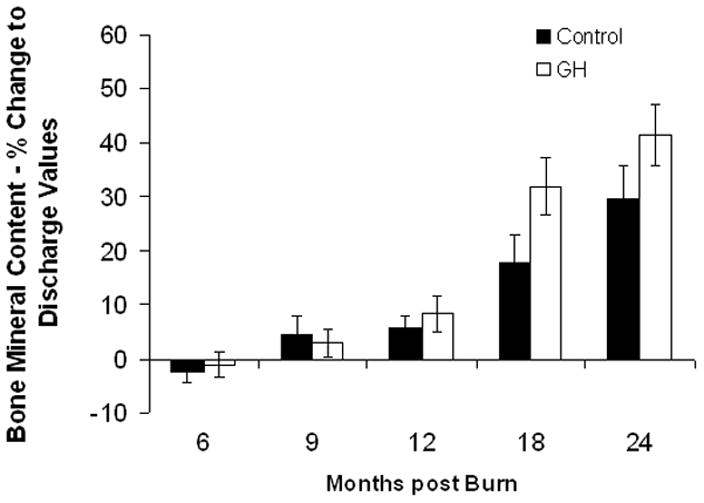
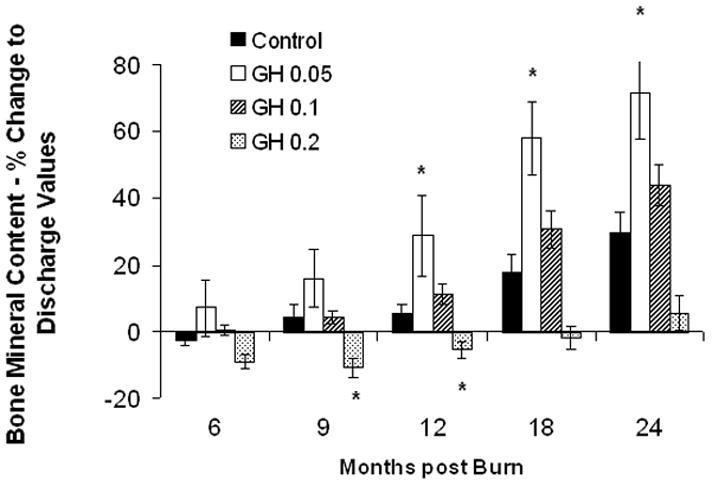
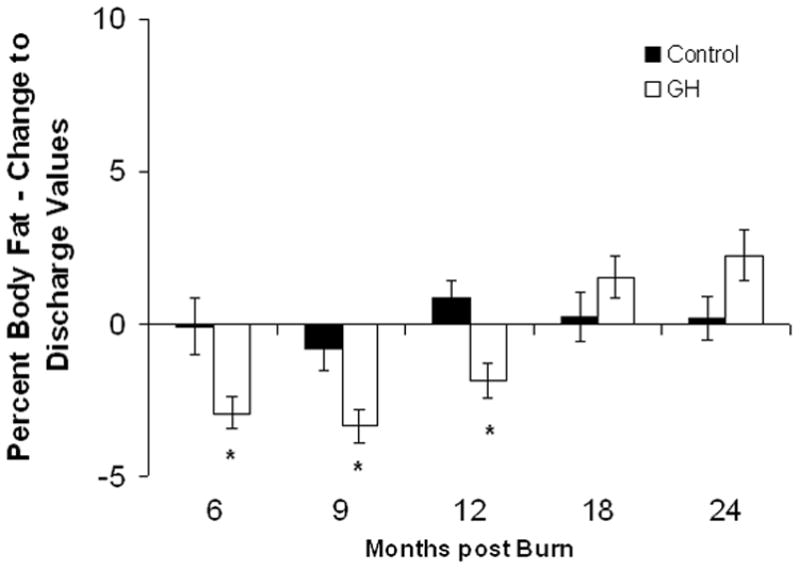
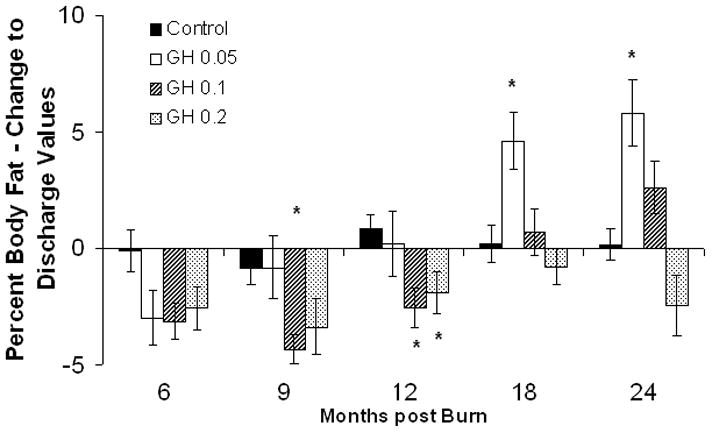
Body composition. Data are shown as percent change from hospital discharge to 24 months after burn. A and B, Lean body mass. C and D, Bone mineral content. E and F, Percent body fat. Values are means±SEM. *Significant difference between rhGH group(s) and placebo, P < 0.05.
