Abstract
The potential anxiolytic effects of a novel positive allosteric modulator (PAM) of the metabotropic glutamate receptor subgroup 2 (mGluR2) were investigated using a self-referencing recording technique with enzyme-based microelectrode arrays (MEAs) that reliably measures tonic and phasic changes in extracellular glutamate levels in awake rats. Studies involved glutamate measures in the rat prefrontal cortex during subcutaneous injections of the following: vehicle, a mGluR2/3 agonist, LY354740 (10 mg/kg), or a mGluR2 PAM, 1-Methyl-2-((cis-(R,R)-3-methyl-4-(4-trifluoromethoxy-2-fluoro)phenyl)piperidin-1-yl)methyl)-1H-imidazo[4,5-b]pyridine ((+)-TFMPIP; 1.0 or 17.8 mg/kg). Studies assessed changes in tonic glutamate levels and the glutamatergic responses to a five minute restraint stress. Subcutaneous injection of (+)-TFMPIP at a dose of 1.0 mg/kg (day 3: −7.1 ± 15.1 net AUC; day 5: −24.8 ± 24.9 net AUC) and 17.8 mg/kg (day 3: −46.5 ± 33.0 net AUC; day 5: 34.6 ± 36.8 net AUC) significantly attenuated the stress-evoked glutamate release compared to vehicle controls (day 3: 134.7 ± 50.6 net AUC; day 5: 286.6 ± 104.5 net AUC), while the mGluR2/3 agonist LY354740 had no effect. None of the compounds significantly affected resting glutamate levels, which we have recently shown to be extensively derived from neurons. Taken together, these data support that systemic administration of (+)-TFMPIP produces phasic rather than tonic release of glutamate that may play a major role in the effects of stress on glutamate neuronal systems in the prefrontal cortex.
Keywords: Anxiolytic, therapeutic targets, neurotransmitter, amperometry, biosensor, mood disorder.
1. Introduction
Anxiety develops from an individual’s interpretation of stressful or fearful stimuli. When this interferes with daily activities, it is considered a pathological condition and is classified as an anxiety disorder (McElligott and Winder, 2009; Pasquini and Berardelli, 2009). Mood and anxiety disorders are characterized by a variety of neuroendrocrine, neurotransmitter, and neuroanatomical disruptions (Martin et al., 2009) and have been characterized in several animal models of stress and anxiety including restraint, tail pinch, and elevated plus maze (Bagley and Moghaddam, 1997; Graeff et al., 1998; Sullivan and Gratton, 1999; Figueiredo et al., 2003; Stevenson et al., 2003; Weinberg et al., 2007; Estanislau et al., 2010; Sutherland et al., 2010). Current anxiety disorder treatments involve pharmacotherapy targeted towards modulating γ-aminobutyric acid and monoamine receptors or transporter proteins (Martin et al., 2009; Swanson et al., 2005). Unfortunately, these drugs have poor efficacy (Martin et al., 2009) and side-effects including sedation, memory impairment, ataxia, and physical dependence (Swanson et al., 2005; Millan, 2004; Schatzberg, 2002). For these reasons, the search for novel therapeutic targets to treat anxiety disorders is an ongoing endeavor.
Glutamate is widely regarded as the major excitatory neurotransmitter in the mammalian central nervous system and has been implicated in several neurodegenerative diseases and neuropsychiatric and anxiety disorders (Danbolt, 2001; Witkin et al., 2007; Riaza Bermudo-Soriano et al., 2011; de la Fuente—Sandoval et al., 2011). Therefore, therapeutics targeting glutamate receptors have pharmacotherapy potential. However, most iGluRs antagonists induce serious side-effects in humans including memory loss, disorientation and hallucinations (Olive, 2009) which has shifted the focus to creating ligands that target mGluR2/3 with some success (Swanson et al., 2005).
One compound that has been developed for potential anxiolytic pharmacotherapy is LY354740. LY354740 is a conformationally constrained analog of L-glutamate that acts as a mGluR2/3 agonist and readily crosses the blood-brain barrier (Schoepp et al., 2003), but does not have selectivity between the two receptor subtypes (Schoepp et al., 1999). It binds at the N-terminus ligand recognition site of Group II receptors with nanomolar potency (Kd: 48 nM and 94 nM in rats and humans, respectively) and no appreciable activity was observed at other glutamate receptor subtypes (Monn et al., 1999, 1997; Schoepp et al., 1999). An initial clinical study in humans evaluating LY354740 indicated a decrease in the number and severity of panic episodes in patients diagnosed with panic disorder (Schoepp et al., 2003). However, a follow-up study reporting adverse events including convulsions and nausea was discontinued (Dunayevich et al., 2008).
mGluR2/3 couples via Gi/Go to inhibit adenylyl cyclase activity and is localized to pre- and post-synaptic neurons while mGluR3 is localized to post-synaptic neurons and inhibits glutamate release (Witkin et al., 2007; Schoepp et al., 2003). These receptors are predominantly located in forebrain regions commonly associated with anxiety and fear including the hippocampus, amygdala, and PFC (Dunayevich et al., 2008).Compounds that act as mGluR2/3 agonists and allosteric modulators have shown anxiolytic-like effects in rodents (Witkin et al., 2007; Johnson et al., 2005; Fell et al., 2011) and humans (Dunayevich et al., 2008). Unfortunately, the high level of conservation at the agonist binding site has hindered the development of mGluR Group II subtype specific drugs (Rowe et al., 2008).Positive allosteric modulators (PAMs), on the other hand, may overcome these obstacles. They can be designed with no agonist activity, act as weak partial agonists, or increase potency and efficacy when an agonist is present (Rowe et al., 2008). Additionally, several PAMs have been identified with specificity for mGluR2 over mGluR3 (Bonnefous et al., 2005; Hu et al., 2004; Johnson et al., 2003; Zhang et al., 2011), such as the novel compound 1-Methyl-2-((cis-(R,R)-3-methyl-4-(4-trifluoromethoxy-2-fluoro)phenyl)piperidin-1-yl)methyl)-1H-imidazo[4,5-b]pyridine ((+)-TFMPIP), showing good brain penetration and excellent pharmacokinetic properties in rodents (Figure 1; (+)-17e in Zhang et al., 2011). In vitro assays using a HEK cell line transfected with human or rat mGluR2 showed (+)-TFMPIP EC50 values of 31 and 35 nM for the human and rat receptor, respectively, and displayed robust in vivo efficacy in two rodent psychosis models (Zhang et al., 2011).
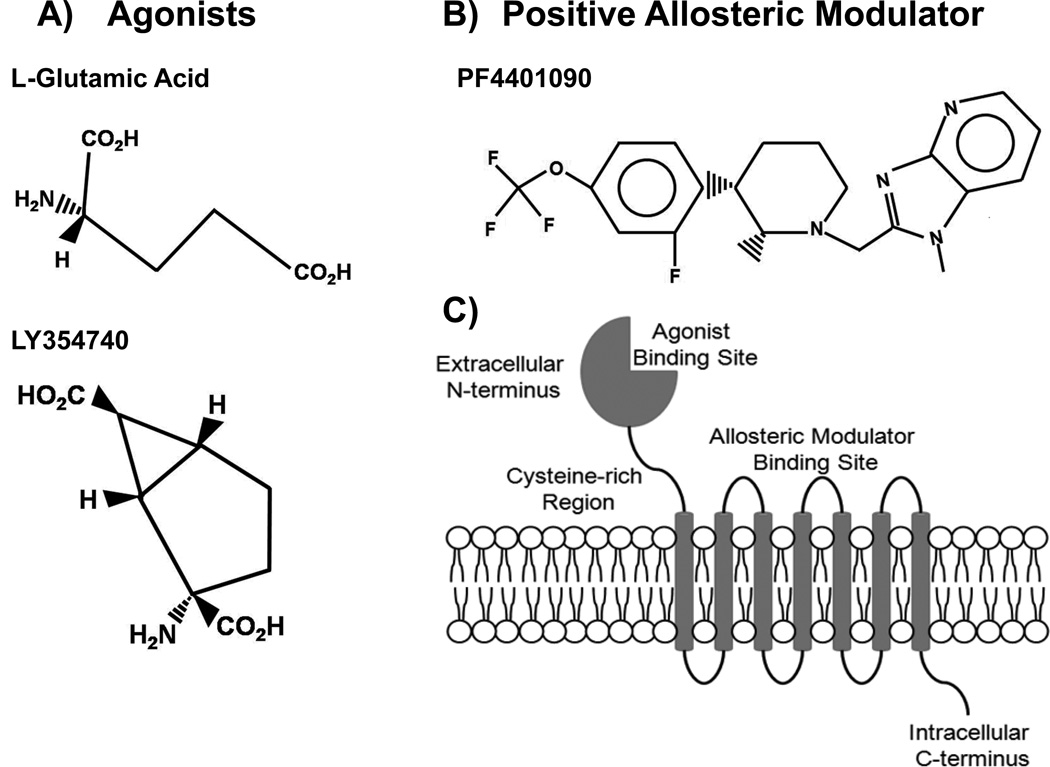
Figure 1.
Stereochemical structures of mGluR2/3 agonists, L-glutamic acid and LY354740 (A), and PAM (+)-TFMPIP (B). Schematic representation of an mGluR2/3 receptor (C) illustrating the extracellular N-terminal agonist binding domain connected to the transmembrane heptahelical domain. The extracellular portion of the heptihelical transmembrane domain contains the binding site for allosteric modulators.
