Significance
Fish appear to be absent from the ocean's greatest depths, the trenches from 8,400–11,000 m. The reason is unknown, but hydrostatic pressure is suspected. We propose that the answer is the need for high levels of trimethylamine oxide (TMAO, common in many marine animals), a potent stabilizer capable of counteracting the destabilization of proteins by pressure. TMAO is known to increase with depth in bony fishes (teleosts) down to 4,900 m. By capturing the world's second-deepest known fish, the hadal snailfish Notoliparis kermadecensis from 7,000 m, we find that they have the highest recorded TMAO contents, which, moreover, yield an extrapolated maximum for fish at about 8,200 m. This is previously unidentified evidence that biochemistry may constrain depth for a large taxonomic group.
Keywords: deep-sea, piezolyte, chemical chaperone, osmoregulation
Abstract
No fish have been found in the deepest 25% of the ocean (8,400–11,000 m). This apparent absence has been attributed to hydrostatic pressure, although direct evidence is wanting because of the lack of deepest-living species to study. The common osmolyte trimethylamine N-oxide (TMAO) stabilizes proteins against pressure and increases with depth, going from 40 to 261 mmol/kg in teleost fishes from 0 to 4,850 m. TMAO accumulation with depth results in increasing internal osmolality (typically 350 mOsmol/kg in shallow species compared with seawater's 1,100 mOsmol/kg). Preliminary extrapolation of osmolalities of predicted isosmotic state at 8,000–8,500 m may indicate a possible physiological limit, as greater depths would require reversal of osmotic gradients and, thus, osmoregulatory systems. We tested this prediction by capturing five of the second-deepest known fish, the hadal snailfish (Notoliparis kermadecensis; Liparidae), from 7,000 m in the Kermadec Trench. We found their muscles to have a TMAO content of 386 ± 18 mmol/kg and osmolality of 991 ± 22 mOsmol/kg. These data fit previous extrapolations and, combined with new osmolalities from bathyal and abyssal fishes, predict isosmotic state at 8,200 m. This is previously unidentified evidence that biochemistry could constrain the depth of a large, complex taxonomic group.
All known marine organisms are vertically constrained within distinct bathymetric strata (1, 2). The factors that set these limitations remain elusive, as the effects of temperature, pressure, salinity, oxygen, and food supply are often covarying and difficult to disentangle at the species level (1, 3). The most conspicuous environmental gradient in the sea is hydrostatic pressure, which increases by 10 atm (about 1 MPa) per 100 m, reaching ∼1,000 atm or 100 MPa in the deepest environment, the hadal zone (6,000–11,000 m, primarily in the tectonic trenches). Identifying the effects of hydrostatic pressure on bathymetric zonation of all individual deep-sea species is currently an unrealistic task; however, examining trends across large groups of species, for example, ray-finned bony fishes (Actinopterygii, infraorder Teleostei), is practical. With scientific investigations now reaching full ocean depth (4), some of the extreme depth limits for life in the marine biosphere are emerging. Two of these limits, founded on historical databases of net captures and recent observational data, are the absence of elasmobranchs (sharks, etc.) from below about 4,000 m (5) and the absence of all fishes including teleosts from below about 8,400 m. Moreover, only two teleost families [snailfish (Liparidae) and cusk-eels (Ophidiidae)] have been found below about 6,000 m (see Discussion for more details) (6–8). Although the elasmobranch limit has been attributed to energy limits (5), the apparent ultimate 8,400-m limit for all fish does not appear to correlate with any environmental factor (e.g., energy, oxygen, temperature, predators) except for pressure (6).
Hydrostatic pressure can have large inhibitory effects on cellular structures, including proteins. Proteins from deep-sea organisms have been found to have structural adaptations that confer some pressure resistance (9, 10). Such genetic adaptations might limit a species' depth range, as proteins adapted to a particular pressure range do not work well at other pressures. Even with such adaptations, many proteins from deep species still retain significant levels of pressure sensitivity in vitro (9, 11–14). Recently, another mechanism for pressure adaptation has been hypothesized involving “piezolytes” (11, 15): small organic solutes that counteract the effects of pressure on proteins, potentially allowing proteins to work over greater depth ranges but possibly also constraining species depths if regulation of these solutes is limited (11, 13–17). Piezolytes are solutes first discovered as organic osmolytes (intracellular chemical effectors that prevent osmotic water loss). Most marine invertebrates are osmoconformers with internal osmolalities about the same as seawater, about 1,100 mOsmol/kg at 35 ppt; however, whereas extracellular fluids are dominated by NaCl, cells accumulate organic osmolytes to achieve osmotic balance. In many shallow-living marine invertebrate taxa, these osmolytes are neutral amino acids such as glycine and taurine and methylamines such as trimethylamine N-oxide (TMAO) (18).