Cardiac Function and Metabolism
Significant decreases in CO were observed at 12 and 18 months post burn in the entire rhGH group as compared with placebo (Table 2), with no differences in CO between the individual treatment groups and placebo at 6, 9, and 24 months postburn; at 12 months postburn, 0.1 and 0.2 mg/kg/d rhGH show a significant decrease versus placebo. A significant decrease in predicted REE was observed in the rhGH patients when compared with placebo at all time-points but 24 months postburn (Fig. 4A). The individual analysis showed the largest decrease in the 0.1 mg/kg/d rhGH group (Fig. 4B).
Table 2.
Cardiac Output, Hormones, and Other Lab Parameters
| Discharge | P | 6 Months | P | 9 Months | P | 18 Months | P | 24 Months | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| IGF-1 (ng/mL) | ||||||||||
| Control | 48.9±8.8 | 114.2±35.2 | 122.4±29.3 | 141.9±42.2 | 151.2±29.2 | |||||
| All GH | 83.4±12.0 | <0.05 | 206.0±30.3 | <0.05 | 276.3±35.3 | <0.05 | 184.4±45.2 | ns | 157.8±22.2 | ns |
| 0.05 GH | 98.3±38.5 | ns | 133.4±38.1 | ns | 147.5±37.6 | ns | 114.8±55.0 | ns | 174.7±24.1 | ns |
| 0.1 GH | 89.3±20.0 | ns | 232.1±33.1 | ns | 346.6±49.4 | <0.01 | 297.6±85.5 | ns | 215.6±33.2 | ns |
| 0.2 GH | 70.6±14.1 | ns | 217.9±67.8 | ns | 272.9±73.7 | ns | 260.2±9.4 | ns | 200.0±13.1 | ns |
| GH (ng/ml) | ||||||||||
| Control | 2.5±0.5 | 1.2±0.2 | 0.8±0.2 | 2.2±0.6 | 1.6±0.5 | |||||
| All GH | 2.8±0.6 | ns | 6.2±1.3 | <0.05 | 6.4±1.6 | <0.05 | 1.9±0.5 | ns | 1.7±0.7 | ns |
| 0.05 GH | 1.3±3.8 | ns | 3.8±1.3 | <0.01 | 2.7±0.9 | ns | 2.0±1.6 | ns | 0.9±0.4 | ns |
| 0.1 GH | 2.1±0.4 | ns | 4.3±1.5 | <0.05 | 5.8±2.0 | <0.05 | 1.8±0.5 | ns | 1.5±0.5 | ns |
| 0.2 GH | 4.1±1.3 | ns | 9.9±3.0 | <0.01 | 10.2±4.0 | <0.01 | 1.9±1.1 | ns | 3.2±3.0 | ns |
| PTH (pg/ml) | ||||||||||
| Control | 12.9±1.2 | 17.3±4.7 | 25.1±6.3 | 31.2±5.8 | 47.5±10.0 | |||||
| All GH | 12.5±5.2 | ns | 19.1±2.6 | ns | 20.4±2.4 | ns | 23.4±4.0 | ns | 30.6±5.3 | ns |
| 0.05 GH | 10.5±2.9 | ns | 24.7±5.0 | ns | 11.6±3.3 | ns | 22.6±9.0 | ns | 43.6 ±17.4 | ns |
| 0.1 GH | 8.8±1.5 | ns | 22.2±3.3 | ns | 32.3±3.3 | ns | 31.1±5.5 | ns | 27.3±4.3 | ns |
| 0.2 GH | 17.6±3.8 | <0.05 | 11.4±2.2 | ns | 12.3±3.6 | ns | 6.2±4.9 | <0.05 | n/a | n/a |
| OCAL (ng/ml) | ||||||||||
| Control | 13.1±1.3 | 38.6±4.3 | 43.8±4.4 | 42.1±3.6 | 37.1±4.6 | |||||
| All GH | 14.4±1.4 | ns | 42.0±3.7 | ns | 49.7±5.2 | ns | 48.9±5.5 | ns | 36.3±5.8 | ns |
| 0.05 GH | 18.3±1.9 | ns | 27.1±3.2 | ns | 23.4±3.2 | ns | 53.2±4.8 | ns | 34.2±4.0 | ns |
| 0.1 GH | 15.0±2.3 | ns | 37.8±4 | ns | 52.5±5.6 | ns | 43.9±8.2 | ns | 40.4±4.8 | ns |
| 0.2 GH | 12.2±2.3 | ns | 61.9±7.4 | <0.01 | 76.1±13.2 | <0.01 | 70.9±12.1 | <0.05 | n/a | n/a |
| CO (% predicted) | ||||||||||
| Control | 144.7±6.7 | 122.7±7.3 | 125.5±7.9 | 127.6±10.1 | 121.4±8.7 | |||||
| All GH | 148.3±6.9 | ns | 131.6±6.0 | ns | 117.9±5.3 | ns | 104.8±5.9 | <0.05 | 112.4±5.5 | ns |
| 0.05 GH | 157.0±12.4 | ns | 129.6±7.6 | ns | 117.5±7.5 | ns | 119.4±9.6 | ns | 110.3±7.3 | ns |
| 0.1 GH | 146.4±9.2 | ns | 138.1±11.1 | ns | 120.8±10.4 | ns | 92.9±9.9 | ns | 118.3±10.0 | ns |
| 0.2 GH | 129.6±12.5 | ns | 127.1±14.1 | ns | 114.1±10.0 | ns | 95.4±9.5 | ns | 105.2±9.9 | ns |
Values expressed as Averages ± SEM. P-values expressed as versus control.