The present studies were carried out to study stress-evoked glutamate release in the PFC of awake rats and the effects of drugs that affect mGluR2/3’s. Changes in both tonic and phasic glutamate release were measured using an enzyme-based MEA that is selective for measuring glutamate with low limits of detection, fast temporal resolution (Burmeister et al., 2004, 2002, 2000; Burmeister and Gerhardt 2001) and minimal damage to surrounding brain tissue (Hascup et al., 2009). MEAs were chronically implanted into the PFC of 3 month-old rats allowing us to reliably measure changes in extracellular glutamate levels over several days (Hascup et al., 2008; Rutherford et al., 2007) without the effects of anesthesia that are known to alter extracellular glutamate levels (Rutherford et al., 2007). The recordings of glutamate were carried out in the infralimbic region of the PFC, a clinically relevant brain area (Dunayevich et al., 2008; Pratt, 1992), to determine how LY354740 or (+)-TFMPIP affect restraint stress-evoked changes in extracellular glutamate.
2. Materials and Methods
2.1 Animals
Male Sprague Dawley rats (351.8 ± 7.7 grams at experiment commencement) were obtained from NOVA-SCB (Scanbur, Sweden). Animals were housed in pairs in a 12 hour light/dark cycle at a constant room temperature of 23 °C with ad libitum access to food and water. Animals were treated in accordance with protocols approved by the animal Ethical Committee of Stockholm, the Federation of European Laboratory Animal Science Associations and all procedures were conducted in conformity with the Karolinska Institutet’s Guidelines. Efforts were made in order to minimize the number of animals used and reduce their suffering. Animals were allowed at least one week to acclimate to the testing environment and researchers prior to experiments. All appropriate animal care was performed by the animal resource center staff. Following implantation surgery, rats were individually housed under the same conditions.
2.2 Microelectrode Arrays for Measures of Glutamate
MEAs were assembled and selected for in vivo recordings as previously described (Burmeister et al., 2002, 2000). Preparation of the MEA for recording has been extensively described in Hascup et al., 2006 and Burmeister et al., 2002. Briefly, the platinum recording sites were dip coated with Nafion® (Sigma-Aldrich Corp., St. Louis, MO) to repel anions (Burmeister and Gerhardt, 2001). Two of the MEA recording sites were coated with an L-glutamate oxidase (EC 1.4.3.11; Seikagaku America, Inc., East Falmouth, MA; GluOx) coating solution (Nickell et al., 2005). In order to induce cross-linking of GluOx and increase adhesion to the MEA, glutaraldehyde and BSA (Sigma-Aldrich Corp., St. Louis, MO) were added to the GluOx solution. The GluOx layer is required to measure glutamate, as it causes the enzymatic break-down of glutamate to α-ketoglutarate and the electroactive reporter molecule, H2O2. When a potential of +0.7 V vs. a Ag/AgCl reference electrode was applied, H2O2 was oxidized yielding two electrons at the MEA platinum recording surface per molecule of glutamate oxidized by the enzyme. The resulting current was amplified and recorded by a FAST16 mkI recording system (Quanteon, LLC, Nicholasville, KY). The remaining two MEA recording sites (self-referencing or sentinel sites) were coated similar to the glutamate recording sites, except the coating solution did not contain GluOx. This meant the sentinel sites could not record glutamate but could record any electroactive interferents not repelled by Nafion®. When the current recorded on the sentinel sites was subtracted from the current on the glutamate recording sites, the resulting signal represents resting glutamate levels in brain tissue or tonic glutamate release (Burmeister and Gerhardt, 2001; Burmeister et al, 2002; Hascup et al., 2010; Hascup et al., 2011). For details on preparing and constructing the head pedestal (consisting of the MEA, miniature connector, connecting wires, and the miniature Ag/AgCl reference electrode) see Hascup et al., 2006, Rutherford et al., 2007, Hascup et al., 2008, Hascup et al., 2009, Hascup et al., 2010, and Hascup et al., 2011.
2.3 MEA Calibration
MEAs were calibrated to determine their sensitivity and selectivity against ascorbic acid as previously described (Nickell et al., 2005). Briefly, constant potential amperometry was performed using a FAST16 mkI system designed for recording simultaneously from the four, independent channels with a final gain of 200 pA/V (at +0.7 V vs. a Ag/AgCl reference electrode). The tip of the modified MEA was placed in a continuously stirred solution of 0.05 M PBS (pH 7.4). A recirculating water bath (Gaymar Co.) maintained a constant calibration temperature of 37°C to allow the enzyme layer to function optimally relative to in vivo conditions. Calibrations were performed using a final buffer concentration of 250 µM ascorbic acid from a stock solution of 20 mM ascorbic acid and stepwise additions of 20 mM glutamate yielding 20, 40, and 60 µM final glutamate concentrations, respectively. Selectivity ratios for glutamate over ascorbic acid were calculated in addition to the slope (sensitivity), limit of detection (LOD, based on a signal-to-noise ration of 3:1), and linearity (R2) for glutamate for all MEAs. The MEAs were also tested with dopamine (2 µM final concentration) and H2O2 (8.8 µM final concentration) as test substances. MEAs were only used if the Pt recording sites had in vitro responses to H2O2 within 20% of each other. For this study, we used 32 MEAs consisting of 62 functioning glutamate recording sites. MEAs had an average sensitivity of 5.3 ± 0.3 pA/µM, selectivity ratio of 84.2 ± 18.9 to 1, and LOD of 0.3 ± 0.04 µM.
2.4 MEA Pedestal Implantation
Following a successful calibration of the MEA, rats were anesthetized with 2–3 % isoflurane, and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Animal body temperature was maintained at 37°C with a heating pad (Braintree Scientific, Braintree, MA). The animals’ eyes were coated with artificial tears (The Butler Company, Columbus, OH) to help maintain moisture and prevent infection. Prior to incision, the skin directly on top of the animals head was wiped with Betadine solution to keep the incision area clean and to prevent infection. The skin on top of the rat’s head was reflected. Three small holes were drilled in the skull in the adjacent quadrants of where the MEA was implanted for placement of stainless steel scull screws. A fourth hole was drilled contralateral from the recording site for insertion of the miniature Ag/AgCl reference electrode. Next, three small stainless steel screws (Small Parts, Inc) were threaded into the skull to serve as anchors and care was taken so that the screw tips did not touch brain tissue. A 2 mm×2 mm craniotomy was performed over the right PFC and a glutamate selective MEA pedestal assembly was implanted in the PFC (MEA tip coordinates: AP: +3.2 mm; ML: 0.8 mm, DV: −5.0 mm vs. bregma) with the incisor bar set so that the skull was level (approximately −2.3 mm), based on the atlas of Paxinos and Watson (2007). The assembly was secured with approximately four layers of dental acrylic (Lang Dental MFG, Wheeling, IL), making sure to cover as much of the MEA as possible. The dental acrylic had a smooth texture and excess acrylic was removed from the skin surface. Rats were allowed to recover for three days prior to initial recordings.
2.5 Recording Protocol
Typical recording sessions involved allowing the rat to freely roam around the recording chamber for 30 minutes to acclimate to the surroundings before connecting the pedestal to the RatHat amplifier and starting data acquisition. Once the recordings were started, the rat was given a minimum additional 90 minute acclimation period, or until a stable baseline was established on the recording and sentinel sites. Once a stable baseline was obtained, rats received s.c. injections (2 ml/kg) of either vehicle (5% DMSO and 5% cremophor in 0.9% physiological saline), LY354740 (10 mg/kg), (+)-TFMPIP (1 mg/kg), or (+)-TFMPIP (17.8 mg/kg) on days 3 and 5 post-implantation (rats received the same injection on day 5 as on day 3). LY354740 and (+)-TFMPIP were synthesized at Pfizer Central Research, Groton, CT. Doses were based on previously published reports in rats (Schoepp et al., 2003; Zhang et al., 2011), and expressed as free base of the drug. One hour following the s.c drug injection, rats underwent a 5 minute restraint stressor, where rats were wrapped in a restrictive towel and held continuously for five minutes. Rats were weighed on days 0, 3, 4, and 5 post-implantation. Following recording sessions on day 5, rats were anesthetized with isoflurane followed by decapitation.
2.6 Data Analysis
The FAST16 mkI system recorded amperometric data averaged to 1 Hz resolution for all four recording channels. Unless otherwise noted, calibration data, in conjunction with a MATLAB graphic user interface program developed by Quanteon, LLC (Lexington, Kentucky), was used to calculate resting glutamate. Resting glutamate levels were calculated by taking a 60 second baseline average prior to drug treatments so fluctuations from spontaneous increases or decreases in glutamate spillover did not lead to inaccurate resting glutamate level determinations. All other analyses were performed using GraphPad Prism® software. A one-way analysis of variance (ANOVA) followed by a Tukey’s post-hoc or a two-way ANOVA followed by a post-hoc Bonferroni correction was used as indicated. Data are shown as mean ± standard error of the mean (SEM) and significance is defined as p < 0.05.
