Significance
These findings indicate that the combination of shorter leukocyte telomeres with high telomerase activity is associated with stress-related impairment of function at both the biological and psychological levels. Slow poststress recovery in cardiovascular activity in inflammatory responses and reduced stress responsivity in blood pressure and cortisol are indicative of a state of heightened allostatic load. Older men with the shorter telomere/high telomerase phenotype also show impoverished resources for dealing with stress, including low levels of social support and optimism and higher levels of hostility. The integrated approach taken in this study advances our understanding of the cellular substrate of stress-related processes and documents the dynamic interplay between social environmental exposures and the mechanisms underlying chromosomal integrity.
Keywords: cellular senescence, allostasis, psychological distress
Abstract
Recent work has linked psychological stress with premature cellular aging as indexed by reduced leukocyte telomere length. The combination of shorter telomeres with high telomerase activity (TA) may be indicative of active cell stress. We hypothesized that older individuals characterized by shorter telomeres with high TA in unstimulated leukocytes would show signs of high allostatic load and low levels of protective psychosocial resources. We studied 333 healthy men and women aged 54–76 y who underwent laboratory testing in which we measured cardiovascular, neuroendocrine, and inflammatory responses to standardized mental stress tasks. The tasks elicited prompt increases in blood pressure (BP), heart rate, cortisol, and mediators of inflammation and reductions in heart rate variability, returning toward baseline levels following stress. However, men having shorter telomeres with high TA showed blunted poststress recovery in systolic BP, heart rate variability, and monocyte chemoattractant protein-1, together with reduced responsivity in diastolic BP, heart rate, and cortisol, in comparison to men with longer telomeres or men with shorter telomeres and low TA. Shorter telomeres with high TA were also associated with reduced social support, lower optimism, higher hostility, and greater early life adversity. These effects were independent of age, socioeconomic status, and body mass index. We did not observe differences among older women. Our findings suggest that active cell stress is associated with impaired physiological stress responses and impoverished psychosocial resources, reflecting an integration of cellular, systemic, and psychological stress processes potentially relevant to health in older men.
Telomeres are nucleoprotein structures composed of tandem hexanucleotide repeats of the sequence TTAGGG that cap the ends of eukaryotic chromosomes, protecting them from end-to-end fusion and degradation during cell division. Human telomeric DNA naturally shortens with age during somatic cell divisions and as a result of oxidative attack (1, 2). At critical shortness, telomeres exhibit impaired function, leading to genomic instability, apoptosis, and cell senescence, often with altered transcriptional programming and mitochondrial dysfunction (1). Telomere attrition represents one of the hallmarks of aging (3). In humans, Mendelian single-gene mutations that directly compromise telomere maintenance cause premature mortality and onset of a spectrum of diseases overlapping with the age-related diseases common in the population (4). In addition, genome-wide association studies show that certain combinations of SNPs at genetic loci encoding known telomere-maintenance factors are associated with increased relative risks for pulmonary and coronary diseases (5). Shorter telomere length (TL) in white blood cells is linked and, in some cases, anticipates aging-related morbidity and mortality from conditions with immune system involvement, such as infectious diseases, cancer, and cardiovascular diseases, as well as risk factors, including hypertension, diabetes, obesity, and smoking (6–9).
One of the factors thought to influence telomere dynamics is psychosocial stress (10, 11). Epel et al. (12) demonstrated that the severe stress experienced by mothers of chronically ill children was correlated with reduced leukocyte TL. Subsequently, other studies have documented associations between early life stress, current perceived stress, mood disorders, and reduced TL in adults (10, 13, 14). There is also limited research on responses to acute mental stress indicating that greater cortisol responses to acute laboratory challenges are associated with shorter telomeres (15). Shorter TL is correlated with negative psychological characteristics, such as pessimism, distress, and hostility (16–18), although results have not all been consistent (19). By contrast, potentially protective psychosocial factors, such as optimism, social support, and greater educational attainment, have been linked with longer telomeres (20–23).
A critical determinant of TL is the enzyme telomerase, which has the capacity to add TTAGGG sequences onto the 3′ ends of telomeric DNA, extending TL and promoting genomic stability (1). Acute mental stress appears to increase telomerase enzymatic activity at least transiently (24), and it has been suggested that high telomerase activity (TA) in conjunction with shorter TL may be indicative of a stressed system (25). This pattern may be particularly relevant to cardiovascular disease; Kroenke et al. (26) reported that individuals in a longitudinal population cohort who showed progression of subclinical coronary atherosclerosis were characterized by shorter leukocyte telomeres with high TA, and our group previously showed that high TA and shorter telomeres were associated with hostility (16), which is a pathogenic factor in cardiovascular disease (27).