IGF-1 indicates insulin-like growth factor-1; GH, growth hormone; PTH, parathyroid hormone; OCAL, osteocalcin; CO, cardiac output; ns, no significant difference.
Figure 4.
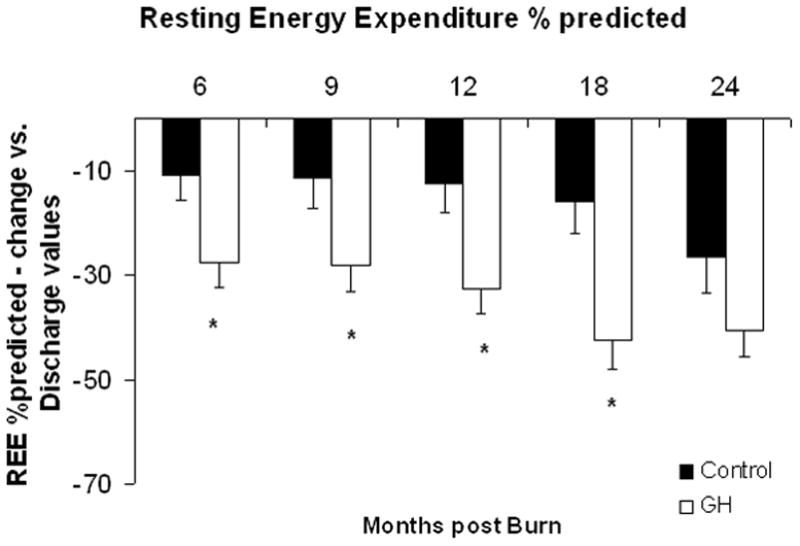
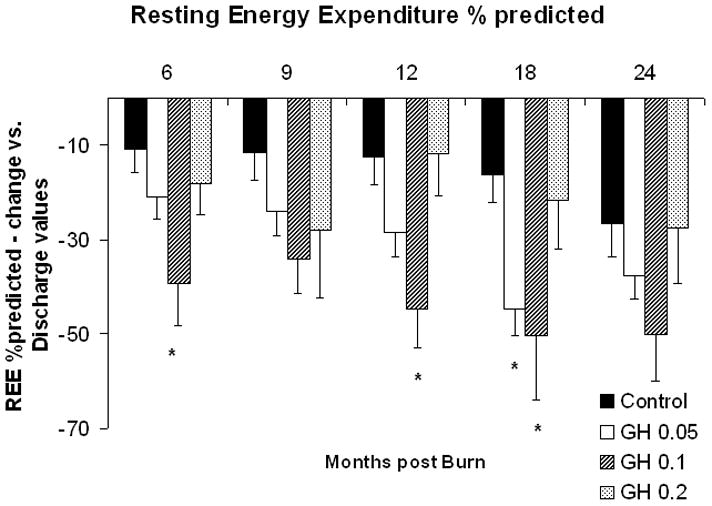
Resting Energy Expenditure. A and B, Resting energy expenditure (REE), depicted as percent of predicted at patient discharge (Dis.), 6, 9, 12, 18, and 24 months postburn. Values are means±SEM. *Significant difference between rhGH group(s) and placebo, P < 0.05.
Hormone Panel and Serum Chemistry
Recombinant human growth hormone administration increased serum GH of the entire rhGH group when compared with placebo during therapy, returning to placebo levels at 18 and 24 months postburn (Table 2). Serum GH levels were highest in the 0.2 mg/kg/d group (Table 2). Serum IGF-1 was increased in the entire rhGH group at 6, 9, and 12 months post burn, returning to placebo levels at 18 and 24 months (Table 2). IGF-1 levels in the 0.1 and 0.2 mg/kg/d subgroups were significantly increased when compared with placebo at 6 months postburn (Table 2). Patients receiving 0.2 mg/kg/d rhGH showed significantly increased serum Osteocalcin levels (Table 2); this increase was not observed in the other treatment groups, resulting in no difference between the entire rhGH group and placebo. Serum PTH levels in the 0.2 mg/kg/d rhGH group, while significantly higher when compared with placebo at discharge, dropped to significantly lower levels by 12 months post burn and remained low at 18 months post burn (Table 2).