Results
Effect of Drug on Rat Body Weight
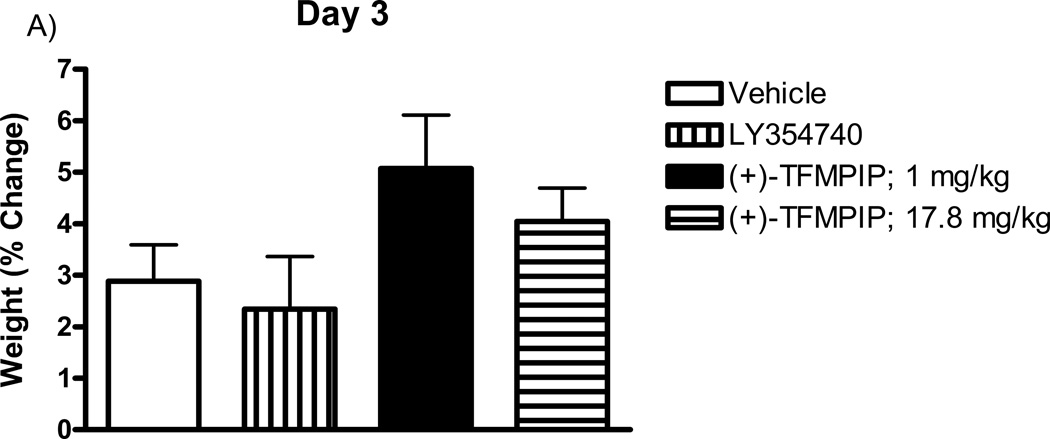
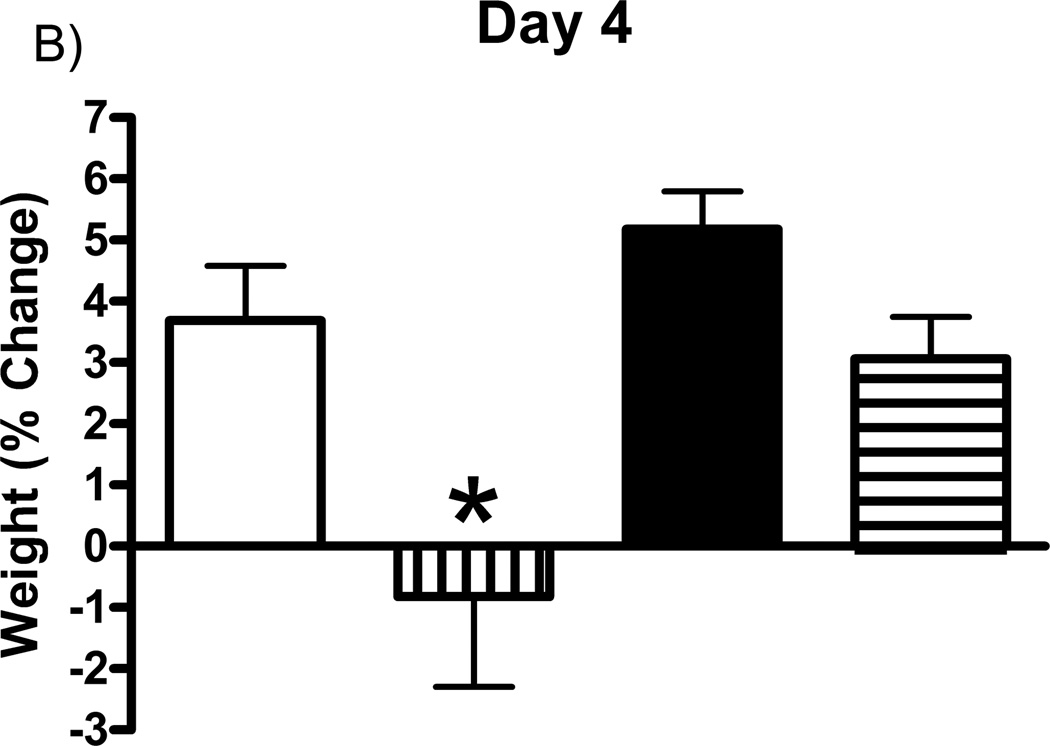
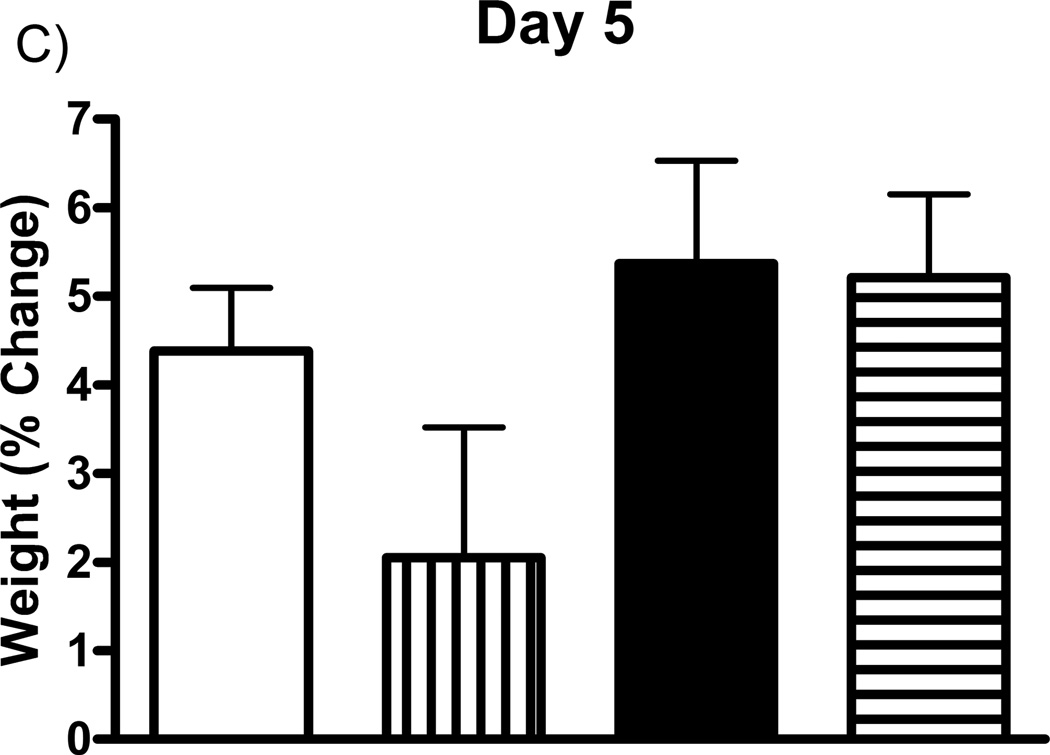
Prior studies have shown that the mGluR2/3 agonists can cause nausea (Dunayevich et al., 2008). Therefore, studies first addressed the effects of the drugs on body weight. Rat weights were recorded on days 3, 4, and 5 post-implantation, prior to glutamate recording sessions and drug administration on each day, and expressed as a percentage of their day 0 weight (immediately following MEA implantation). Each group (one drug per group) of rats received subcutaneous injections of either vehicle (5% cremophor, 5% DMSO, in 0.9% physiological saline), LY354740 (10 mg/kg), (+)-TFMPIP (1 mg/kg), or (+)-TFMPIP (17.8 mg.kg) on days 3 and 5 post-implantation. There were no significant differences in weight observed in rats receiving either dose of (+)-TFMPIP (1 mg/kg or 17.8 mg/kg) compared to vehicle on any of the three days tested (Figure 2). There was also no difference in weight on day 3 post-implantation between vehicle and LY354740 rats (Figure 2A). However, there was a significant (*p<0.05) decrease in weight on day 4 post-implantation in rats receiving the LY354740 drug (−0.8 ± 1.5%) approximately 24 hours prior compared to vehicle treated rats (3.7 ± 0.9%; Figure 2B). However, on day 5 post-implantation, approximately 48 hours after drug administration, the weight loss was regained and was within normal levels compared to vehicle treated rats (Figure 2C).
Figure 2.
Effects of systemic drug administration on whole animal weights. Male Sprague-Dawley Rats were weighed immediately following MEA implantation on day 0 and prior to recording sessions on days 3, 4, and 5. On days 3 and 5, rats received a single subcutaneous injection (2 ml/kg) of either vehicle (n=8), LY354740 (10 mg/kg; n=9), (+)-TFMPIP (1 mg/kg, n=7), or (+)-TFMPIP (17.8 mg/kg, n=9). Weights on days 3 (A), 4 (B), and 5 (C) were expressed as a percentage of their day 0 weight. Rats treated with LY354740 showed significant weight loss on day 4. *p<0.05 based on a one-way ANOVA with a Tukey’s post-hoc test.
Effects of Drugs on Tonic or Resting Levels of Glutamate
Stable baseline recordings were established in all rats prior to subcutaneous administration of either vehicle, LY354740 (10 mg/kg), (+)-TFMPIP (1 mg/kg), or (+)-TFMPIP (17.8 mg.kg) on days 3 and 5 post-implantation. By subtracting the self-referencing site values from the glutamate recording site values we were able to determine tonic, or resting, glutamate levels. Sixty second averages immediately prior to drug injection were used to determine tonic glutamate (day 3: 11.2 ± 1.7 µM; day 5: 10.2 ± 2.1 µM). These values were then used to determine the change in glutamate levels compared to a 60 second average 1 hour following drug administration. No significant changes were observed in any of the treatment groups (Figure 3). Thus, we observed no changes in tonic glutamate release following drug administrations.