These findings suggest that the shorter TL/high TA phenotype may be associated with stressed psychobiological regulatory processes. One of the cardinal features of disrupted stress processes is increased allostatic load, characterized by disturbed responses to stress and impaired poststress recovery (28). In the present study, we therefore hypothesized that short TL/high TA would be associated with markers of allostatic load (assessed by impaired cardiovascular recovery following acute mental stress); heightened inflammatory responses to stress; and low levels of psychosocial resources, including social support and optimism. Because childhood adversity has been associated with shorter TL (13), we also assessed the relationship of the short TL/high TA phenotype with early life loss and chronic adversity.
Results
We distinguished three groups of healthy older participants on the basis of the distribution of leukocyte TL and TA, dividing the sample of 333 by median split into long and shorter telomere groups, and then dividing the shorter telomere sample into those participants with high and low TA, again by median split. The result was a long TL group (n = 167, 79 men and 88 women), a short TL/low TA group (n = 83, 44 men and 39 women), and a short TL/high TA group (n = 83, 42 men and 41 women). Our comparisons of these groups (Table 1) indicated that they did not differ in sex distribution, age, socioeconomic status (SES) as defined by grade of employment or education, body mass index (BMI), or smoking status. The average age of participants was 63 y. There were no differences between the three telomere phenotype groups in baseline cardiovascular, neuroendocrine, or inflammatory factors (Table 1; effects for men and women separately are shown in Table S1).
Table 1.
Characteristics of the three telomere phenotypes
| Sociodemographic/biological variable | Long telomeres (n = 167) | Short TL/low TA (n = 83) | Short TL/high TA (n = 83) | P, trend |
| Sex | 0.54 | |||
| Men | 79 (47.3%) | 44 (53.0%) | 42 (50.3%) | |
| Women | 88 (52.7%) | 39 (47.0%) | 41 (49.4%) | |
| Age, y | 63.3 ± 5.5 | 63.3 ± 5.5 | 62.8 ± 5.5 | 0.78 |
| Grade of employment | 0.63 | |||
| Higher | 64 (38.3%) | 30 (36.1%) | 31 (37.3%) | |
| Intermediate | 60 (35.9%) | 38 (45.8%) | 35 (42.2%) | |
| Lower | 43 (25.7%) | 15 (18.1%) | 17 (20.5%) | |
| Educational attainment | 0.75 | |||
| No qualifications | 12 (7.7%) | 9 (11.7%) | 6 (7.7%) | |
| Basic qualifications | 42 (26.9%) | 24 (31.2%) | 18 (23.1%) | |
| High school graduation | 44 (28.2%) | 19 (24.7%) | 29 (37.2%) | |
| College or higher | 58 (37.2%) | 25 (32.5%) | 25 (32.1%) | |
| BMI | 25.6 ± 4.0 | 25.8 ± 4.1 | 26.5 (3.8) | 0.13 |
| Current smoker | 9 (5.4%) | 7 (8.4%) | 9 (10.8%) | 0.11 |
| Systolic BP, mmHg | 126.5 ± 14.7 | 127.8 ± 14.6 | 125.6 ± 14.2 | 0.64 |
| Diastolic BP, mmHg | 73.2 ± 9.1 | 73.4 ± 8.1 | 72.4 ± 8.9 | 0.88 |
| Heart rate, bpm | 66.6 ± 8.8 | 66.8 ± 9.3 | 67.2 ± 8.7 | 0.88 |
| Heart rate variability, log ms | 3.07 ± 0.52 | 3.11 ± 0.48 | 3.03 ± 0.43 | 0.61 |
| Cortisol, log nmol/L | 1.90 ± 0.46 | 1.87 ± 0.37 | 1.93 ± 0.45 | 0.70 |
| C-reactive protein, mg/L | 1.87 ± 2.49 | 1.63 ± 1.70 | 1.90 ± 2.42 | 0.94 |
| IL-6, pg/mL | 1.42 ± 0.87 | 1.34 ± 0.83 | 1.33 ± 0.72 | 0.41 |
| MCP-1, log pg/mL | 4.88 ± 0.28 | 4.89 ± 0.36 | 4.90 ± 0.31 | 0.65 |
| TL (T/S ratio) | 1.05 ± 0.042 | 0.94 ± 0.042 | 0.93 ± 0.074 | 0.001 |
| TA (per 1,000 live cells) | 10.87 ± 7.16 | 5.12 ± 1.75 | 14.82 ± 6.89 | 0.001 |
bpm, beats per minute; T/S ratio, telomere/beta-globin gene ratio.