Inorganic ions are not elevated intracellularly above basal levels to serve as osmolytes because they perturb macromolecules; in contrast, organic osmolytes are said to be “compatible” (nonperturbing). However, beyond simple compatibility, many of theses solutes have protective properties (18, 19). In particular, TMAO is a potent protein stabilizer that is able to counteract the effects of destabilizers, including temperature and urea, and to enhance protein folding in general (18–20). The latter property has led to the term “chemical chaperone” for TMAO and related stabilizers, with potential medical and biotechnological applications (21). Importantly, TMAO (but not glycine or other common osmolytes) has been found to counteract the effects of hydrostatic pressure on cnidarian, fish, and mammalian enzyme kinetics and protein stability, as well as on yeast growth (11–14, 19). The latest study suggests that TMAO alters water structure in a way that reduces the tendency of pressure to force water molecules into protein interiors (22).
We propose that this property of TMAO is highly relevant to the distribution of teleost fishes in the deep sea. Although most marine organisms are osmoconformers, most vertebrates are osmoregulators, using osmoregulatory organs to maintain a relatively homeostatic internal osmolality. These vertebrates are very hypoosmotic to seawater (e.g., shallow marine teleost fishes have internal osmolalities around 350 mOsmol/kg and only low levels of organic osmolytes, mainly TMAO at 40–50 mOsmol/kg) (11). Deep-sea teleosts, however, have only recently been found to violate this textbook characterization of fishes because they exhibit a striking correlation between TMAO and depth of capture: Muscle TMAO contents among numerous species and families of teleosts were found to increase up to 261 mmol/kg at 4,850 m, concomitantly raising internal osmolalities (11, 13, 16, 17). Moreover, a preliminary extrapolation of osmolalities with depth predicted that teleosts would become isosmotic with seawater at 8,000–8,500 m (6, 17), which corresponds very closely with the apparent depth limit for teleosts noted earlier. To live deeper would require TMAO contents above isosmotic levels, necessitating significant physiological reorganization of osmoregulatory systems because the fish would be hyperosmotic, rather than isosmotic or hypoosmotic. Species that can switch (as salmon during migration) between hyper- and hypoosmotic states are not common and typically require extended time to acclimatize. We propose that it is unlikely that a hadal fish could evolve the ability to migrate down a trench slope and then wait for hours or days at 8,400 m while altering physiological systems for hyperosmotic regulation to migrate deeper (see Discussion for more details).
In this study, we set out to test two hypotheses: the piezolyte hypothesis, that TMAO is needed in teleosts down to their greatest depths, and the depth-limit hypothesis, which predicts that TMAO accumulation results in a physiological osmotic maximum near the observed depth limit for fishes. To test these hypotheses, we obtained a variety of deep-sea teleosts from a greater variety of species and depths than used in previous studies and analyzed TMAO contents and osmolalities of dorsal white muscle tissue. In part, we used frozen tissues from bathyal and abyssal species originally collected for another study (23) in Monterey Bay, California, between 493 and 3,200 m: eelpouts (zoarcids), grenadiers (macrourids), a morid from 3 different depths, and a snailfish (liparid).
However, the most important new data were obtained from new specimens from the hadal zone. After several attempts with a free-fall, retrievable baited trap deployed from a surface vessel, we successfully caught a record number of intact snailfishes (n = 5) from a depth of 7,000 m (24). This liparid, Notoliparis kermadecensis (Fig. 1) from the Kermadec Trench north of New Zealand, is the deepest known fish in the southern hemisphere, the second deepest fish ever seen alive (8), and had not been captured for 59 y until this study (25).
Fig. 1.

The hadal snailfish N. kermadecensis (25). The image shows the snailfish alive at 7,199 m, photographed by baited camera close to the site where the samples were retrieved in this study (8). N. kermadecensis is endemic to the Kermadec Trench off New Zealand and occupies a very narrow depth band of 6,472 (24) to 7,561 m (8). The samples recovered for this study represent the second time this fish has ever been captured (the first time in 59 y) and represents the second deepest fish ever seen alive (7).
Results
Tissue Integrity.