Figure 3.
Effects of mGluR drugs on resting glutamate levels. Resting glutamate levels in PFC were recorded for 60 minutes following a single subcutaneous injection (2 ml/kg) of either vehicle (n=6), LY354740 (10 mg/kg; n=8 (day 3), 6 (day 5)), (+)-TFMPIP (1 mg/kg, n=7 (day 3), 6 (day 5)), or (+)-TFMPIP (17.8 mg/kg, n=6). The difference in pre-injection and 60 minute post-injection glutamate values were examined on days 3 and 5 post implantation. No significant differences were observed for any treatment group compared to vehicle on either day or between days 3 and 5 within a treatment group (based on a 2-way ANOVA).
Effect of Drugs on Glutamate Release Caused by Restraint Stress
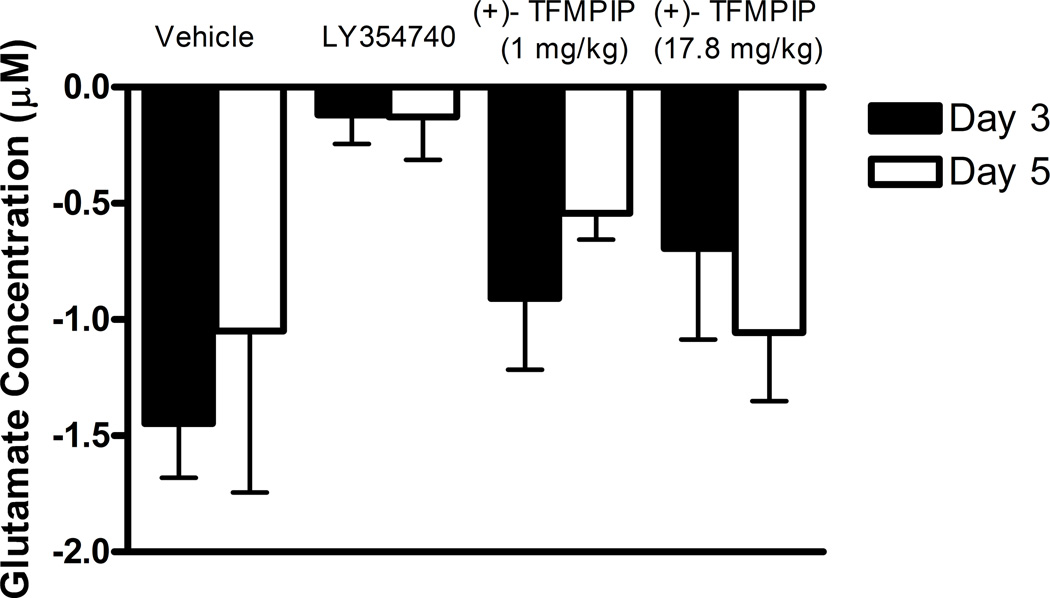
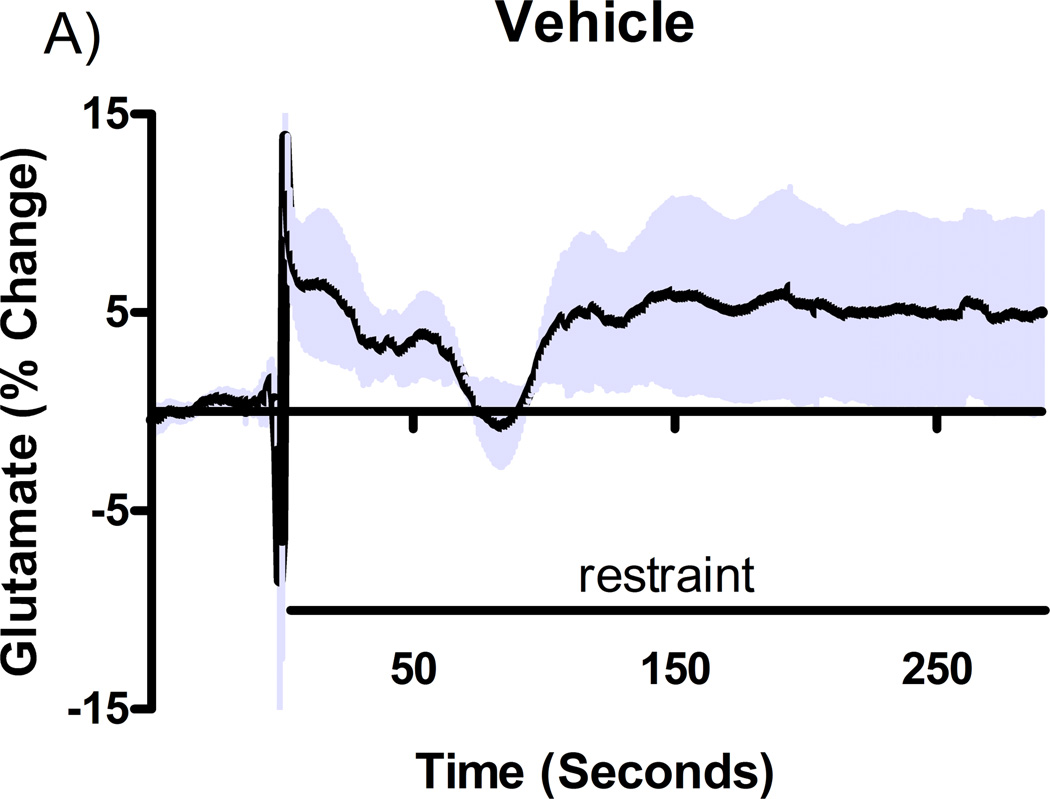
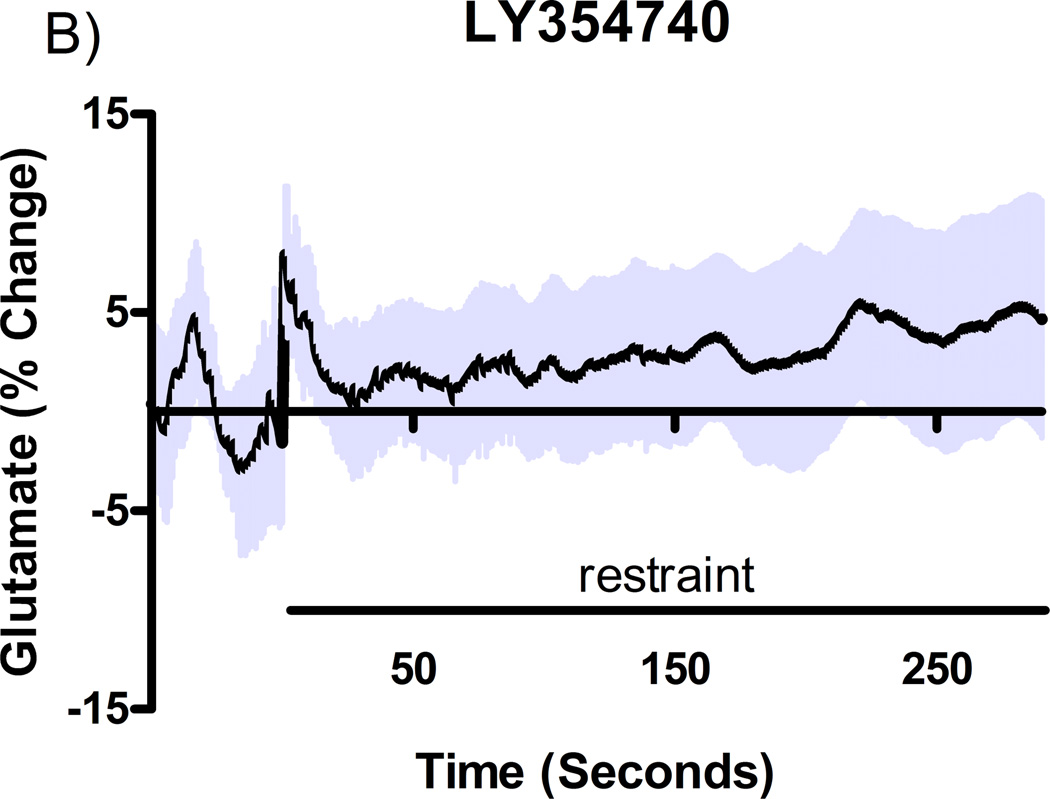
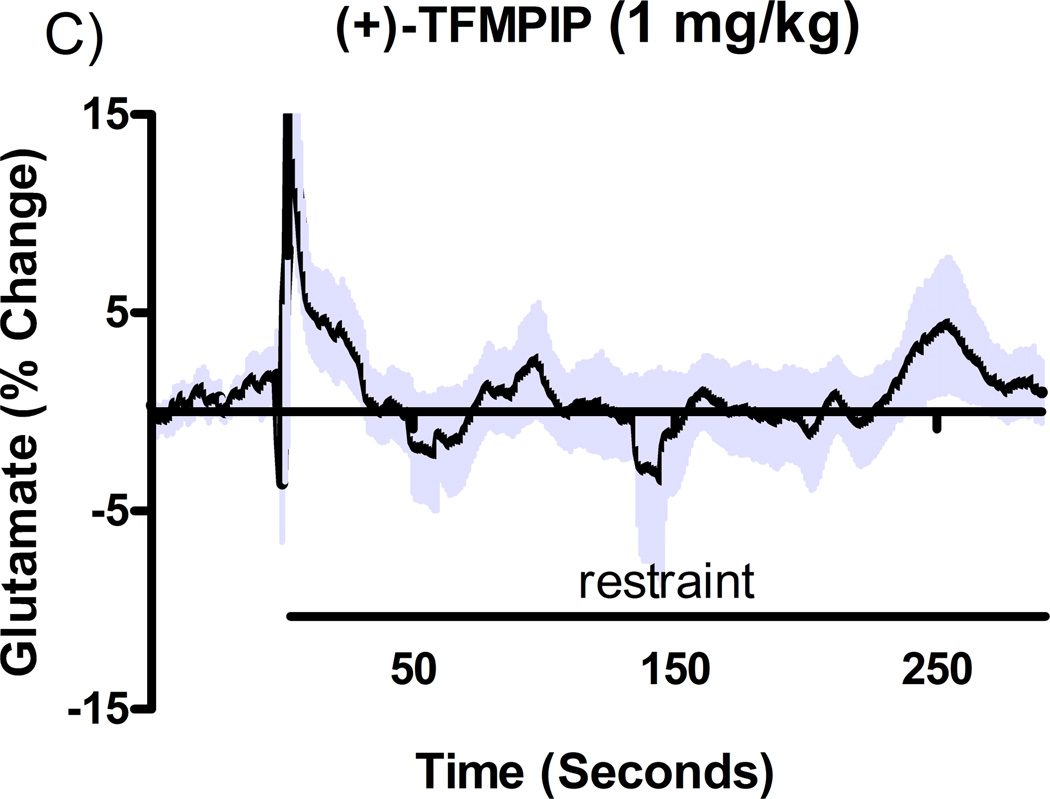
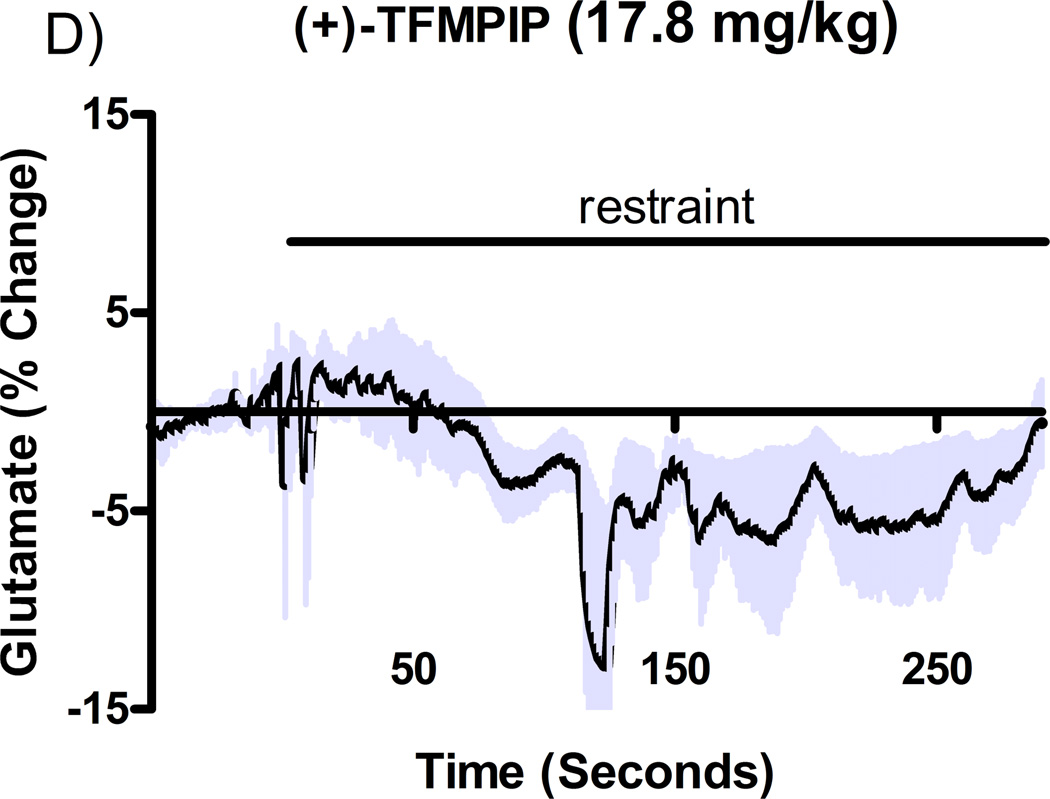
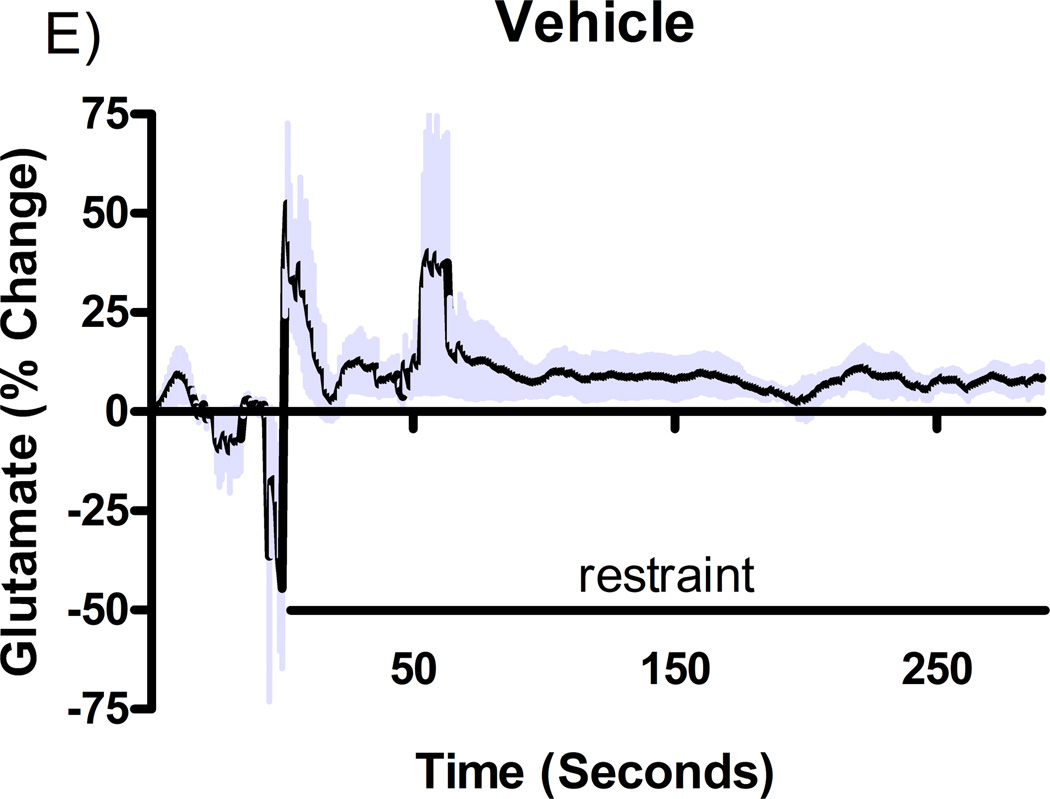
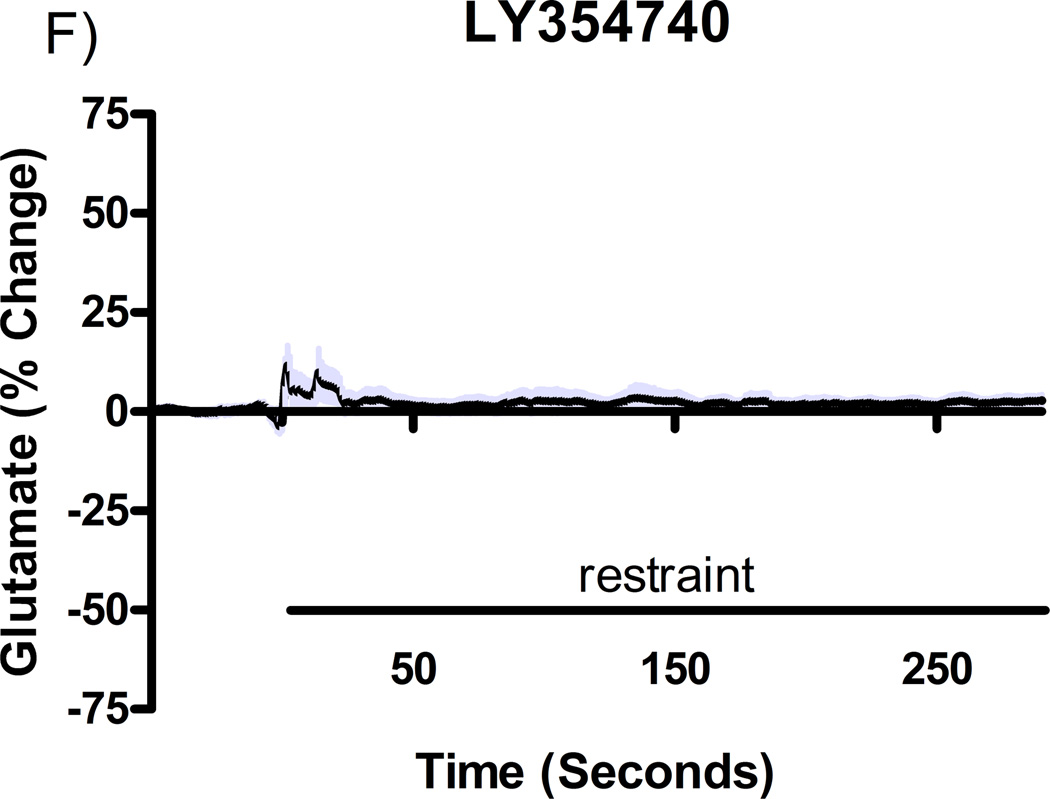
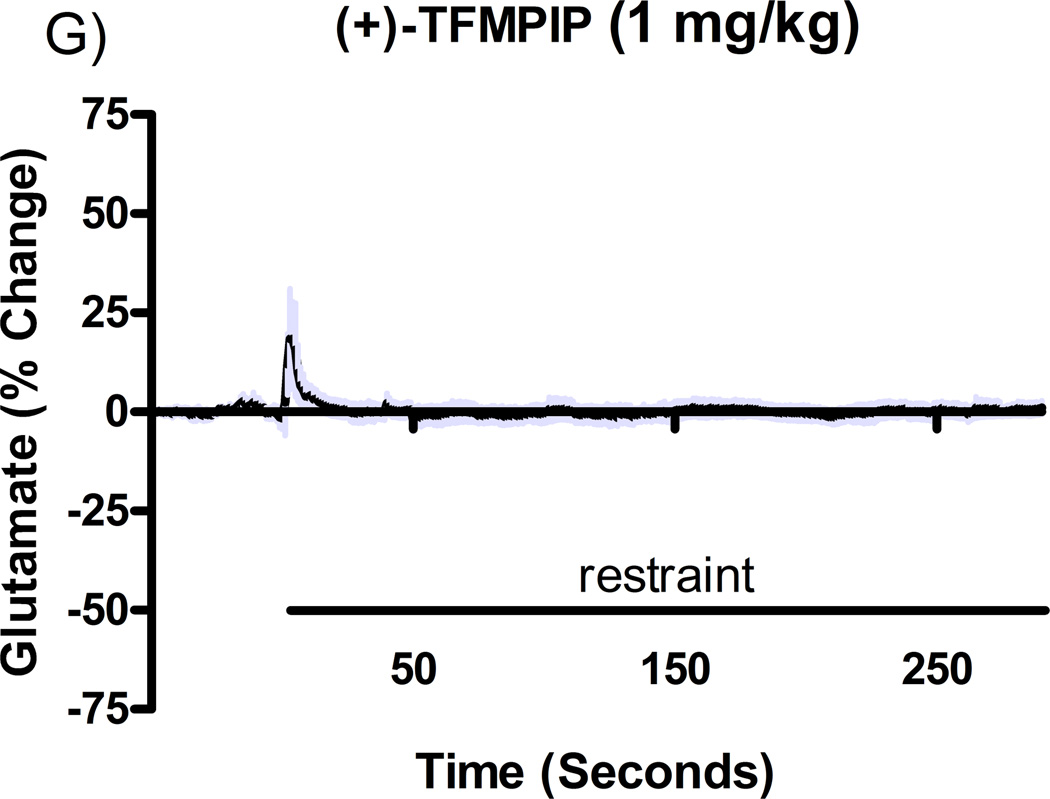
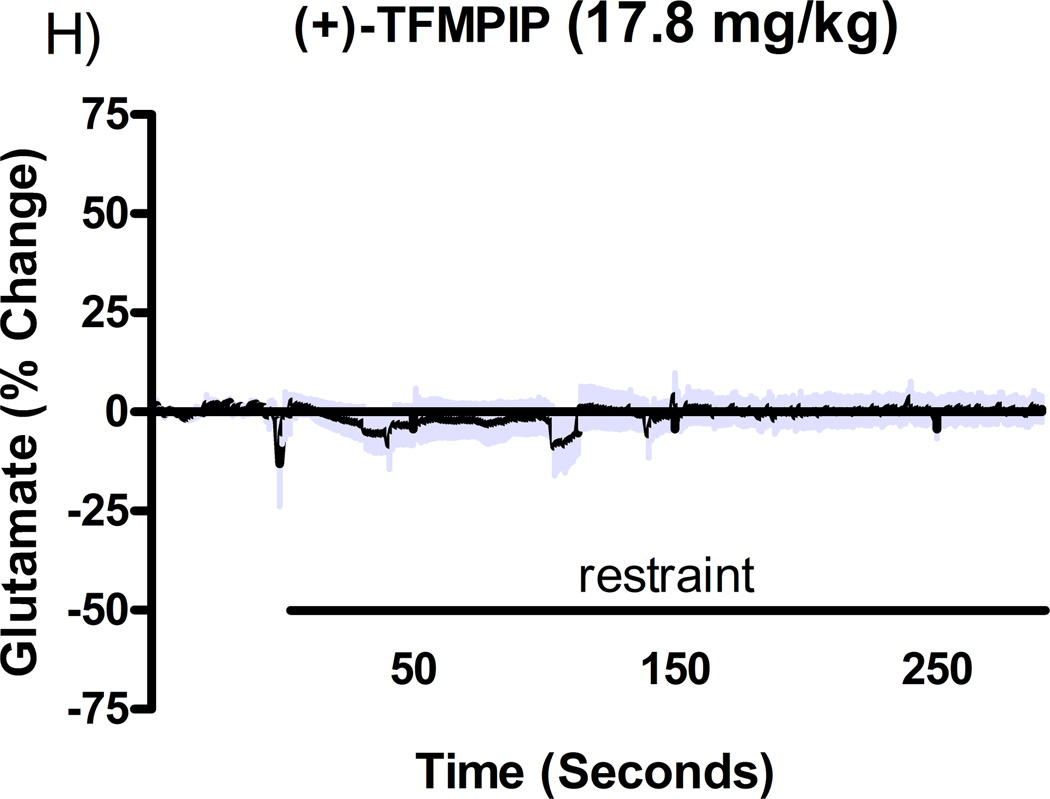
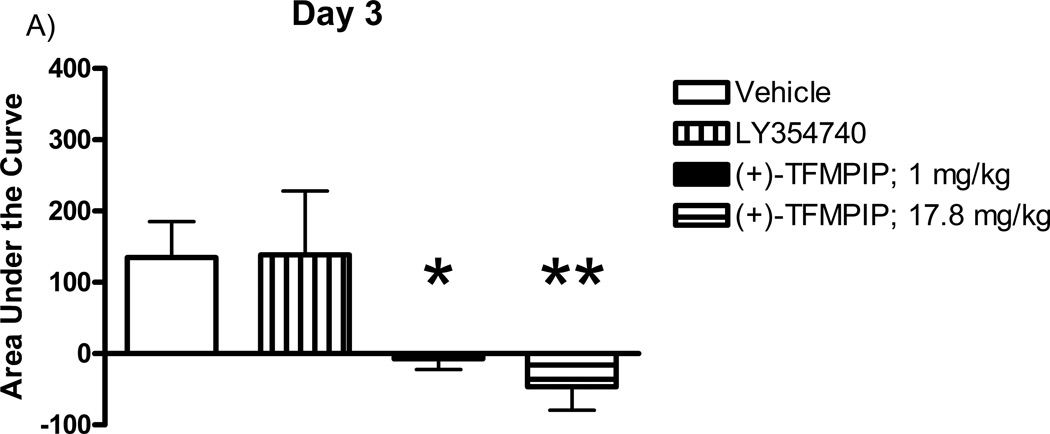
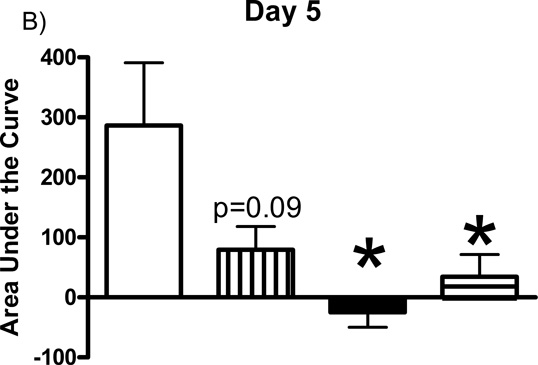
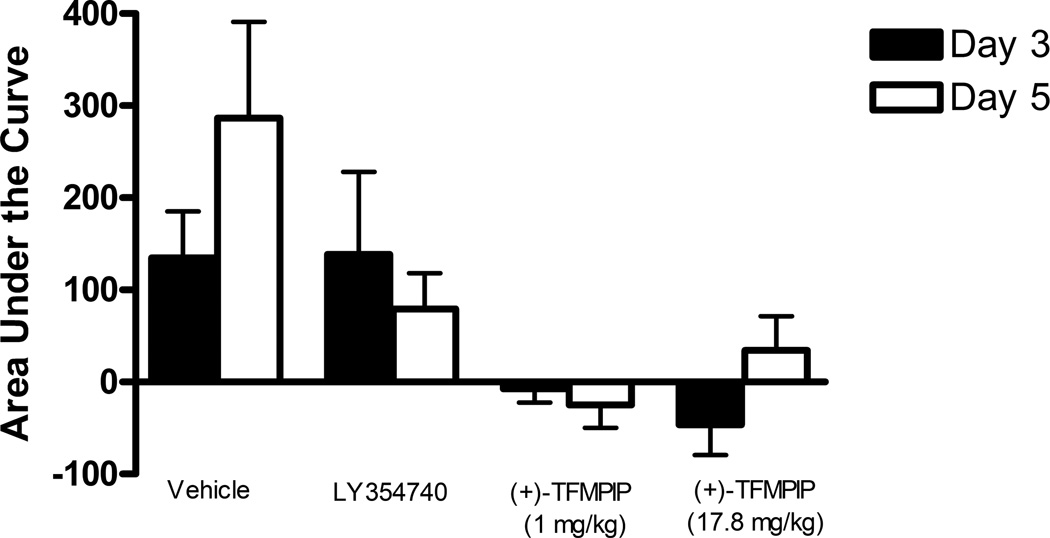
Additional studies were carried out to determine if the mGluR2/3 drugs had effects on restraint stress-induced glutamate release. Following measures of the baseline or tonic release of glutamate, we performed a five minute restraint stress 1 hour after subcutaneous injections of either vehicle, LY354740 (10 mg/kg), (+)-TFMPIP (1 mg/kg), or (+)-TFMPIP (17.8 mg.kg) on days 3 and 5 post-implantation. Separate animal groups were studied for the 4 treatment groups. Figures 4 and 5 show the average glutamate signals (solid line) and SEM (shaded area) from each of the four groups on days 3 and 5 post-implantation, respectively. There was an obvious difference in the dynamics of extracellular glutamate levels in the control animals as compared to the LY354740 and (+)-TFMPIP (17.8 mg/kg) treated rats on day 3 post-implantation (Figure 4A, 4B, 4D), with the highest dose of (+)-TFMPIP showing the most dramatic decrease compared to vehicle control. On day 5 post-implantation, the glutamate dynamics during the restraint stress appear different in all three experimental drug groups compared to vehicle (Figure 4E-H). Notably, a large decrease was observed in control animals on day 3 immediately prior to the restraint stress. This corresponds temporally with the experimenter opening the recording