Cosmetic Outcome
Scar scoring results showed attenuated formation of hypertrophic scarring in rhGH patients when compared with placebo at 12 months postburn (Fig. 5).
Figure 5.
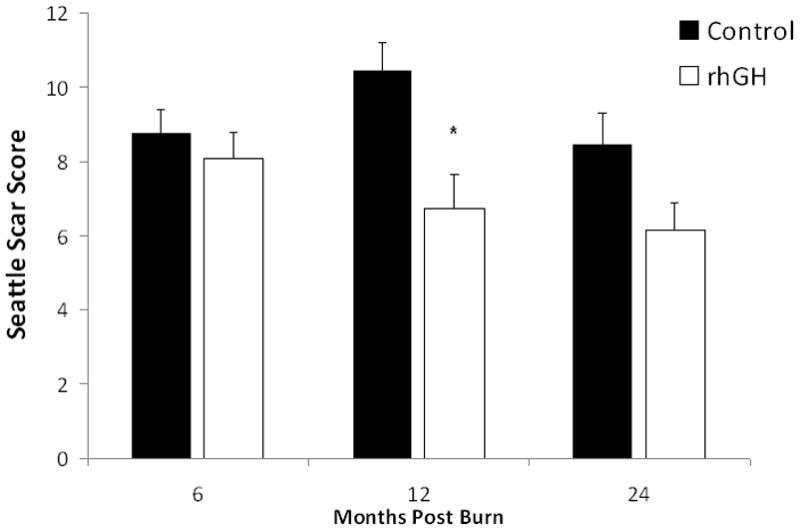
Scarring. Scar quality was assessed in both patient groups using the modified Seattle Scar Score. Scar surface, thickness, border height, and color differences were assessed from representative patient photographs; each parameter is scored from 0 to 4, 0 being the normal value and 4 the most severe, for a total possible score from 0 to 16. Values are means of total scar scores±SEM, measured at 6, 12 to 18, and 24 months postburn. *Significant difference between rhGH group(s) and placebo, P<0.05.
Exercise
Forty-one patients in the rhGH group and 30 patients in the Control group took part in the 12-week exercise program. In terms of strength measurements, both rhGH and control patients who were part of the exercise program showed a similar increase between discharge and 12 months postburn (Table 3). Exercise had an impact on lean body mass - in the subgroups of exercise patients, the differences between rhGH and placebo were lower than in the no-exercise subgroups (Table 3). Both exercise and no-exercise patients in the rhGH groups, however, showed significant LBM increases in comparison to their respective control groups at 12 months post burn. Height percentile increase and percentage body fat decrease was similar in both exercise and no-exercise rhGH groups as compared with their respective controls at 12 months postburn (Table 3).
Table 3.
Exercise Data
| LBM 6 mo (Percent change to discharge values) | LBM 12 mo (Percent change to discharge values) | PFat 6 mo (Change to discharge values) | PFat 12 mo (Change to discharge values) | Height Discharge (Percentile) | Height 6 mo (Percentile) | Height 12 mo (Percentile) | Strength Discharge (Peak Torque, Nm) | Strength 12 mo (Peak Torque, Nm) | |
|---|---|---|---|---|---|---|---|---|---|
| Control-exercise | 4.4 ± 3.9 | 9.9 ± 4.4 | 0.3 ± 1.3 | 2.1 ± 1.0 | 39.8 ± 6.8 | 26.9 ± 5.1 | 29.5 ± 5.2 | 42.1 ± 6.8 | 74.5 ± 8.1 |
| rhGH-exercise | 10.6 ± 1.7 | 20.7 ± 2.1* | −2.4 ± 0.7* | −2.1 ± 0.7* | 44.5 ± 4.8 | 38.0 ± 5.0 | 52.1 ± 5.4* | 44.4 ± 5.1 | 71.8 ± 7.8 |
| Control-No exercise | 0.7 ± 2.1 | 8.4 ± 1.9 | −0.4 ± 1.4 | 0.3 ± 0.8 | 35.8 ± 4.4 | 32.1 ± 4.4 | 30.7 ± 3.7 | n/a | n/a |
| rhGH-No exercise | 10.0 ± 1.4† | 25.6 ± 1.9† | −4.1 ± 0.8† | −2.5 ± 0.8† | 41.9 ± 5.2 | 41.2 ± 5.3 | 48.0 ± 5.4† | n/a | n/a |
Discharge, 6 months and 12 months are the follow-up time points postburn injury. Strength measurements only performed in exercise patients.
P < 0.05 versus control-exercise.
P < 0.05 versus control-no exercise. Values expressed as averages ± SEM.
LBM indicates lean body mass; PFat, percentage body fat.
