Abstract
Delayed or failed engraftment remains a concern after cord blood transplantation (CBT) even when using double-unit grafts. Therefore, we analyzed the association between bone marrow (BM) assessment performed approximately 21 days after transplantation and the speed and success of sustained donor-derived neutrophil engraftment in 56 myeloablative double-unit CBT (DCBT) recipients. Overall, the cumulative incidence of sustained neutrophil engraftment was 95% (95%CI:89–100). Of the percentage of myeloid precursors, the BM cellularity, and the total donor chimerism, the total donor chimerism percentage had the most critical association with the speed and success of engraftment. DCBT recipients who were 100% donor achieved a 98% engraftment rate at a median of 22 days. This compared with 100% engraftment in patients who were 90–99% donor but at a delayed median of 29 days, and only 68% engraftment in patients < 90% donor at a median of 37 days (p = 0.001). Multivariate analysis was performed in the sub-group of patients who had not engrafted at the time the BM analysis was performed, the sub-group of most clinical concern. This confirmed donor chimerism was predictive of subsequent neutrophil recovery (p = 0.004). These findings demonstrate the importance of the day 21 BM chimerism determination after DCBT.
INTRODUCTION
Cord blood (CB) is an important alternative hematopoietic stem cell source. However, while CB may have advantages, delayed or failed engraftment remains a concern even with double-unit grafts. Total nucleated cell (TNC), CD34+ cell, and colony-forming unit (CFU) doses as well as human leukocyte antigen match (HLA)-match have been shown to influence neutrophil engraftment in single-unit CB transplantation1–5. In double-unit CB transplantation (DCBT), the infused CD34+ cell and CFU dose of the engrafting unit dictates the speed and success of neutrophil engraftment, although engraftment is also influenced by the total TNC dose (both units combined)6. However, in individual patients it is usually not possible to predict from the graft characteristics on transplantation day whether a patient’s engraftment will be successful. We, therefore, routinely perform bone marrow (BM) analyses approximately 21 days after DCBT to assess engraftment, and now report the association between day 21 BM aspirate and biopsy composition and donor chimerism and the speed and success of sustained donor-derived neutrophil engraftment in 56 myeloablative DCBT recipients.
METHODS
All CBT recipients transplanted during the study period received double-unit grafts7, 8. All consecutive eligible patients were included in this analysis. Eligible patients had hematological malignancies consisting of acute leukemia in morphologic remission, myelodysplasia with < 5% blasts, and Non-Hodgkins lymphoma without morphologic BM involvement at pre-transplant work-up. In addition, they were recipients of first allograft using myeloablative conditioning and underwent BM analysis approximately 21 days after DCBT. Units were selected according to total TNC dose ≥ 1.5 × 107/kilogram (kg)/unit, 4–6/6 HLA-A,-B antigen,-DRB1 allele match, and CB bank6, 9. All patients received fludarabine-based myeloablative conditioning except 3 in whom clofarabine was substituted (as summarized in Table 1), a calcineurin inhibitor with mycophenolate mofetil as immunosuppression, granulocyte colony-stimulating factor, and no anti-thymocyte globulin as previously described6, 9. Patients were transplanted between October 2005 and October 2009. Median follow-up of survivors was 23 months (range 7–57). Informed consent to transplantation and analysis of transplant outcome was obtained.
Table 1.
Patient demographics and graft characteristics.
| Characteristics | N = 56 |
|---|---|
| Median Age (range) | 29.5 years (2–64) |
| N (%) Male | 30 (54%) |
| Median Weight (range) | 68 kg (13–118) |
| N (%) Recipient CMV Seropositive | 28 (50%) |
| N (%) Diagnosis | |
| AML* | 24 (43%) |
| ALL | 15 (27%) |
| MDS or CML | 4 (7%) |
| Lymphoma | 13 (23%) |
| Conditioning | |
| TBI 1320–1375 with Cy/Flu or Thio/Flu | 32 (57%) |
| Cy/Flu/Thio/TBI 400 | 15 (27%) |
| Clo/Mel/Thio** | 3 (5%) |
| Mel/Flu** | 6 (11%) |
| Donor-recipient HLA-Match (N = 112 units) | |
| 6/6 | 3 |
| 5/6 | 59 |
| 4/6 | 50 |
| Median Infused TNC × 107/kg (range) | |
| Larger Unit | 2.7 (1.5–7.3) |
| Smaller Unit | 1.9 (0.9–5.3) |
| Median Infused CD34+ × 105/kg (range) | |
| Larger Unit | 1.1 (0.3–6.4) |
| Smaller Unit | 0.7 (0.1–1.5) |
Abbreviations: N, number; Kg, kilogram; CMV, cytomegalovirus; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; TBI, total body irradiation; Cy, cyclophosphamide; Flu, fludarabine; Thio, thiotepa; Clo, clofarabine; Mel, melphalan; HLA, human leukocyte antigen.