chamber door. The decrease was also observed in control animals on day 5, however, the decrease was much smaller compared to day 3. Quantitation of these signals involved examination of the net area under the curve for the entire 5 minutes of the restraint stress. On day 3, we observed significantly decreased net area in (+)-TFMPIP (1 mg/kg; −7.1 ± 15.1; *p<0.05) and (+)-TFMPIP (17.8 mg/kg; −46.5 ± 33.0; **p<0.01) treated rats compared to vehicle treated animals (134.7 ± 50.6) (Figure 5A). We also observed significant decreases in net area stress-induced glutamate release on day 5 from the low dose of (+)-TFMPIP (1 mg/kg; −24.8 ± 24.9; *p<0.05) and the higher dose of (+)-TFMPIP (17.8 mg/kg; 34.6 ± 36.8; *p<0.05) compared to vehicle (286.6 ± 104.5), and a trend of decreased net area with LY354740 (p=0.09) compared to vehicle (Figure 5B). Interestingly, similar to tonic glutamate levels, the effects of restraint stress tended to be larger, but were not significantly different, in the vehicle treated group at day 5 compared to day 3, as well as in the high (+)-TFMPIP (17.8 mg/kg) treated rats (Figure 6). This could be due to a sensitization or priming effect. However, no significance was observed between days 3 and 5 within treatment groups (based on a within group paired t-test for each treatment group, α=0.05).
Figure 4.
Phasic glutamate release after stress #1 (A-D) and after stress #2 (E-H). Shown are average traces (solid line) ± SEM (shaded area) of the glutamate response to a 5 minute restraint stress (indicated by solid bar) applied 60 minutes following a single subcutaneous injection (2 ml/kg) of either vehicle (A, E; n=6), LY354740 (B, F; 10 mg/kg; n=8), (+)-TFMPIP (C, G; 1 mg/kg; n=6), or (+)-TFMPIP (D, H; 17.8 mg/kg; n=6, 5, respectively) on day 3 post-implantation (A-D) or day 5 post-implantation (E-H). On day 3, glutamate release during the stressor was inhibited by the rats treated with (+)-TFMPIP (17.8 mg/kg). On day 5, glutamate release was affected in all treatment groups compared to vehicle control.
Figure 5.
Phasic glutamate release area for the duration of the 5 minute restraint stress, which occurred 60 minutes following a single subcutaneous injection (2 ml/kg) of either vehicle (n=6), LY354740 (10 mg/kg; n=8 (day 3), n=6 (day 5)), (+)-TFMPIP (1 mg/kg, n=6), or (+)-TFMPIP (17.8 mg/kg, n=6) on day 3 (A) and 5 (B) post-implantation. There was no change in stress response in the LY354740 treated rats. However, both doses of the (+)-TFMPIP drug produced significant decreases in the stress response on days 3 and 5. *p<0.05, **p<0.01 based on a one-way ANOVA with a Tukey’s post-hoc test.