Side Effects
Three patients in the rhGH group showed side-effects. Two patients had the dose of rhGH adjusted from 0.2 to 0.1 mg/kg/d due to increased serum calcium levels at the 9-month follow-up time point. One patient had an occurrence of hyperglycemia and glucose intolerance, as measured by the oral glucose tolerance test, at the 9-month follow-up time point. Growth Hormone was discontinued. No other adverse reactions that are typically associated with long term administration of GH, such as hirsutism or virilization, epiphyseal closure or advancement of bone age, or pseudotumor cerebri, were observed in the rhGH group.
Discussion
Significant improvements have been made in the acute treatment of pediatric burn injuries over the past 3 decades, resulting in a dramatic decrease in mortality.1 The persistent catabolism during convalescence, however, remains a major contributor to long-term morbidity. Children with more than 40% total body surface area burn, demonstrate a negative lean body mass balance for at least 9 months after trauma,16 and a continuous hypermetabolic state for up to 3 years post burn injury.18 Additional studies conducted in this patient population have indicated that the hypermetabolic and catabolic response continues up to 2 years after burn, significantly delaying the recovery of pediatric burn victims.19
Several treatment options for diseases causing similar clinical problems, such as loss of LBM and growth delay, have been developed in the past. RhGH given at 0.05 mg/kg/d has a beneficial effect on growth in children suffering from Turner syndrome.20 Long-term rhGH treatment of patients with Turner syndrome resulted in an increased final height and weight. Additionally, hormone replacement therapy with rhGH improved the quality of life in these patients when compared with patients without hormone therapy.21 Studies in similar conditions, such as children born small for gestational age, confirmed the efficacy of rhGH as a growth promoting agent.22 All these studies have demonstrated a wide safety margin for rhGH; however, patients treated with rhGH should be monitored long-term for possible side effects such as pseudotumor cerebri, gynecomastia, altered lipid profiles, and hyperglycemia.
The effect of rhGH administration in severely burned children has been initially examined during the acute burn phase. A significant increase in net protein synthesis and an improved wound healing have been observed in children treated with 0.2 mg/kg/d rhGH without side effects.6,7,23 These positive findings of a short-term administration of rhGH and the fact that the hypermetabolic and catabolic response continues up to 3 years after burn, encouraged us to investigate effects of an extended rhGH treatment after discharge in massively burned children.9 In this study, treatment with 0.05 mg/kg rhGH from discharge to 1 year after burn caused significant increases in growth and muscle mass when compared with placebo. Increases in height improved during and after treatment. The effects on LBM, however, were observed only during treatment, although IGF-1 continued to be elevated compared with placebo 6 months after the treatment was discontinued. Catabolic serum cortisol levels were significantly lower with rhGH even after therapy. Furthermore, low-dose rhGH did not affect the hypermetabolic response.9
The current study summarizes our experience with 3 different doses of rhGH—0.05, 0.1, and 0.2 mg/kg/d, administered in a dose finding design from patient discharge until 1 year after severe burn compared to randomized placebo controls. We found significant differences between the entire rhGH treatment group in comparison to placebo with regard to growth, body composition, hormones, cardiac output, energy expenditure, and scarring. Some effects observed with rhGH administration were dose-related. Whereas a significant increase in LBM was observed at all time-points apart from 18 months postburn in the entire rhGH group, the 0.05 and 0.1 mg/kg/d groups, when analyzed separately, showed an increase only at 9 and 12 month postburn and did not significantly differ from placebo once the drug was discontinued. Only with 0.2 mg/kg rhGH could persistent improvement in LBM be achieved. With regards to percent body fat, a significantly lower body fat content was observed in the entire rhGH during treatment, and no difference was observed at the post-therapy 18 and 24 month follow-up time points. When assessing the rhGH groups separately, separately, the 0.2 mg/kg/d group is characterized by a prolonged decrease in body fat content. These general effects of rhGH therapy on body composition–increase in muscle mass and decrease in body fat content have been well documented in long-term studies that examined rhGH therapy in GH-deficient adults.24–26 No studies, however, have been performed with long-term high-dose rhGH (0.2 mg/kg/d). This study shows a prolonged effect after cessation of therapy. Interestingly, the observed changes in percent body fat mass were not associated with changes in triglycerides, free fatty acids, or cholesterol.
Bone mineral content levels in patients treated with rhGH were decreased in patients who received the highest dose of rhGH. This effect is associated with a sustained suppression in PTH levels and an increase in serum osteocalcin, indicating a higher bone mineral turnover in the 0.2 rhGH group. Interestingly, no differences in urinary or serum calcium levels were observed between groups. In previous studies with low-dose rhGH an increase in BMC was associated with an increase in PTH27; short-term administration of 0.2 mg/kg/d rhGH was shown not to increase osteocalcin levels during acute patient stay.28 The dissociation of increase in LBM and decrease in BMC, as observed in the 0.2 mg/kg/d rhGH subgroup in this study, may be caused by direct effects of rhGH on bone and not by decreased skeletal loading of muscle, resulting in bone resorption and secondary suppression of PTH. An additional intake of calcium may be required to offset these stimulatory effects of rhGH on tissue growth.