Includes 2 patients with biphenotypic acute leukemia.
The melphalan doses in these regimens were 140 mg/m2.
BM aspirates and biopsies were obtained from the iliac crests at a median of 21 days (range 19–27) after transplantation as clinically appropriate. Wright-Giemsa stained aspirate smears had a 200 cell differential (unless acellular). Biopsies were fixed in buffered formalin followed by decalcification, paraffin embedding, hematoxylin-eosin staining, and analyzed for percentage cellularity (0%, 1%, 5%, 10% and above in 10% increments) and the presence of megakaryocytes. Donor chimerism was determined serially on BM and blood using semi-quantitative polymerase chain reaction (PCR) assays of informative polymorphic short tandem repeats7, 10, 11. BM analyses were performed by hematopathologists blinded to engraftment outcome.
Standard engraftment definitions were used9. Sustained engraftment was defined as sustained donor-derived neutrophil recovery (i.e initial engraftment and no secondary graft failure) with achievement of chimerism ≥ 90% (both units combined). Neutrophil and platelet engraftment were estimated using cumulative incidence with 95% confidence intervals (CI). Death prior to engraftment was the competing risk. The dominant unit was the only one detected or contributed > 50% total chimerism in serial testing. For the purposes of analysis the percentages of myeloid precursors in the aspirate were divided into 0%, 1–50%, and > 50%, and 0%, 1%, and ≥ 5% for biopsy cellularity. The Log Rank statistic was used to estimate statistical significance in univariate analyses, and Cox regression was used to estimate the hazard ratio (HR) and significance of differences between groups in the multivariate analysis.
RESULTS
Patient and graft demographics are summarized in Table 1. The 56 patients (median age 29 years) were transplanted predominantly for acute leukemia or lymphoma. The median infused TNC doses were 2.7 × 107/kg for the larger unit, and 1.9 × 107/kg for the smaller unit, with a donor-recipient HLA-match that was predominantly 5/6 or 4/6.