Figure 6.
Phasic glutamate area for the duration of the 5 minute restraint stress, which occurred 60 minutes following a single subcutaneous injection (2 ml/kg) of either vehicle (n=6), LY354740 (10 mg/kg; n=8 (day 3), n=6 (day 5)), (+)-TFMPIP (1 mg/kg, n=6), or (+)-TFMPIP (17.8 mg/kg, n=6) comparing days 3 and 5 post-implantation. Paired t-tests were performed to determine changes within treatment groups. No significant differences were observed within treatment groups for any of the four treatments on days 3 and 5.
Discussion
Stress is a complicated phenomenon in animals and humans and glutamate neurotransmission is clearly involved with the CNS manifestations of stress. In these studies we used a novel recording technique that allows for accurate and reliably measurements of both tonic and phasic changes in glutamate in the PFC of behaving animals. We used this improved technology to investigate the potential anxiolytic effects of (+)-TFMPIP, a novel PAM of the mGluR2. Further, the effects of (+)-TFMPIP were compared to the mGluR2/3 agonist LY354740. Similar to results reported on another mGluR group II, LY487379 (Nikiforuk et al., 2010), resting glutamate levels were unchanged 1 hour following injections of drugs or vehicle on days 3 and 5, indicating that neither LY354740 nor (+)-TFMPIP had a significant effect on tonic glutamate levels in the PFC when given systemically. We also examined the effects of LY354740 and (+)-TFMPIP on the phasic glutamate release by applying a five minute restraint stressor one hour following the subcutaneous injection of either of the compounds. Our data support that (+)-TFMPIP (1 or 17.8 mg/kg) produced a significant reduction in stress-induced glutamate that was the greatest on day 5 of our studies. Our recent studies have shown as well that tail pinch stress-induced release of glutamate is abolished following locally-applied tetrodotoxin, supporting the neuronal origin of the glutamate signals recorded by the MEA technology (Hascup et al., 2010). Thus, these data suggest that the novel PAM drug is affecting transient glutamate regulation and not the resting or tonic levels of glutamate.