Serum concentrations of IGF-1 and GH differed depending on the dose of rhGH. Administration of 0.1 and 0.2 mg/kg/d rhGH increased IGF-1 and GH concentrations more than the 0.05 mg/kg/d rhGH and placebo groups. It is well accepted that rhGH mediates its effect through up-regulation of IGF-1 and increase in endogenous GH.29,30 Receptors for these anabolic hormones are expressed in various tissues.30 Stimulation of these receptors in the bone stimulates growth, whereas activation of these receptors in the muscle increases protein synthesis and decreases glucose uptake.30 Additionally, stimulation of the GH-IGF-1 cascade induces lipolysis in fat tissue and increases gluconeogenesis in the liver. The significant up-regulation of insulin with rhGH administration might cause a reduction in insulin sensitivity at the used dose of rhGH.30 A decrease in insulin sensitivity may therefore be of some concern; however, there was no alterations in serum glucose apart from 1 patient, as reported in the side effect summary. More importantly, insulin itself has significant anabolic effects and has been used successfully to attenuate catabolism in severely burned children during acute hospitalization.31,32
The observed changes in cardiac output are indicating a significant decrease in hypermetabolism, and this finding is confirmed by the results during indirect calorimetry measurements. Previous studies have shown, however, that long-term rhGH administration results in an increase rather then decrease in CO33; others found that long-term rhGH increases cardiac contractility.34 No studies, however, have been performed up to date to assess rhGH effects on cardiac function in severely burned patients who display a prolonged state of hypermetabolism that is associated with longterm increases in CO and resting energy expenditure. We speculate that the beneficial effects of long-term rhGH therapy on lowering cardiac work and attenuating hypermetabolism might be associated with decreases in cortisol levels, an effect we have seen after therapy with 0.05 mg/kg/d rhGH.
Results of scar scores indicate that rhGH favorably influenced scarring. It has been previously shown that rhGH directly stimulates collagen synthesis in the wound and increases tensile strength.35–37 However, if growth hormone improves wound healing by collagen synthesis in the wound, one might also expect it to contribute to the formation of hypertrophic scars. In previous studies, we showed that administration of rhGH during the acute hospitalization showed no adverse clinical effects at 2 years postburn. There was no evidence of epiphyseal closure or increased scar formation in any of the longitudinal evaluations of children treated acutely with rhGH.38 An analysis of the long-term application of 0.05 mg/kg rhGH also showed no adverse effects, but also no improvement in scarring and collagen deposition as evaluated by immunohistochemistry.39 The current study now demonstrates that with long-term application of 0.1 and 0.2 mg/kg rhGH, scarring is decreased at 12 months post burn. We can only speculate on the underlying molecular mechanisms of improved scarring. One explanation might be that the enhanced wound repair with rhGH consequently leads to accelerated wound maturation with a higher collagen deposition and neovascularization ratio. Another explanation might be that the decreased cardiac output and curbed hypermetabolism lead to diminished scar blood flow and therefore decreased hypertrophic scarring.
A confounding factor in the evaluation of strength and lean body mass is the participation of burn patients in exercise programs after discharge from acute hospital stay. We have previously reported the benefits of a 12-week structured exercise training program during the rehabilitation of burned children, which include improvements in LBM, muscle strength, and power.40,41 Lean body mass was additionally found to improve 3 months following completion of supervised exercise training.40 We therefore separately analyzed the subgroups of rhGH and control patients who took part in the 12-week exercise program, as well as the nonexercise patients of both groups. The increase in peak torque measurements between discharge from acute stay and the 12-month follow-up time point was similar in the exercise groups. This finding is not surprising, considering that a period of at least 3 months passed between the end of the program and the 12 month measurements, in which no structured exercise training occurred. Indeed, these results reflect one of our previous publications,40 in which exercise induced benefits in strength are not significantly different between groups 3 months after cessation of the program. As seen in the comparison of the entire patient cohorts, lean body mass was significantly increased at 12 months post burn in rhGH exercise-patients versus the exercise controls. This difference is even more pronounced between rhGH and control patients who were not part of the exercise program. With regards to growth, both exercise and no-exercise groups who received rhGH reached the 50th percentile at 12-months postburn, while their respective controls remained significantly lower. With respect to these findings, it is important to mention that the average age of patients in the exercise groups was higher than in the nonexercise groups. Structured exercise programs were usually performed by children 7 years or older, as they require coordinated use of exercise equipment and cooperation in obtaining the strength measurements. At our institution, however, younger children have been taking part in a program using music and movements specifically chosen to increase strength, flexibility, and endurance.42 All patients, regardless of participation in the structured exercise program, were instructed to perform a home-based physical rehabilitation program, which was directed at enhancing strength, range of motion, and minimizing scar deformities and contractures. It included range of movement exercises, positioning and splinting routines, and functional strength assessments, as well as wound and scar management techniques involving pressure garments, inserts, and physical agent modalities.43 In regular intervals, therapists assessed and confirmed that a patient’s parent or guardian was able to comply with these instructions.