Two patients had primary graft failure and one had early secondary graft failure (initial engraftment on day 16 and secondary graft failure onset 24 days post-transplant). Two were recipients of high-dose myeloablative conditioning and one was a recipient of intermediate intensity but myeloablative conditioning. Thus, the cumulative incidence of sustained neutrophil engraftment was 95% (95%CI:89–100) and neutrophil recovery occurred at a median of 24 days (range 13–40) after transplantation. The cumulative incidence of day 180 platelet engraftment ≥ 50 × 109/l was 79% (95%CI:68–90).
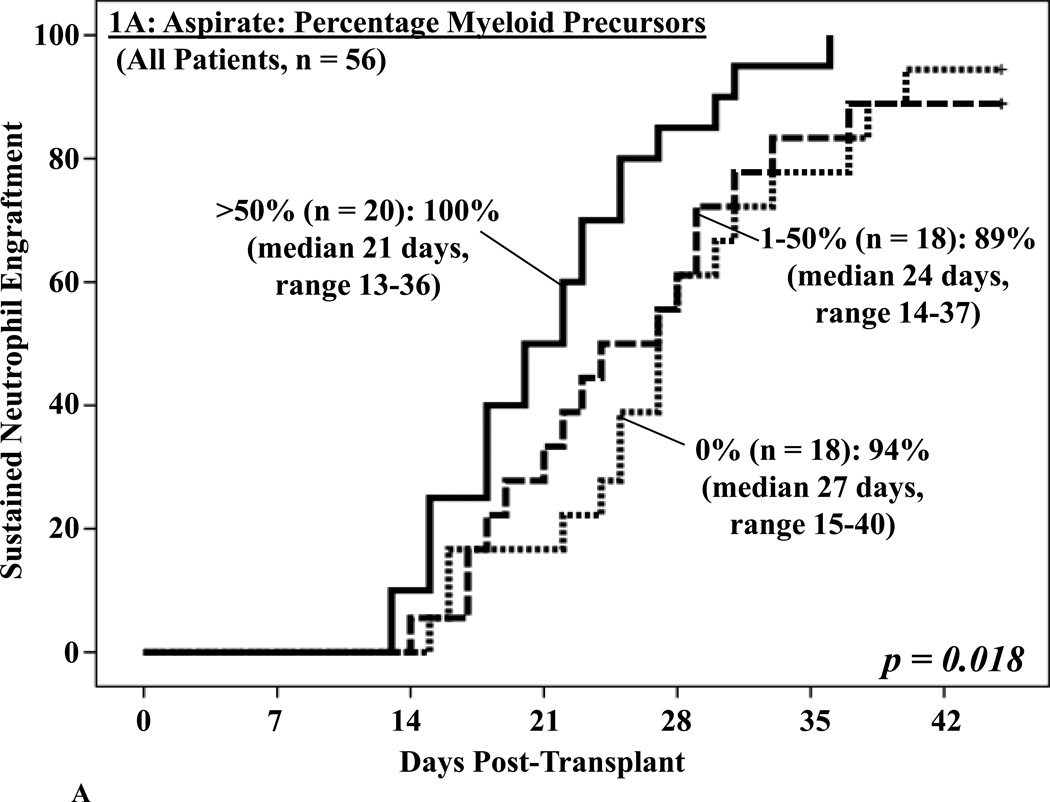
Association between percentage of myeloid precursors in the BM aspirate and neutrophil engraftment
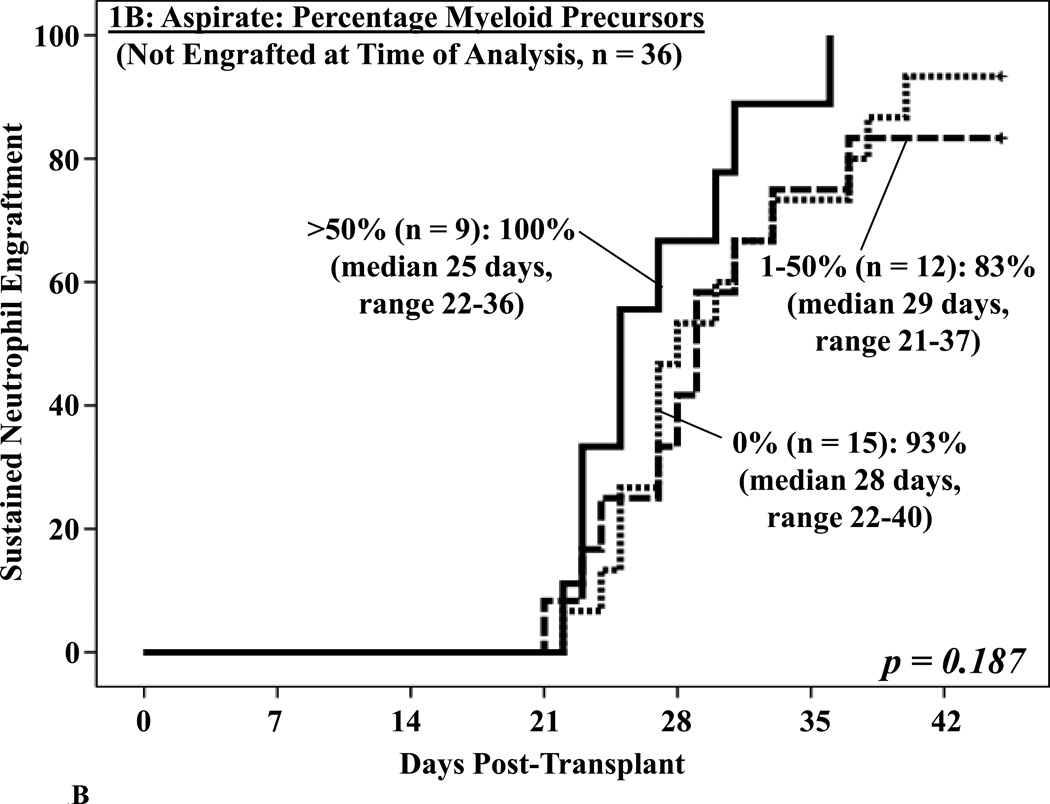
Day 21 aspirates were frequently hypocellular with 18 (32%) being acellular. While the percentages of blasts or lymphoid cells were not associated with engraftment, an aspirate myeloid precursor percentage (granulocytes and granulocyte precursors excluding blasts, median 40%, range 0–87) of > 50% was associated with improved neutrophil engraftment on univariate analysis (p = 0.018, Figure 1A). While high rates of sustained engraftment were also seen in patients with lower percentages of myeloid precursors, the rate of neutrophil recovery was slower. In the 36 patients without neutrophil recovery by the day the BM biopsy was performed, a sub-group of particular clinical concern, the aspirate did not predict subsequent engraftment (p = 0.187, Figure 1B).
Figure 1. Cumulative incidence of sustained donor neutrophil engraftment after myeloablative DCBT according to the percentage myeloid precursors in the day 21 BM aspirate in all patients (1A) and in patients who had not engrafted at the time the BM analysis was performed (1B).