We also examined the stress response quantitatively using the net area of the stress-induced glutamate signal. There were no significant changes in area under the curve in rats receiving LY354740 on days 3 or 5 compared to vehicle controls. However, both doses of the novel PAM drug, (+)-TFMPIP (1.0 and 17.8 mg/kg) resulted in significant decreases in phasic glutamate on days 3 and 5 compared to vehicle controls, further supporting the potential role of (+)-TFMPIP as an anxiolytic treatment. Interestingly, similar to tonic glutamate findings, (+)-TFMPIP (17.8 mg/kg) tended to produce a greater decrease in the total amount of stress induced glutamate release on day 5 compared to day 3. These increases observed in glutamate peak area, or the change in glutamate associated with the stress, were much more dramatic than those seen from tonic glutamate. Although not significant, LY354740 and (+)-TFMPIP (1.0 mg/kg) were both slightly more effective at diminishing the stress response on day 5 compared to the same treatments on day 3. Taken together, these data support that the mGluR2 PAM, (+)-TFMPIP, was more effective than the mGluR2/3 agonist, LY354740, at alleviating stress-induced glutamate release in the PFC of awake rats.
Concerns with new pharmacotherapies are adverse events, which can be difficult to monitor in animal models. One common adverse event is weight loss.We observed a significant weight loss with 10 mg/kg dose of LY354740 (−0.8 ± 1.5%) compared to vehicle treated rats (*p<0.05, 3.7 ± 0.9%) 24 hours post-treatment. To our knowledge, this post-treatment weight loss has not been previously reported in animals. But, a recent double blind, placebo-controlled clinical trial of LY354740 found that nausea was the most prevalent treatment-emergent adverse event in patients suffering from generalized anxiety disorder (Dunayevich et al., 2008). The prevalence of nausea increased with dose and was double to triple that of patients receiving placebo. Unfortunately, the weight loss in rats treated with 10 mg/kg of LY354740 was an unexpected finding and food consumption was not monitored in this study. We hypothesize that rats treated with LY354740 were nauseous and would not eat thus contributing to the weight loss. No weight loss was observed in rats receiving either the low or high dose of (+)-TFMPIP. Regardless, the fact that rats receiving subcutaneous injection of (+)-TFMPIP showed significantly reduced stress-induced glutamate release in the PFC coupled with no change in weight indicates this mGluR2 PAM may provide anxiolytic activity without nausea as an adverse event.