Dorsal white muscle samples of the hadal snailfish were analyzed in several ways. We first tested for changes that might have occurred during the fish trap's 3 h 11 min ascent by analyzing water, sodium, potassium, and trimethylamine (TMA) contents. The muscles had a mean water content of 84.8 ± 1.1% (wt/wt), very similar to those in bathyal and abyssal species from 2,000–3,000 m (26). Sodium and potassium contents were 199 ± 19 and 48.2 ± 5.1 mmol/kg, respectively, differing considerably from seawater, which at 35 ppt has sodium and potassium concentrations of about 470 and 10 mM, respectively. Levels of TMA, a product of bacterial breakdown of TMAO and a common indicator of spoilage, were low (< 3 mM) in the hadal fish (as well as in all other species used). These results suggest there was little change in muscle contents during recovery. Fish skin is largely impermeable to water and solutes, with most ion exchange occurring at the gills (presumably not functioning, as the fish die during ascent).
Muscle TMAO Contents.
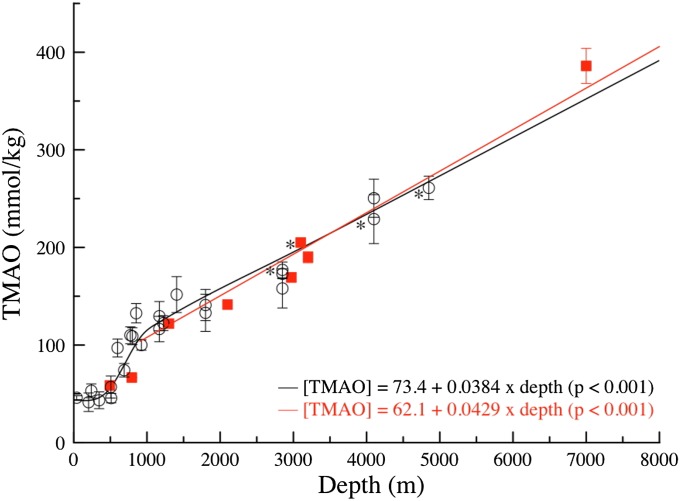
We next analyzed TMAO contents (per kilogram wet mass of tissue) and osmolalities (mOsmol/kg) of muscles. For TMAO, we first plotted muscle contents against depth for fish from previous studies (Fig. 2, circles). Although the pattern appears sigmoidal at lower depths, with a threshold at about 500 m, it is linear from about 900–4,850 m (Fig. 2, black line and equation). We then added the bathyal and abyssal fish from Monterey Bay; these fit well with the regression (Fig. 2, red squares without error bars). For the abyssal grenadier Coryphaenoides armatus, the data from specimens caught at different depths are noted with an asterisk in Fig. 2. Finally, we plotted the hadal snailfish; most strikingly, it had much higher TMAO contents, at 386 ± 18 mmol/kg (Fig. 2, red square with error bars). This value fell within the error range of the original linear fit, changing that fit only slightly (Fig. 2, red line and equation).
Fig. 2.
Muscle TMAO contents (mmol/kg wet mass) vs depth of capture for teleosts. Circles, with standard deviation bars, are published data (11, 13, 16, 17), with a linear fit (black line) from 900–4,850 m. Solid red squares without standard deviation bars are new data (n = 1 each) for a snailfish C. melanurus (793 m), four eelpout, and two grenadier species. The solid red square with standard deviation bars is the hadal snailfish N. kermadecensis from 7,000 m (n = 5), with a new linear fit (the red line) for all new and old data for 900–7,000 m. *C. armatus (abyssal grenadier) at four depths (note that the specimen at 4,850 m was from the northeast Atlantic, whereas the others were from the northeastern Pacific).
Muscle Osmolalities.