While there was a significant association between the BM aspirate and neutrophil engraftment when all patients were analyzed (1A), the percentage of myeloid precursors was not predictive of subsequent engraftment (1B).
Association between biopsy cellularity and neutrophil engraftment
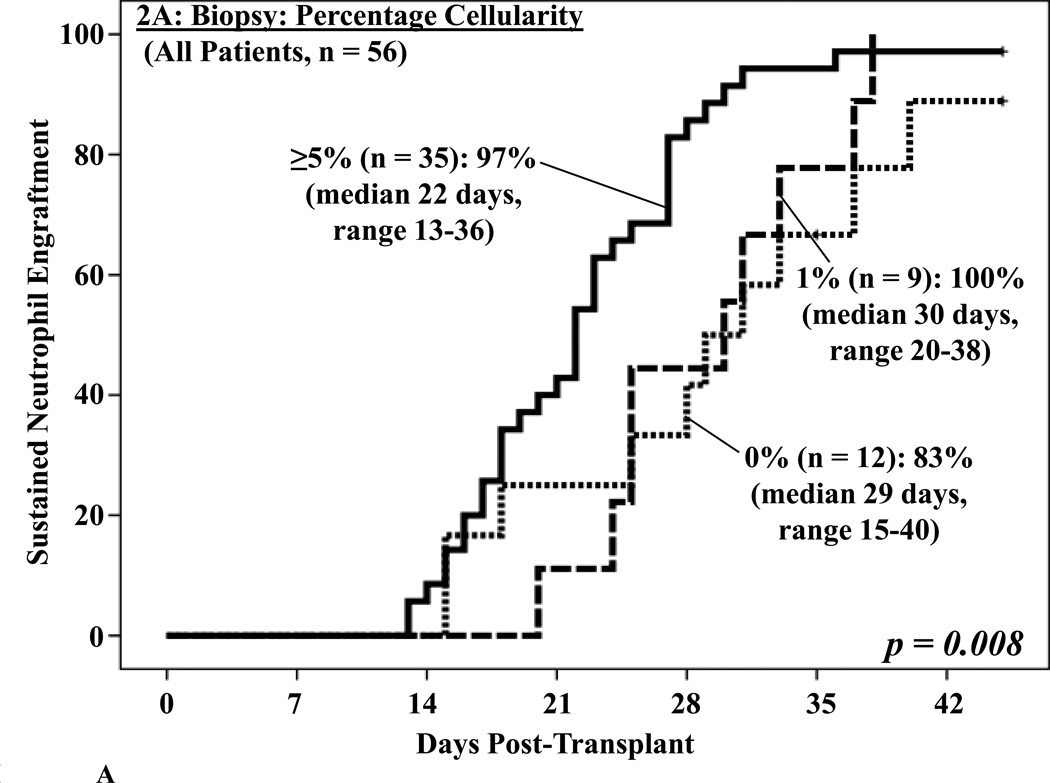
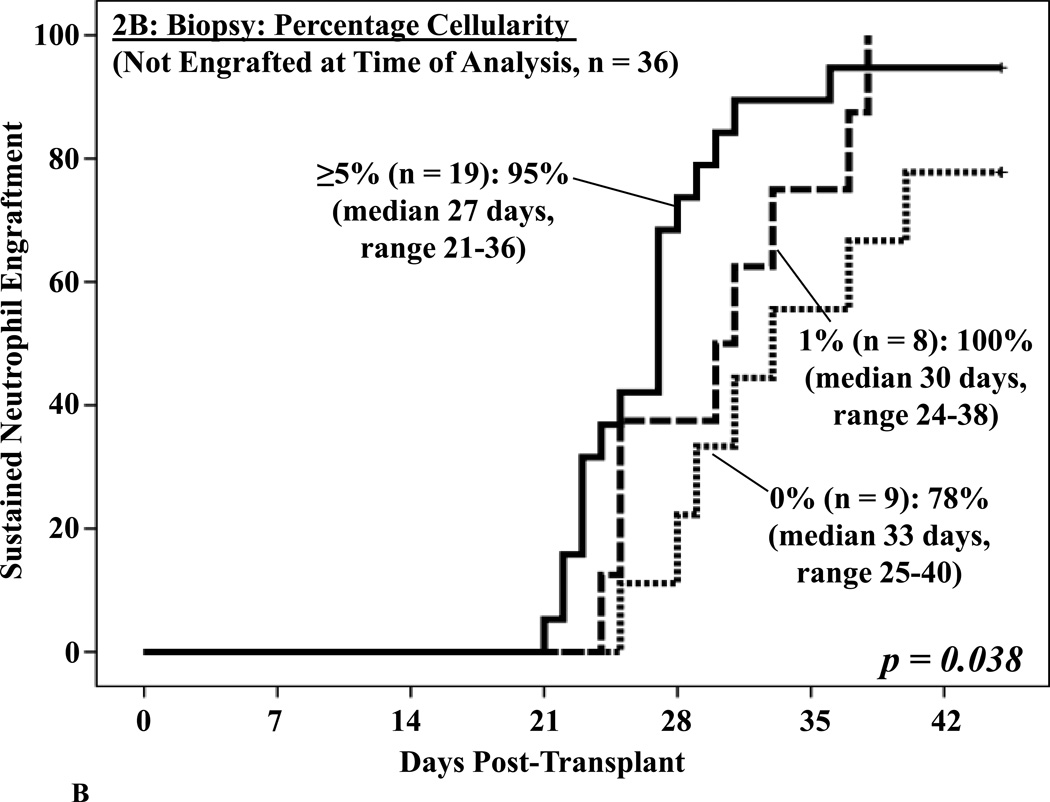
Biopsies were also often markedly hypocellular (median cellularity 5%, range 0–80), and a morphological appearance suggestive of acute myeloid leukemia was reported in 4 patients. This correlated with prominent myeloid activity in the aspirates (40–69%), but the immature cells were of donor origin. A BM cellularity of ≥ 5% was associated with enhanced neutrophil engraftment on univariate analysis (p = 0.008, Figure 2A). BM cellularity was also predictive of subsequent neutrophil engraftment in those who had not yet engrafted when the BM analysis was performed (p = 0.038, Figure 2B).