This was the first study using second-by-second recording methods to examine the effects of LY354740 on the in vivo extracellular concentrations of glutamate in the rat PFC. Our data showed that subcutaneous injection of LY354740 (10 mg/kg) did not affect resting glutamate levels compared to controls nor did it significantly attenuate glutamate release from immobilization stress (a rodent model of anxiety), which is in contrast to other reports. Marek and colleagues (2000) demonstrated that LY354740 could block evoked excitatory glutamate responses in layer V pyramidal cell of the PFC in a dose-dependent manner and this was thought to underlie its effect in behavioral models of anxiety and stress (Schoepp et al., 2003). Also, Helton and colleagues (1998) demonstrated that oral administration of LY354740 suppresses expression of fear-potentiated startle (a human model of anxiety) in rats without disrupting acquisition or retention of memory. However, Linden and colleagues (2004) showed that subcutaneous injection of LY354740 increased c-fos expression in the PFC and several other brain regions associated with stress and sensory perception. They explained that the c-fos induction was due to an undetermined effect of LY354740 on the central nervous system (Linden et al., 2004). Since subcutaneous injection of LY354740 induces c-fos expression, this may explain why stress-induced glutamate release after administration of LY354740 was similar to vehicle controls.
In summary, we found that LY354740 treatment did not significantly affect tonic glutamate levels or restraint stress-induced release of glutamate in the prefrontal cortex of awake rats. However, the fact that either dose of (+)-TFMPIP reduced stress-evoked glutamate release, without altering resting glutamate levels, may indicate that this compound is a potential candidate for use as an anxiolytic that may affect anxiety without producing unwanted effects on glutamate signaling in cortical areas. Future studies are needed to better understand the effects of novel glutamate drugs, such as the mGluR2 PAM, (+)-TFMPIP and their potential use for the treatment of anxiety and other disorders of the CNS.
Acknowledgements
Disclosures
This project was funded by Quanteon, LLC and Pfizer, Inc. Erin R. Hascup has no disclosures. Kevin N. Hascup was employed by Quanteon, LLC. Francois Pomerleau and Pete Huettl have received compensation for services provided for Quanteon, LLC and Pfizer, Inc. Eva Hajos-Korcsok is employed by Pfizer, Inc. Jan Kehr is the sole proprieter of Pronexus Analytical, AB and the company has received financial compensation from Pfizer, Inc. Greg A. Gerhardt is the sole proprietor of Quanteon, LLC, which manufactures and sells the FAST-16 recording system used to measure glutamate in these studies. Greg A. Gerhardt has received compensation from Pfizer, Inc.
Abbreviations
- PFC
prefrontal cortex
- MEA
microelectrode area
- (+)-TFMPIP
1-Methyl-2-((cis-(R,R)-3-methyl-4-(4-trifluoromethoxy-2-fluoro)phenyl)piperidin-1-yl)methyl)-1H-imidazo[4,5-b]pyridine
References
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Bonnefous C, Vernier JM, Hutchinson JH, Gardner MF, Cramer M, James JK, et al. Biphenyl-indanones: allosteric potentiators of the metabotropic glutamate subtype 2 receptor. Bioorg Med Chem Lett. 2005;15:4354–4358. doi: 10.1016/j.bmcl.2005.06.062. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. Ceramic-based multisite microelectrodes for electrochemical recordings. Anal Chem. 2000;72:187–192. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J Neurosci Methods. 2002;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Coates TD, Gerhardt GA. Multisite microelectrode arrays for measurements of multiple neurochemicals. Conf Proc IEEE Eng Med Biol Soc. 2004;7:5348–5351. doi: 10.1109/IEMBS.2004.1404493. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, et al. Higher Levels of Glutamate in the Associative-Striatum of Subjects with Prodromal Symptoms of Schizophrenia and Patients with First-Episode Psychosis. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2008;33:1603–1610. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- Estanislau C, Ramos AC, Ferraresi PD, Costa NF, de Carvalho HM, Batistela S. Individual differences in the elevated plus-maze and the forced swim test. Behav Processes. 2011;86:46–51. doi: 10.1016/j.beproc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Witkin JM, Falcone JF, et al. N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther. 2011;3361:165–177. doi: 10.1124/jpet.110.172957. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Netto CF, Zangrossi H., Jr The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev. 1998;23:237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Hascup ER, af BS, Hascup KN, Pomerleau F, Huettl P, Stromberg I, et al. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009;1291:12–20. doi: 10.1016/j.brainres.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A, et al. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell J, Pomerleau F, et al. : Second-by-Second Measures of l-glutamate and other neurotransmitters using enzyme-based microelectrode arrays. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton: CRC Press; 2006. pp. 407–450. [PubMed] [Google Scholar]
- Hascup KN, Hascup ER, Pomerleau F, Huettl P, Gerhardt GA. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J Pharmacol Exp Ther. 2008;324:725–731. doi: 10.1124/jpet.107.131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Hascup ER, Stephens ML, Glaser PE, Yoshitake T, Mathe AA, et al. Resting Glutamate Levels and Rapid Glutamate Transients in the Prefrontal Cortex of the Flinders Sensitive Line Rat: A Genetic Rodent Model of Depression. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- Hu E, Chua PC, Tehrani L, Nagasawa JY, Pinkerton AB, Rowe BA, et al. Pyrimidine methyl anilines: selective potentiators for the metabotropic glutamate 2 receptor. Bioorg Med Chem Lett. 2004;14:5071–5074. doi: 10.1016/j.bmcl.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Baez M, Jagdmann GE, Jr, Britton TC, Large TH, Callagaro DO, et al. Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2- trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J Med Chem. 2003;46:3189–3192. doi: 10.1021/jm034015u. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, et al. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology (Berl) 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Linden AM, Greene SJ, Bergeron M, Schoepp DD. Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology. 2004;29:502–513. doi: 10.1038/sj.npp.1300321. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32:549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LT, Christopoulos A. Allosteric modulators of G-protein-coupled receptors. Curr Opin Pharmacol. 2003;3:551–556. doi: 10.1016/s1471-4892(03)00107-3. [DOI] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–1335. doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The role of monoamines in the actions of established and"novel" antidepressant agents: a critical review. Eur J Pharmacol. 2004;500:371–384. doi: 10.1016/j.ejphar.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, et al. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Nickell J, Pomerleau F, Allen J, Gerhardt GA. Age-related changes in the dynamics of potassium-evoked L-glutamate release in the striatum of Fischer 344 rats. J Neural Transm. 2005;112:87–96. doi: 10.1007/s00702-004-0151-x. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P, Drescher KU, et al. Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J Pharmacol Exp Ther. 2010;335(3):665–673. doi: 10.1124/jpet.110.170506. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–989. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini M, Berardelli I. Anxiety levels and related pharmacological drug treatment: a memorandum for the third millennium. Ann Ist Super Sanita. 2009;45:193–204. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: stereotaxic coordinates. New York: Academic Press; 2007. [Google Scholar]
- Pratt JA. The neuroanatomical basis of anxiety. Pharmacol Ther. 1992;55:149–181. doi: 10.1016/0163-7258(92)90014-q. [DOI] [PubMed] [Google Scholar]
- Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. New perspectives in glutamate and anxiety. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Rowe BA, Schaffhauser H, Morales S, Lubbers LS, Bonnefous C, Kamenecka TM, et al. Transposition of three amino acids transforms the human metabotropic glutamate receptor (mGluR)-3-positive allosteric modulation site to mGluR2, and additional characterization of the mGluR2-positive allosteric modulation site. J Pharmacol Exp Ther. 2008;326:240–251. doi: 10.1124/jpet.108.138271. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Stromberg I, Gerhardt GA. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J Neurochem. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF. Pharmacological principles of antidepressant efficacy. Hum Psychopharmacol. 2002;17(Suppl 1):S17–S22. doi: 10.1002/hup.399. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Monn JA, Marek GJ, Aghajanian GK, Moghaddam B. LY354740: A systemically active mGlu2/3 receptor agonist. CNS Drug Rev. 1999;5:1–12. [Google Scholar]
- Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- Stevenson CW, Sullivan RM, Gratton A. Effects of basolateral amygdala dopamine depletion on the nucleus accumbens and medial prefrontal cortical dopamine responses to stress. Neuroscience. 2003;116:285–293. doi: 10.1016/s0306-4522(02)00553-5. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JE, Burian LC, Covault J, Conti LH. The effect of restraint stress on prepulse inhibition and on corticotropin-releasing factor (CRF) and CRF receptor gene expression in Wistar-Kyoto and Brown Norway rats. Pharmacol Biochem Behav. 2010;97:227–238. doi: 10.1016/j.pbb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Girotti M, Spencer RL. Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration. Neuroscience. 2007;150:478–486. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- Zhang L, Brodney MA, Candler J, Doran AC, Duplantier AJ, Efremov IV, et al. 1-[(1-methyl-1H-imidazol-2-yl)methyl]-4-phenylpiperidines as mGluR2 positive allosteric modulators for the treatment of psychosis. J Med Chem. 2011;54:1724–1739. doi: 10.1021/jm101414h. [DOI] [PubMed] [Google Scholar]