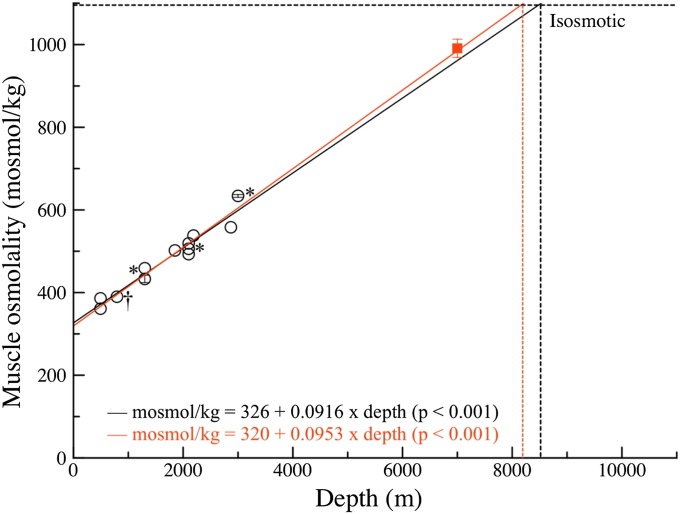
Last, we measured and plotted osmolalities of muscle fluids against depth, first for the fish from 490–3,000 m (Fig. 3, circles). Values increased linearly with depth to 3,000 m (Fig. 3, solid black line and equation). Finescale moras (Antimora microlepis) caught at different depths are noted by an asterisk in Fig. 3. The fitted line extrapolated to an isosmotic value of 1,100 mOsmol/kg at 8,450 m (Fig. 3, dotted black line). We then added the new value for the hadal snailfish. Similar to TMAO, the value was much higher, plotting close to the extrapolated line, at 991 ± 22 mOsmol/kg (Fig. 3, red square), changing the linear fit only slightly (Fig. 3, solid red line and equation). Including the hadal snailfish data yielded an extrapolated isosmotic state at about 8,200 m (Fig. 3, dotted red line), an even closer fit to the maximum depths observed or estimated from meta-analytic approaches (6).
Fig. 3.
Osmolalities (mOsmol/kg) of muscle fluid vs depth of capture for teleosts. Circles (n = 1 each; for the two osmolalities with standard deviation bars, n = 3) are new data for Monterey Bay fish: eelpouts, grenadiers, a morid, and a snailfish C. melanurus († at 793 m). A linear fit (the solid black line) extrapolates to isosmotic state at 8,450 m (dotted vertical black line). The red square with standard deviation bars is the hadal snailfish N. kermadecensis from 7,000 m (n = 5), included in a new linear fit (the solid red line) extrapolating to isosmotic state at about 8,200 m (dotted vertical red line). *A. microlepis (finescale mora) at three depths.
Discussion
Implications for Depth Hypotheses.
The osmotic and TMAO data clearly support both of our hypotheses; that is, teleosts appear to need TMAO (presumably as a piezolyte) at all depths in proportion to hydrostatic pressure, and any teleosts approaching about 8,200 m depth would become isosmotic. We propose that fish would find it physiologically difficult to migrate readily to greater depths (see Reasons for an Osmoregulatory Limit below). The ability to regulate TMAO with depth may also determine whether a particular species can migrate over a wide depth range or not. Some species, such as C. armatus and A. microlepis (points with asterisks in Figs. 2 and 3), can regulate TMAO over a wide range, but other species may not be able to do so. Both up- and down-regulation are important because excess TMAO in the absence of a perturbant (such as urea or pressure) can inhibit protein function as a result of overstabilization (19, 27). For example, a recent finding is that elevated TMAO in human blood is associated with cardiovascular disease (28). Negative effects of TMAO may explain why TMAO is not elevated in shallow teleost species, even though high TMAO might reduce osmoregulatory costs (19).
Alternative Hypotheses and Explanations.
Although the data support our hypotheses, there are other possibilities. First, of course, there may be deeper-living fish species that simply have not been found. However, we regard the evidence against this possibility to be compelling (6). To date, the Hadal Environment and Educational Program project (7, 8) has deployed baited cameras and traps successfully 47 times at depths from 6,000–10,000 m across five Pacific Trenches. Fish have been consistently observed in all deployments made up to a depth of 7,700 m (7). No fish have been observed in the 14 deployments deeper than this. The only two major trawling efforts undertaken below abyssal depths were on the Danish Galathea and Soviet Vityaz expeditions of the 1950s to many trenches. The Galathea performed 150 trawls or dredges, of which 28 were greater than 6,000 m (29). The deepest fish trawled in these 28 stations were from 6,660–6,770 m and 7,160 m (30). The Vitjaz performed 106 trawls from depths greater than 6,000 m (down to nearly 11,000 m) in most of the trenches of the Western Pacific Ocean, and the deepest fish recovered were from 7,210–7,230 m and 7,587 m. Various other catches have been made that are reviewed in Fujii and colleagues (7), but all fish are recorded from depths less than the current record of 8,370 m (25). Nevertheless, we recognize that a future discovery of a fish thriving at 10,000 m, for example, would disprove our second hypothesis. The piezolyte hypothesis, however, would not necessarily be challenged.