In summary, rhGH therapy results in an attenuation of hypermetabolism, improvement in lean body mass, a decrease in cardiac output, and a more rapid scar maturation. Some of the effects are dose dependent, such as increases in LBM and decreases in percent body fat. These increases occur independently from the participation in structured exercise programs. Although long-term therapy with 0.2 mg/kg rhGH is most effective in achieving lean body mass increase, it causes significant loss of BMC and higher bone mineral turnover, as documented by a marked PTH suppression and increased levels of osteocalcin, and is associated with few side effects. As a result, and summarizing the outcome of this and previous studies from our institution, we support the use of 0.2 mg/kg rhGH in the acute phase postburn, and 0.1 mg/kg rhGH for at least 1 year during the convalescence period.
Acknowledgments
The authors thank Debrah Benjamin, Wes Benjamin, Michael Buffalo, Mario Celis, Joana Huddleston, Lupe Jecker, Mary Kelly, Jane Martinez, Charles Mitchell, Sylvia Ojeda, and Joana Wilkins for their assistance in obtaining the study measurements. Recombinant human growth hormone was kindly provided from the Eli Lilly Corp. (Indianapolis, IN).
Supported by National Institutes of Health Grants #P50-GM60338-06, #T32-GM08256-15, and #R01-HD049471; National Institute for Disability and Rehabilitation Research Grant #H133A020102-05 and #H133A070026, and Shriners Hospital for Children Grants #8952, #8741, #8760, and #8480.
References
- 1.Herndon DN, Tompkins RG. Support of the metabolic response to burninjury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 2.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. doi: 10.1001/archsurg.1990.01410150114021. [DOI] [PubMed] [Google Scholar]
- 3.Jeschke MG, Mlcak RP, Finnerty CC, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on glucose utilization in burn patients. J Surg Res. 1991;51:518–523. doi: 10.1016/0022-4804(91)90175-l. [DOI] [PubMed] [Google Scholar]
- 5.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991;126:38–43. doi: 10.1001/archsurg.1991.01410250042006. [DOI] [PubMed] [Google Scholar]
- 6.Herndon DN, Barrow RE, Kunkel KR, et al. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg. 1990;212:424–429. doi: 10.1097/00000658-199010000-00005. discussion 430–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578–1584. doi: 10.1097/00003246-200005000-00053. [DOI] [PubMed] [Google Scholar]
- 8.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study. Lancet. 1999;354:1789. doi: 10.1016/s0140-6736(99)02741-5. [DOI] [PubMed] [Google Scholar]
- 9.Przkora R, Herndon DN, Suman OE, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Ann Surg. 2006;243:796–801. doi: 10.1097/01.sla.0000219676.69331.fd. discussion 801–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkins JP, Suman OE, Benjamin DA, et al. Comparison of self-reported and monitored compliance of daily injection of human growth hormone in burned children. Burns. 2003;29:697–701. doi: 10.1016/s0305-4179(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 11.Hart DW, Wolf SE, Herndon DN, et al. Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann Surg. 2002;235:152–161. doi: 10.1097/00000658-200201000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC Growth Charts: United States. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Advance Data. 2000;314:1–28. [PubMed] [Google Scholar]
- 13.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 14.Mlcak RP, Jeschke MG, Barrow RE, et al. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–130. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart DW, Wolf SE, Chinkes DL, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–764. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 16.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 17.Yeong EK, Mann R, Engrav LH, et al. Improved burn scar assessment with use of a new scar-rating scale. J Burn Care Rehabil. 1997;18:353–355. doi: 10.1097/00004630-199707000-00014. discussion 352. [DOI] [PubMed] [Google Scholar]
- 18.Jeschke MG, Przkora R, Suman OE, et al. Sex differences in the long-term outcome after a severe thermal injury. Shock. 2007;27:461–465. doi: 10.1097/01.shk.0000238071.74524.9a. [DOI] [PubMed] [Google Scholar]
- 19.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–971. doi: 10.1097/01.ta.0000214580.27501.19. discussion 971. [DOI] [PubMed] [Google Scholar]
- 20.Rongen-Westerlaken C, Wit JM, De Muinck Keizer-Schrama SM, et al. Dutch Growth Hormone Working Group. Growth hormone treatment in Turner syndrome accelerates growth and skeletal maturation. Eur J Pediatr. 1992;151:477–481. doi: 10.1007/BF01957747. [DOI] [PubMed] [Google Scholar]
- 21.Kanaka-Gantenbein C. Hormone replacement treatment in Turner syndrome. Pediatr Endocrinol Rev. 2006;3(suppl 1):214–218. [PubMed] [Google Scholar]