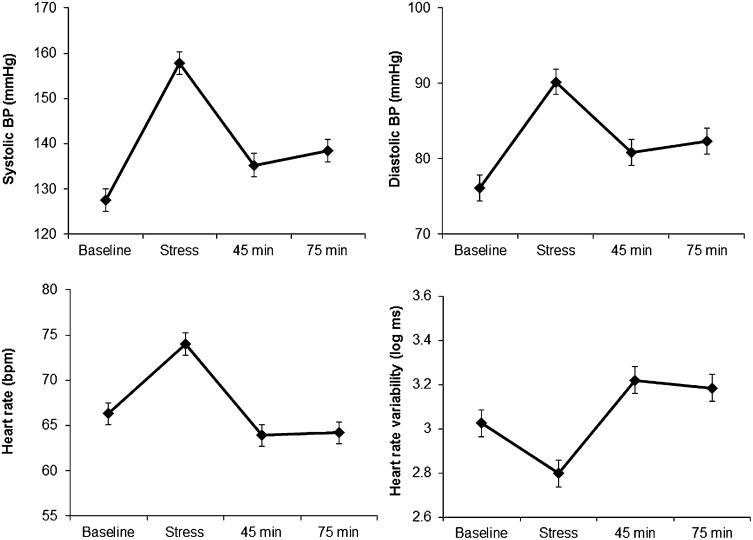
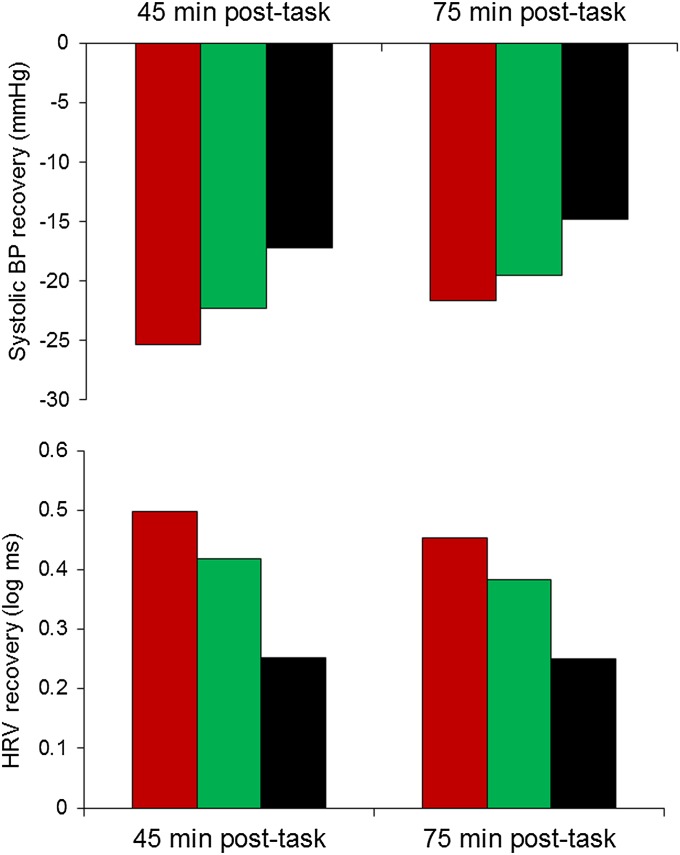
The mental stress tasks elicited substantial cardiovascular responses, with increases in systolic and diastolic blood pressure (BP) and heart rate, together with reduced heart rate variability (P < 0.001; Fig. 1). These responses were accompanied by increases in subjective stress (on a scale from 1 to 7) from an average rating of 1.42 ± (SD) 0.8 at baseline to 4.15 ± (SD) 1.4 following tasks. BP showed a partial recovery to baseline values at 45 and 75 min following stress, whereas heart rate and heart rate variability recovered to levels below and above baseline, respectively. However, the variation between people in stress reactivity and recovery was substantial. We found that in men, poststress recovery in systolic BP was associated with telomere group. The reduction in systolic BP between tasks and recovery measures was greatest in the long TL group and least in the short TL/high TA group, with an intermediate pattern in the short TL/low TA group, after statistical adjustment for age, SES, BMI, and smoking status (P = 0.008 and P = 0.026 for 45 and 75 min, respectively; Fig. 2). This result indicates that the short TL/high TA group exhibited impaired poststress recovery. Heart rate variability recovery following stress was also reduced in men in the short TL/high TA group (P = 0.003 and P = 0.047 for 45 and 75 min, respectively); as shown in Fig. 2, the increase in heart rate variability between task and recovery periods was greatest in the long TL group but 50% smaller in the short TL/high TA participants. Subjective stress responses did not differ between telomere groups. The corresponding effects in women were not significant (Table S2).