Second, if the depth limit for fishes is real, it might not be a result of osmoregulatory difficulties. One alternative possibility is inadequate food supply. In turn, that might limit a variety of internal processes, including the ability to make or ingest sufficient TMAO. Another possibility is that TMAO itself is toxic above 400 mM or so, regardless of pressure. As noted earlier, TMAO in the absence of a perturbant can have negative effects; perhaps such effects at depths below 8,200–8,400 m become greater than positive effects in counteracting pressure. Finally, it may simply be that TMAO cannot adequately counteract pressure effects below that depth and that fish have not evolved other mechanisms to cope with higher pressures.
Reasons for an Osmoregulatory Limit.
Our hypothesis of an osmotic limit for fishes is based on the extrapolated value for isosmotic state at 8,200–8,400 m, coinciding with the observed depth distributions of fishes. If this hypothesis is correct, what would prevent fish from evolving mechanisms to pass this barrier? To understand this, we must examine the evolutionary history of bony fishes. There is very strong phylogenetic, fossil, and physiological evidence that the ancestors of bony fishes evolved in fresh water (31), which can approach 0 mOsmol/kg. Freshwater fish are therefore hyperosmotic at about 300 mOsmol/kg internally. To cope with this osmotic imbalance, these fish have (among other adaptations) kidneys with glomeruli, filtering structures that remove excess water from vertebrate bodies (32). In evolving for seawater, shallow teleosts have become hypoosmotic at about 350 mOsmol/kg internally, with the opposite physiological processes, including inactive glomeruli or, in some groups, complete absence of glomeruli. These so-called aglomerular species include some gelatinous deep-sea fishes (33). Many marine fish lineages have never returned to fresh water, including the Liparidae (no species of which have been found in low-salinity waters) (34). Those that can reacquire hyperosmotic regulation during their life cycles, including euryhaline fish such as salmon, pause at an intermediate estuarine salinity as their gills reorganize and glomerular filtration is reactivated. This “reprogramming” of osmoregulation takes many hours or days (35).
We do not know whether the deepest-living marine teleosts are aglomerular, but they are gelatinous, similar to known aglomerular deep-sea species (33), with N. kermadecensis having a thick subdermal gelatinous layer. Regardless, they would need to switch from a hypo- to a hyperosmotic state were they to accumulate TMAO at levels much greater than 400 mOsmol/kg. A fish migrating down the slope while it accumulates TMAO might have to wait many hours or days around 8,200–8,400 m while acclimatizing for greater depths. The fish would need reactivated glomeruli or other mechanisms to cope with water influx. If our hypothesis is correct, it may be that there is insufficient selective pressure or genetic variation for physiological switching and waiting behavior to evolve. Another possibility is that there has not been enough time for these mechanisms to evolve; indeed, there is strong evidence that deep-sea lineages have only recently and gradually (in geological terms) recolonized the deep after an anoxic extinction event in the Cretaceous (36). A slow recolonization of the deep is also supported by observations that the number of fish families declines with depth and that only ophidiids and liparids have been found below about 6,000 m (6–8, 36).
Implications for Other Taxa.
Although TMAO may explain the depth limit for teleosts, other taxa (e.g., archaea, bacteria, foraminifera, and among animals, anemones, holothurians, and amphipods), which are isosmotic even in shallow species, exist at the ocean's greatest depths, including the Mariana Trench at nearly 11,000 m. We do not yet know how these organisms survive at higher pressures. Do piezolytes play an important role, and/or have these organisms evolved proteins more pressure-resistant than those of teleosts? Although anemones, mollusks, and holothurians cannot make TMAO, species analyzed from 3,000 m have other osmolytes, including scyllo-inositol and glycerophosphocholine, that are not found in their shallow relatives (37). These solutes are chemical chaperones (13, 19), although they have not yet been tested with hydrostatic pressure. Future investigations into depth zonation should provide great insight from examining the upper end of the pressure gradient, which the hadal zone provides.
Materials and Methods
Fish Capture and Equipment.