Figure 2. Cumulative incidence of sustained donor neutrophil engraftment after myeloablative DCBT according to the percentage BM cellularity in the day 21 BM biopsy in all patients (2A) and in patients not engrafted at the time the BM analysis was performed (2B).
BM cellularity was associated with neutrophil engraftment (2A) and predictive of subsequent engraftment (2B).
Ninety-three percent (95%CI:75–100) of patients with megakaryocytes present (n = 14) had platelet engraftment ≥ 50 × 109/l by day 180. This compared with 74% (95%CI:60–87) of patients (n = 42) without them (HR 0.58, p = 0.103).
Association between total donor chimerism and neutrophil engraftment
Donor hematopoiesis was detected in all patients on day 21 (one unit in 49 and two in 7 patients). In the 53 patients with sustained engraftment, the median total donor chimerism (unit#1 + unit#2) was 100% (range 65–100). The 3 patients with graft failure had a dominant unit (27%, 80%, and 100% donor), but donor hematopoiesis did not result in sustained recovery. The pattern of chimerism was not affected by the conditioning regimen (data not shown).
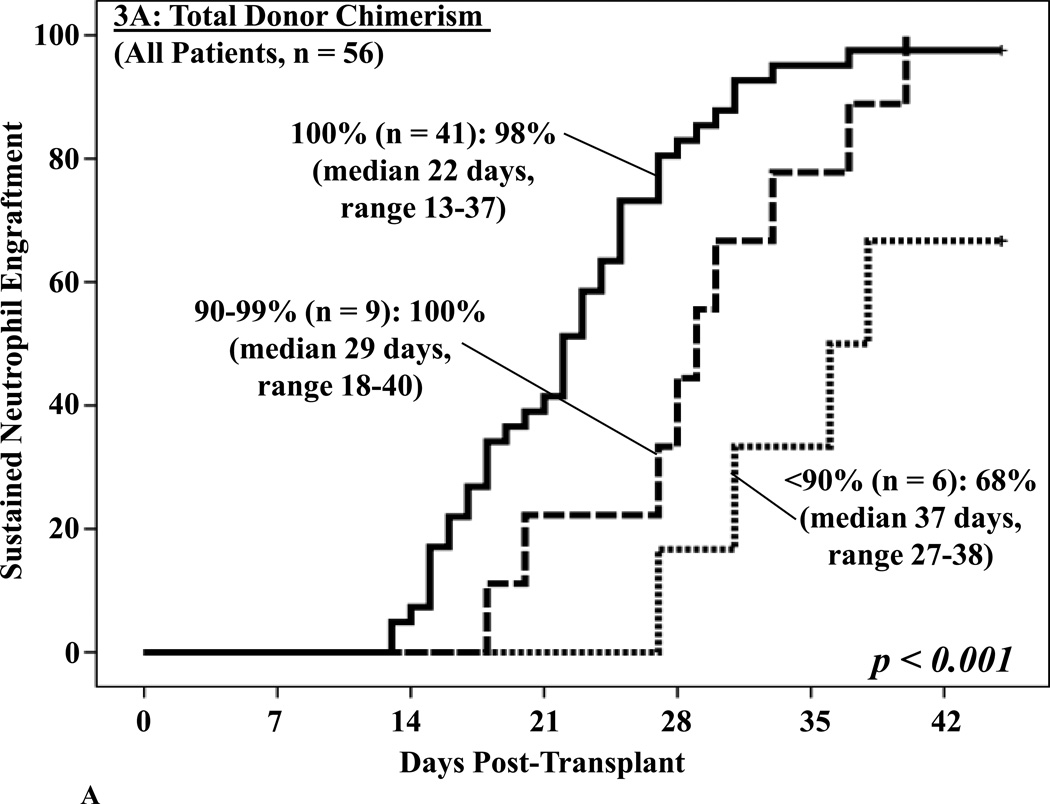
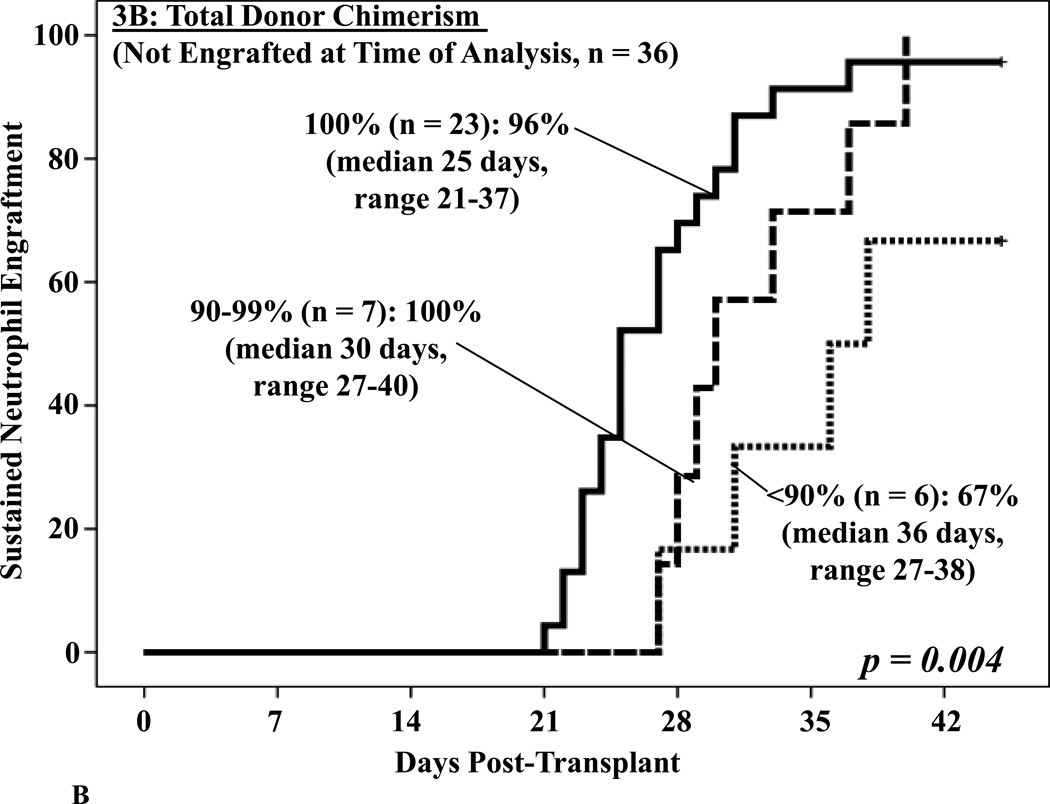
No differences in neutrophil recovery were detected in patients engrafting with 1 versus 2 units (data not shown). However, the 41 patients who were 100% total donor had a significant advantage with a 98% engraftment incidence at a median of 22 days. This compared with 100% engraftment but at a delayed median of 29 days in those who were 90–99% donor, and only 68% if < 90% donor at a median of 37 days (p = 0.001, Figure 3A). Furthermore, total donor chimerism was the most critical predictor of subsequent neutrophil engraftment in the 36 patients who had not engrafted at the time the BM analysis was performed (p = 0.004, Figure 3B).
Figure 3. Cumulative incidence of sustained donor neutrophil engraftment after myeloablative DCBT according to the total donor chimerism in the day 21 BM biopsy in all patients (3A) and in patients not engrafted at the time the BM analysis was performed (3B).
Total donor chimerism had a highly significant association with engraftment (3A) and was the most critical factor predicting subsequent engraftment (3B).