Fig. 1.
Mean levels of systolic BP. Diastolic BP, heart rate, and heart rate variability (log values) during baseline trials, stress tasks, and 40–45 min and 70–75 min following stress are shown. Error bars are SEMs.
Fig. 2.
Differences between systolic BP during tasks (Upper) and heart rate variability during tasks (Lower) and values recorded 40–45 min and 70–75 min following tasks in men. The red bar represents the long telomere group, the green bar represents the short TL/low TA group, and the black bar represents the short TL/high TA group. Values are adjusted for age, grade of employment, BMI, and smoking status.
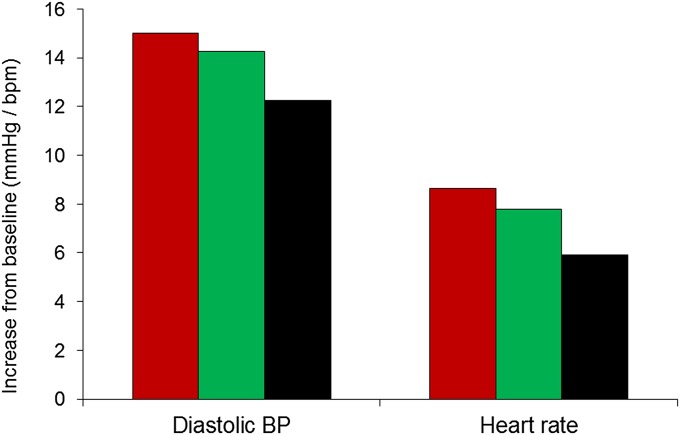
Diastolic BP and heart rate recovery after stress did not vary with telomere group. Instead, we found evidence for a relationship with stress reactivity because both diastolic BP and heart rate reactivity to the mental stress tasks were attenuated in the short TL/high TA group compared with other groups (P = 0.037 and P = 0.027, respectively). The mean diastolic BP and heart rate stress responses were 18% and 32% lower in the short TL/high TA group compared with the long TL group, with an intermediate pattern in the short TL/low TA group (Fig. 3). Neither association was significant in women (Table S2).
Fig. 3.
Differences between diastolic BP and heart rate during tasks and baseline in men in the long telomere group (red bar), short TL/low TA group (green bar), and short TL/high TA group (black bar). Values are adjusted for age, grade of employment, BMI, and smoking status.
The laboratory stress protocol elicited small but significant salivary cortisol responses, as described previously (29). We found that responses to stress varied across telomere groups in men (P = 0.02) but not women. Cortisol increased from baseline to the poststress task sample in the long TL (adjusted mean log increase of 1.06 ± 3.9 nmol/L) and in the short TL/low TA group (mean of 1.07 ± 5.2) but fell, on average, in the short TL/high TA group (mean of −0.74 ± 2.8) after adjustment for age, SES, BMI, smoking status, and time of stress testing. This finding indicates that the male short TL/high TA group displayed blunted cortisol responses to acute mental stress.
We did not observe associations between telomere group and the magnitude of IL-6 responses to short-term stress, but we did see differences in monocyte chemoattractant protein-1 (MCP-1) responses. The concentration of MCP-1 increased following the stress and returned toward baseline in samples taken 45 min after the tasks (P < 0.001), and we found that the extent of poststress recovery varied with telomere group in men. The decrease in MCP-1 concentration between samples taken immediately after tasks and 45 min later was greater in the long TL group (adjusted log mean change of 0.079 ± 0.18 pg/mL) and in the short TL/low TA group (0.069 ± 0.20 pg/mL) than in the short TL/high TA group, where there was actually a small increase on average (−0.020 ± 0.17 pg/mL; P = 0.011). There were no associations in women (Table S2).