For the Monterey Bay fish, bathyal fishes were collected in 2009 using otter trawls from the R/V Point Sur (23). Trawls were fished along depth contours using an acoustic pinger so that depth could be monitored in real time. For the Kermadec Trench hadal fish, the free-falling fish trap (24) included two fish cages (40 × 40 × 100 cm cuboid) with a square funnel opening of 14 × 14 cm recessed 25 cm into the trap. Each trap was lined with 1 cm mesh and baited with ∼500 g Jack mackerel (Trachurus declivis) and attached to either side of a 1 m−3 aluminum frame. Each trap was also equipped with a temperature and pressure sensor (SBE-39; SeaBird Electronics) that recorded at 30-s intervals throughout the deployment. The traps were positioned such that they were in contact with the sea floor on landing. Each trap was deployed in free fall, whereby it descended to the seafloor at a speed of 36 m⋅min−1 and remained static on the seafloor for 12 h 39 min. After this period, ballast weights were jettisoned by acoustic command from the surface vessel [R/V Kaharoa; National Institute of Water and Atmospheric Research (NIWA), New Zealand] which initiated the trap’s ascent to the surface at 40 m⋅min−1.
Confirmation that the hadal snailfish were collected from 7,000 m, and not during the trap's ascent, is given by the use of a baited camera deployed in the same vicinity. This provides unequivocal observations that the fish are benthic; moreover, this species is known to be benthic from other trawl and camera deployments (8, 25). Furthermore, the traps ascend to the surface at 40 m⋅min−1 and are therefore extremely unlikely to capture fish. For the fish from Monterey Bay, the trawl caught specimens at bottom depths measured with pingers (accurate acoustic transmitters that continuously show height above the seafloor). Although animals could be captured in the water column during ascent, this is not likely, as acoustic studies often show very little biomass in the water column above about 100 m off the seafloor. Moreover, the species we analyzed are known from camera work to be benthic or benthopelagic, and no species were caught that are known to be pelagic from other studies using midwater nets well above the seafloor.
Other Fish Species.
For Fig. 2, the older (11, 13, 16, 17) data were from bathyal and abyssal species caught by otter trawls off the Oregon, California, and Ireland coasts: hake (merluciid), flatfishes (pleuronectids), a sablefish (anoplopomatid), rockfishes (scorpaenids), eelpouts (zoarcids), a mora (morid), and grenadiers (macrourids). Species from Monterey Bay, again caught by otter trawl (23), for new TMAO data were a snailfish (liparid) Caraproctus melanurus from 793 m, eelpouts Lyocdes diapterus from 493 m, Bothrocara molle from 2,100 m, Pachycara gymninium from 2,972 m, Pachycara sp. from 3,200 m, and grenadiers Coryphaenoides acrolepis from 1,300 m and C. armatus from 3,100 m.
For Fig. 3, species from Monterey Bay (23) used for initial osmolality extrapolation (circles) were a snailfish (liparid) C. melanurus from 793 m; eelpouts L. diapterus and L. cortezianus from 493 m; B. molle from 2,100 m; a morid A. microlepis from 1,300, 2,100, and 2,910 m; and grenadiers C. acrolepis from 1,300 m and C. yaquinae from 2,870 m. The species used for Figs. 2 and 3 overlap only partly because for some there was insufficient muscle tissue left from other studies to perform both analyses.
Tissue Integrity Analyses.
On the ship, on ice, white muscle samples were rapidly dissected from the anterior dorsal side of each fish and flash-frozen in liquid nitrogen and then transferred to −80 °C freezers on shore. They were stored in tightly capped cryovials to prevent hydration changes. The following were analyzed as indicators of changes that might have occurred during ascent of the capture device: TMA was analyzed by the TMAO method discussed earlier, omitting the iron reagent; water contents were determined by weighing tissues before and after heating for 3 d at 60 °C on tared aluminum-foil “boats”; and sodium and potassium contents were measured in the dried material dissolved in 1% nitric acid and analyzed by atomic absorption.
Tissue TMAO and Osmolality Analyses.
Frozen weighed muscle samples were homogenized in 7% (vol/vol) perchloric acid to remove proteins by centrifugation, and then TMAO concentrations of the supernatants were measured using an iron-EDTA reagent and a colorimetric reaction with picric acid, with appropriate standards, as previously described (16). TMAO and TMA standards show that the precision of the colorimetric test is about ±3%. Osmolalities of tissue fluid were measured with Wescor vapor-pressure osmometers in fluid obtained from muscle pieces mechanically homogenized with a Teflon pestle in a microcentrifuge tube, which was then centrifuged at maximum speed for 30 min at 4 °C. Ten microliters of supernatant were then taken for the osmometer. The osmometer standards show that the precision of the machines is ±1%.