The achievement of 100% total donor on day 21 also predicted platelet recovery by day 180. Eighty-three percent (95%CI:71–95) of patients who were 100% donor had platelet recovery as compared with 67% (95%CI:42–91) if < 100% donor (RR 0.43, p = 0.028).
Multivariate analysis
To confirm the importance of total donor chimerism a multivariate analysis to test the ability of the 3 BM variables to predict subsequent neutrophil engraftment was done in the 36 patients who had not engrafted by the time of the BM analysis was performed (usually day 21). We included the infused viable CD34+ cell dose/kg of the unit that was dominant in engraftment as we have previously identified this is a critical determinant of the speed and success of engraftment in this patient population6, 12. We confirmed that a total donor chimerism < 90% was an independent predictor of impaired engraftment (p = 0.002, Table 2). A BM biopsy showing 0% cellularity was also significant whereas the percentage of myeloid precursors in the aspirate was not.
Table 2.
Multivariate analysis of the influence of day 21 BM composition and chimerism on neutrophil engraftment in patients who had not engrafted by the day the BM was performed* incorporating the infused viable CD34+ dose/kg of the unit dominant in engraftment# (n = 36).
| Day 21 BM Characteristic Engraftment RR (95% CI) by BM Characteristic (p value) | ||
|---|---|---|
| % Total Myeloid Precursors in Aspirate |
% Cellularity in Biopsy |
% Total Donor Chimerism |
|
> 50% (n = 9) Reference |
≥ 5% (n = 19) Reference |
100% (n = 23) Reference |
|
1–50% (n = 12) HR 0.52 (0.19–1.38) (p = 0.189) |
1% (n = 8) HR 0.64 (0.34–1.68) (p = 0.366) |
90–99% (n = 7) HR 0.69 (0.24–1.98) (p = 0.490) |
|
0% (n = 15) HR 0.43 (0.16–1.15) (p = 0.094) |
0% (n = 9) HR 0.29 (0.09–0.87) (p = 0.028) |
< 90% (n = 6) HR 0.13 (0.04–0.47) (p = 0.002) |
In these 36 patients the BM analysis was performed a median of 21 days post-transplant (range 19–26).
The infused viable CD34+ cell dose/kg of the dominant unit analyzed as a continuous variable had a HR of 2.09 (0.84–5.16, p = 0.111).
Abbreviations: HR, hazard ratio; CI, confidence interval; BM, bone marrow.
DISCUSSION
The association between BM morphologic and chimerism analyses and the success of CB engraftment has not been systematically addressed, and existing studies have been restricted to single-unit CBT13,14. As reported following single-unit CBT13, 15, we found BM aspirates and biopsies performed early after transplantation were often profoundly hypocellular and some patients had a prominence of very immature cells in the day 21 BM which were of donor origin. This latter finding reinforces the need for careful correlation of BM morphology with cytogenetic and molecular analyses after DCBT in leukemic patients. More importantly, we found aspirate myeloid precursors < 50% and biopsy cellularity < 5% are associated with impaired engraftment after DCBT. However, while an aspirate without myeloid activity or a near empty biopsy was associated with delayed engraftment, it did not necessarily portend graft failure. Notably, in patients who had not engrafted by the time the BM analysis was performed, the aspirate did not predict subsequent engraftment, and 93% of patients without myeloid precursors on the aspirate subsequently engrafted. Similarly, nearly all patients with at least 1% cellularity on the biopsy subsequently engrafted.
Most critical, however, was the chimerism analysis. In the transplantation of hematopoietic stem cells from adult donors, the focus in chimerism testing has been in the potential ability of lineage-specific analysis of the peripheral blood to predict graft rejection and graft-versus-host disease after non-myeloablative and reduced intensity conditioning16, 17. Traditionally chimerism has not been recommended in recipients of myeloablative conditioning when transplanting unmodified grafts from adult donors16, and two recent studies have suggested that chimerism testing of the BM is of limited usefulness in the myeloablative setting18, 19. In DCBT, the focus in chimerism analysis has been on the pattern of unit dominance (reviewed in reference20) without detailed analysis of the determinants of engraftment success. In our study, total donor chimerism was significantly associated with the speed and success of neutrophil recovery, and was the most predictive of subsequent engraftment in those patients who had not engrafted by the time the BM analysis was performed. The lack of host, and not whether the patient engrafted with 1 versus 2 units, was critical. This may suggest that the ability of the graft to eradicate host hematopoiesis determines allogeneic engraftment. Total donor chimerism also predicted subsequent platelet recovery.