- 22.de Zegher F, Ong KK, Ibanez L, et al. Growth hormone therapy in short children born small for gestational age. Horm Res. 2006;65(suppl 3):145–152. doi: 10.1159/000091520. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez RJ, Wolf SE, Barrow RE, et al. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg. 1998;228:439–448. doi: 10.1097/00000658-199810000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Arnao J, Jabbar A, Fulcher K, et al. Effects of growth hormone replacement on physical performance and body composition in GH deficient adults. Clin Endocrinol (Oxf) 1999;51:53–60. doi: 10.1046/j.1365-2265.1999.00737.x. [DOI] [PubMed] [Google Scholar]
- 25.Christiansen JS, Jorgensen JO. Beneficial effects of GH replacement therapy in adults. Acta Endocrinol (Copenh) 1991;125:7–13. doi: 10.1530/acta.0.1250007. [DOI] [PubMed] [Google Scholar]
- 26.Hansen TB, Vahl N, Jorgensen JO, et al. Whole body and regional soft tissue changes in growth hormone deficient adults after one year of growth hormone treatment: a double-blind, randomized, placebo-controlled study. Clin Endocrinol (Oxf) 1995;43:689–696. doi: 10.1111/j.1365-2265.1995.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 27.Hart DW, Herndon DN, Klein G, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827–834. doi: 10.1097/00000658-200106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein GL, Wolf SE, Langman CB, et al. Effects of therapy with recombinant human growth hormone on insulin-like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. J Clin Endocrinol Metab. 1998;83:21–24. doi: 10.1210/jcem.83.1.4518. [DOI] [PubMed] [Google Scholar]
- 29.Cioffi WG, Gore DC, Rue LW, III, et al. Insulin-like growth factor-1 lowers protein oxidation in patients with thermal injury. Ann Surg. 1994;220:310–316. doi: 10.1097/00000658-199409000-00007. discussion 316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller EE, Rigamonti AE, Cella SG. Mechanisms of action of GH. J Endocrinol Invest. 2003;26(suppl 10):2–15. [PubMed] [Google Scholar]
- 31.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg. 2004;239:553–560. doi: 10.1097/01.sla.0000118569.10289.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas SJ, Morimoto K, Herndon DN, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002;132:341–347. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 33.Le Corvoisier P, Hittinger L, Chanson P, et al. Cardiac effects of growth hormone treatment in chronic heart failure: a meta-analysis. J Clin Endocrinol Metab. 2007;92:180–185. doi: 10.1210/jc.2006-1313. [DOI] [PubMed] [Google Scholar]
- 34.Cho GY, Jeong IK, Kim SH, et al. Effect of growth hormone on cardiac contractility in patients with adult onset growth hormone deficiency. Am J Cardiol. 2007;100:1035–1039. doi: 10.1016/j.amjcard.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 35.Garrel DR, Gaudreau P, Zhang LM, et al. Chronic administration of growth hormone-releasing factor increases wound strength and collagen maturation in granulation tissue. J Surg Res. 1991;51:297–302. doi: 10.1016/0022-4804(91)90111-x. [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen PH, Oxlund H. Growth hormone increases the biomechanical strength and collagen deposition rate during the early phase of skin wound healing. Wound Repair Regen. 1996;4:40–47. doi: 10.1046/j.1524-475X.1996.40108.x. [DOI] [PubMed] [Google Scholar]
- 37.Herndon DN, Hawkins HK, Nguyen TT, et al. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg. 1995;221:649–656. doi: 10.1097/00000658-199506000-00004. discussion 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barret JP, Dziewulski P, Jeschke MG, et al. Effects of recombinant human growth hormone on the development of burn scarring. Plast Reconstr Surg. 1999;104:726–729. doi: 10.1097/00006534-199909030-00017. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira GV, Chinkes D, Mitchell C, et al. Objective assessment of burn scar vascularity, erythema, pliability, thickness, and planimetry. Dermatol Surg. 2005;31:48–58. doi: 10.1111/j.1524-4725.2005.31004. [DOI] [PubMed] [Google Scholar]
- 40.Suman OE, Herndon DN. Effects of cessation of a structured and supervised exercise conditioning program on lean mass and muscle strength in severely burned children. Arch Phys Med Rehabil. 2007;88(12 Suppl 2):S24–S29. doi: 10.1016/j.apmr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Suman OE, Spies RJ, Celis MM, et al. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 42.Neugebauer CT, Serghiou MA, Marvin J. A comprehensive 8–12 week group music and movement program for severely burned children. Proceedings of the ABA. J Burn Care Rehabil. 2005;26:S85. [Google Scholar]
- 43.Celis MM, Suman OE, Huang TT, et al. Effect of a supervised exercise and physiotherapy program on surgical interventions in children with thermal injury. Burn Care Rehabil. 2003;24:57–61. doi: 10.1097/00004630-200301000-00014. discussion 56. [DOI] [PubMed] [Google Scholar]