Acknowledgments
We thank the crew of the R/V Kaharoa (NIWA, New Zealand) for their assistance in sampling the fish and Andrew Stewart (Te Papa Tongarewa, Museum of New Zealand) for species identification confirmation and housing the specimens. P.H.Y., M.E.G. and J.C.D. acknowledge support from National Science Foundation (grants OCE-1130712 and OCE-0727135), and A.J. and A.A.R. acknowledge support from the Total Foundation (France) and Ministry for Business, Innovation and Employment (New Zealand). P.H.Y. developed the hypotheses and designed the experiments; M.E.G. and P.H.Y. performed the tissue analyses; A.J. designed the hadal sampling gear; A.J., A.A.R., and M.E.G. organized the Kaharoa expedition and sampling of the hadal fish; and J.C.D. collected and dissected the Monterey Bay samples. All authors were involved in the writing and editing of the paper.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Carney RS. Zonation of deep biota on continental margins. Oceanogr Mar Biol Annu Rev. 2005;43:211–278. [Google Scholar]
- 2.Childress JJ. Are there physiological and biochemical adaptations of metabolism in deep-sea animals? Trends Ecol Evol. 1995;10(1):30–36. doi: 10.1016/s0169-5347(00)88957-0. [DOI] [PubMed] [Google Scholar]
- 3.Seibel BA, Drazen JC. The rates of metabolism in marine animals: Environmental constraints or energetic opportunities? Phil Tran R Soc Lond B. 2007;362(1487):2061–2078. doi: 10.1098/rstb.2007.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson AJ, Fujii T, Mayor DJ, Solan M, Priede IG. Hadal trenches: The ecology of the deepest places on Earth. Trends Ecol Evol. 2010;25(3):190–197. doi: 10.1016/j.tree.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Priede IG, et al. The absence of sharks from abyssal regions of the world’s oceans. Proc Biol Sci. 2006;273(1592):1435–1441. doi: 10.1098/rspb.2005.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamieson AJ, Yancey PH. On the validity of the Trieste flatfish: Dispelling the myth. Biol Bull. 2012;222(3):171–175. doi: 10.1086/BBLv222n3p171. [DOI] [PubMed] [Google Scholar]
- 7.Fujii T, Jamieson AJ, Solan M, Bagley PM, Priede IG. A large aggregation of liparids at 7703 m depth and a reappraisal of the abundance and diversity of hadal fish. Bioscience. 2010;60(7):506–515. [Google Scholar]
- 8.Jamieson AJ, et al. Bait-attending fauna of the Kermadec Trench, SW Pacific Ocean: Evidence for an ecotone across the abyssal-hadal transition zone. Deep Sea Res Part I Oceanogr Res Pap. 2011;58(1):49–62. [Google Scholar]
- 9.Siebenaller JF, Somero GN. Biochemical adaptation to the deep sea. CRC Crit Rev Aquat Sci. 1989;1:1–25. [Google Scholar]
- 10.Morita T. Structure-based analysis of high pressure adaptation of alpha-actin. J Biol Chem. 2003;278(30):28060–28066. doi: 10.1074/jbc.M302328200. [DOI] [PubMed] [Google Scholar]
- 11.Gillett MB, Suko JR, Santoso FO, Yancey PH. Elevated levels of trimethylamine oxide in muscles of deep-sea gadiform teleosts: A high-pressure adaptation? J Exp Zool. 1997;279(4):386–391. [Google Scholar]
- 12.Yancey PH, Fyfe-Johnson AL, Kelly RH, Walker VP, Auñón MT. Trimethylamine oxide counteracts effects of hydrostatic pressure on proteins of deep-sea teleosts. J Exp Zool. 2001;289(3):172–176. doi: 10.1002/1097-010x(20010215)289:3<172::aid-jez3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Yancey PH, Rhea MD, Kemp KM, Bailey DM. Trimethylamine oxide, betaine and other osmolytes in deep-sea animals: Depth trends and effects on enzymes under hydrostatic pressure. Cell Mol Biol (Noisy-le-grand) 2004;50(4):371–376. [PubMed] [Google Scholar]
- 14.Yancey PH, Siebenaller JF. Trimethylamine oxide stabilizes teleost and mammalian lactate dehydrogenases against inactivation by hydrostatic pressure and trypsinolysis. J Exp Biol. 1999;202(Pt 24):3597–3603. doi: 10.1242/jeb.202.24.3597. [DOI] [PubMed] [Google Scholar]
- 15.Martin DD, Bartlett DH, Roberts MF. Solute accumulation in the deep-sea bacterium Photobacterium profundum. Extremophiles. 2002;6(6):507–514. doi: 10.1007/s00792-002-0288-1. [DOI] [PubMed] [Google Scholar]