Our findings concerning chimerism are in contrast to those in myeloablative transplantation of adult stem cell sources but consistent with those of Moscardo et al after single-unit CBT14, and have not previously been reported in double-unit CBT. We conclude patients transplanted in remission with early neutrophil recovery by day 21 may only need chimerism performed to confirm donor mediated hematopoiesis. This could potentially be done on the peripheral blood. By contrast, those without neutrophil recovery should have aspirate, biopsy, and expedited chimerism analyses. Patients without subsequent count recovery or who have < 90% total donor chimerism should have repeat BM analyses on day 28, and a back-up graft should be secured in case engraftment does not ensue. Future studies should investigate the mechanism underlying the engraftment advantage of complete donor chimerism.
ACKNOWLEDGMENTS
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research, the Memorial Sloan-Kettering Cancer Center Society, the Translational and Integrative Medicine Research Grant, and P01 CA23766 from the National Cancer Institute, National Institutes of Health.
Footnotes
Author Contributions: S.A. and C.E.S. analyzed and interpreted the data and wrote the manuscript. M.V., A.G., M.L. analyzed the data. N.K. and A.S. wrote the manuscript. H.C.M. and C.H. analyzed the BM specimens and wrote the manuscript. J.N.B. directed the research, interpreted the data, and wrote the manuscript.
Financial Disclosure: The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 3.Rocha V, Gluckman E. Eurocord-Netcord registry and European Blood and Marrow Transplant group. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147(2):262–274. doi: 10.1111/j.1365-2141.2009.07883.x. [DOI] [PubMed] [Google Scholar]
- 4.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page KM, Zhang L, Mendizabal A, Wease S, Carter S, Gentry T, et al. Total colony-forming units are a strong, independent predictor of neutrophil and platelet engraftment after unrelated umbilical cord blood transplantation: a single-center analysis of 435 cord blood transplants. Biol Blood Marrow Transplant. 2011;17(9):1362–1374. doi: 10.1016/j.bbmt.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Avery S, Shi W, Lubin M, Gonzales AM, Heller G, Castro-Malaspina H, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117(12):3277–3285. doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 8.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker JN, Abboud M, Rice RD, Hawke R, Schaible A, Heller G, et al. A "no-wash" albumindextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15(12):1596–1602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85(7):1954–1963. [PubMed] [Google Scholar]
- 11.Schichman SA, Suess P, Vertino AM, Gray PS. Comparison of short tandem repeat and variable number tandem repeat genetic markers for quantitative determination of allogeneic bone marrow transplant engraftment. Bone Marrow Transplant. 2002;29(3):243–248. doi: 10.1038/sj.bmt.1703360. [DOI] [PubMed] [Google Scholar]
- 12.Scaradavou A, Smith KM, Hawke R, Schaible A, Abboud M, Kernan NA, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16(4):500–508. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda T, Shiozawa E, Mayumi H, Usui T, Nakashima H, Hattori N, et al. Histopathology of bone marrow reconstitution after umbilical cord blood transplantation for hematological diseases. Pathol Int. 2008;58(2):126–132. doi: 10.1111/j.1440-1827.2007.02200.x. [DOI] [PubMed] [Google Scholar]
- 14.Moscardo F, Sanz J, Senent L, Cantero S, de la Rubia J, Montesinos P, et al. Impact of hematopoietic chimerism at day +14 on engraftment after unrelated donor umbilical cord blood transplantation for hematologic malignancies. Haematologica. 2009;94(6):827–832. doi: 10.3324/haematol.2008.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna DH, Rupp C, Wagner J, McGlennen R, Hirsch B, Dolan M, et al. Increased lymphoblast-like cells following umbilical cord blood stem cell transplantation do not predict recurrent acute leukemia. Leukemia. 2002;16(10):2171–2172. doi: 10.1038/sj.leu.2402603. [DOI] [PubMed] [Google Scholar]
- 16.Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T, et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2001;7(9):473–485. doi: 10.1053/bbmt.2001.v7.pm11669214. [DOI] [PubMed] [Google Scholar]
- 17.Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006;20(10):1690–1700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- 18.Mossallam GI, Kamel AM, Storer B, Martin PJ. Prognostic utility of routine chimerism testing at 2 to 6 months after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(3):352–359. doi: 10.1016/j.bbmt.2008.12.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mickelson DM, Sproat L, Dean R, Sobecks R, Rybicki L, Kalaycio M, et al. Comparison of donor chimerism following myeloablative and nonmyeloablative allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46(1):84–89. doi: 10.1038/bmt.2010.55. [DOI] [PubMed] [Google Scholar]
- 20.Sideri A, Neokleous N, De La Grange PB, Guerton B, Le Bousse Kerdilles MC, Uzan G, et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96(8):1213–1220. doi: 10.3324/haematol.2010.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]