- 16.Kelly RH, Yancey PH. High contents of trimethylamine oxide correlating with depth in deep-sea teleost fish, skates, and decapod crustaceans. Biol Bull. 1999;196(1):18–25. doi: 10.2307/1543162. [DOI] [PubMed] [Google Scholar]
- 17.Samerotte AL, Drazen JC, Brand GL, Seibel BA, Yancey PH. Correlation of trimethylamine oxide and habitat depth within and among species of teleost fish: An analysis of causation. Physiol Biochem Zool. 2007;80(2):197–208. doi: 10.1086/510566. [DOI] [PubMed] [Google Scholar]
- 18.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: Evolution of osmolyte systems. Science. 1982;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 19.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208(Pt 15):2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 20.Street TO, Bolen DW, Rose GD. A molecular mechanism for osmolyte-induced protein stability. Proc Natl Acad Sci USA. 2006;103(38):13997–14002. doi: 10.1073/pnas.0606236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J-H, et al. Chemical chaperone and inhibitor discovery: Potential treatments for protein conformational diseases. Perspect Medicin Chem. 2008;1:39–48. doi: 10.4137/pmc.s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarma R, Paul S. Crucial importance of water structure modification on trimethylamine N-oxide counteracting effect at high pressure. J Phys Chem B. 2013;117(2):677–689. doi: 10.1021/jp311102v. [DOI] [PubMed] [Google Scholar]
- 23.Yeh J, Drazen JC. Baited-camera observations of deep-sea megafaunal scavenger ecology on the California slope. Mar Ecol Prog Ser. 2010;424:145–156. [Google Scholar]
- 24.Jamieson AJ, Lacey NC, Lörz A-N, Rowden AA, Piertney SB. The supergiant amphipod Alicella gigantea (Crustacea: Alicellidae) from hadal depths in the Kermadec Trench, SW Pacific Ocean. Deep Sea Res Part II Top Stud Oceanogr. 2013;92:107–113. [Google Scholar]
- 25.Nielson JG. Fishes from depths exceeding 6000 meters. Galathea Rep. 1964;7:113–124. [Google Scholar]
- 26.Drazen JC. Depth related trends in proximate composition of demersal fishes in the eastern North Pacific. Deep Sea Res Part I Oceanogr Res Pap. 2007;54:203–219. [Google Scholar]
- 27.Kumemoto R, Yusa K, Shibayama T, Hatori K. Trimethylamine N-oxide suppresses the activity of the actomyosin motor. Biochim Biophys Acta. 2012;1820(10):1597–1604. doi: 10.1016/j.bbagen.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Tang WHW, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruun AF. General introduction to the reports and list of deep-sea stations. Galathea Reports. 1957;1:7–48. [Google Scholar]
- 30.Wolff T. The deepest recorded fishes. Nature. 1961;190:283–284. [Google Scholar]
- 31.Carrete Vega G, Wiens JJ. Why are there so few fish in the sea? Proc Biol Sci. 2012;279(1737):2323–2329. doi: 10.1098/rspb.2012.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beyenbach KW. Kidneys sans glomeruli. Am J Physiol Renal Physiol. 2004;286(5):F811–F827. doi: 10.1152/ajprenal.00351.2003. [DOI] [PubMed] [Google Scholar]
- 33.Ozaka C, Yamamoto N, Somiya H. The aglomerular kidney of the deep-sea fish, Ateleopus japonicus (Ateleopodiformes: Ateleopodidae): Evidence of wider occurrence of the aglomerular condition in Teleostei. Copeia. 2009;(3):609–617. [Google Scholar]
- 34.Chernova NV. Systematics and phylogeny of fish of the genus Liparis (Liparidae, Scorpaeniformes) J Ichthyol. 2008;48(10):831–852. [Google Scholar]
- 35.Miles HM. Renal function in migrating coho salmon. Comp Biochem Physiol. 1971;38A:787–826. [Google Scholar]
- 36.Priede IG, Froese R. Colonization of the deep sea by fishes. J Fish Biol. 2013;83(6):1528–1550. doi: 10.1111/jfb.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yancey PH, Blake WR, Conley J. Unusual organic osmolytes in deep-sea animals: Adaptations to hydrostatic pressure and other perturbants. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(3):667–676. doi: 10.1016/s1095-6433(02)00182-4. [DOI] [PubMed] [Google Scholar]