In addition to stress-related biological processes, we analyzed relationships between telomere groups and psychosocial factors contributing to stress resistance. As can be seen in Table 2, we found differences in both psychological and social resources, although not in depressive symptoms. In each case, the short TL/high TA group had fewer resources; thus, social support measured with the Enriched Social Support Instrument was significantly lower and optimism measured with the Life Orientation Test (LOT) was lower, whereas hostility assessed with the Cook Medley cynical hostility scale was higher in the short TL/high TA group. All these differences were evident only in men and not in women (Table S2).
Table 2.
Telomere phenotypes and psychosocial resources in men
| Psychosocial factor | Long telomeres | Short TL/low TA | Short TL/high TA | P, trend |
| Social support | 27.95 ± 4.9 | 26.09 ± 6.3 | 25.28 ± 6.1 | 0.013 |
| Optimism | 16.62 ± 3.7 | 15.70 ± 4.3 | 14.89 ± 3.8 | 0.020 |
| Hostility | 2.42 ± 2.3 | 3.12 ± 2.6 | 3.75 ± 3.0 | 0.006 |
| Depressive symptoms | 6.11 ± 5.9 | 7.27 ± 7.0 | 6.42 ± 7.1 | 0.80 |
Values are adjusted for age and grade of employment.
Because the number of participants who experienced major early life adversity was small, our analyses combined men and women. We found that the proportion reporting that their mothers had died before they were 16 y of age was greater in the short TL/high TA group (13.2%) than in the long TL (6.0%) or short TL/low TA (2.4%) group. Compared with respondents in the long TL group, the odds of having experienced such maternal loss were significantly greater in the short TL/high TA group (odds ratio = 2.55, 95% confidence interval: 1.02–6.39; P = 0.046) after adjustment for age, sex, SES, and BMI. There was no association of telomere/telomerase phenotype with paternal loss or with a combined measure of acute and chronic early life adversity.
We carried out additional analyses to check whether responses varied among individuals with long TL in relation to TA. No differences associated with TA were apparent in the physiological or psychological variables assessed in this study.
Discussion
Our analyses of this sample of healthy older men and women identified a distinctive pattern of aberrant responses to mental stress and impaired psychosocial resources in men characterized by shorter leukocyte telomeres coupled with high levels of TA. In comparison to men with long telomeres or shorter telomeres coupled with low TA, the short TL/high TA group showed blunted poststress recovery in systolic BP, heart rate variability, and plasma MCP-1 concentration, combined with reduced stress responses in diastolic BP, heart rate, and salivary cortisol. The short TL/high TA group also reported less social support, greater hostility, and less optimistic dispositions than the other groups, together with greater early life adversity in the form of death of their mothers before they were 16 y of age. These associations were independent of relevant covariates, such as age, SES, and BMI. The differences in biology were not apparent in measures taken at rest, but only when physiological regulation was challenged by mental stress. According to the allostatic load model, impairment of physiological regulation is likely to be manifest first as disturbances of dynamic regulatory processes, including failure in poststress recovery and reduced capacity to mount effective stress responses. If the conditions eliciting these responses persist, they may subsequently result in alterations of resting function (27, 30). In contrast to men, women did not exhibit any differences between telomere phenotype groups in cardiovascular, neuroendocrine, or inflammatory factors during or after acute mental stress. Allostatic load is generally assessed by measuring a range of markers across physiological systems. However, it is apparent that the different systems investigated here varied in their dynamics and time course of response, so analysis of separate measures was warranted.
Acute mental stress elicits brisk increases in BP, heart rate, hemostatic and inflammatory markers, and cortisol concentration, together with reduced heart rate variability, with levels rapidly returning to baseline soon after the stress ends. Slow recovery following stress is a cardinal sign of chronic allostatic load, indicative of the inability of regulatory processes to engender prompt restoration of homeostasis following challenge (30). Delayed poststress BP and heart rate variability recovery is more common in adults with a lower SES (31), and it has been associated with social isolation, poor mental health, and impaired hemostatic responses to stress (32). It also predicts future increases in BP and abdominal adiposity (33, 34). Sustained reductions in heart rate variability following stress may have adverse effects on cardiovascular disease risk (35). We also showed slow recovery of MCP-1 concentration after stress. The chemokine MCP-1 is involved in the pathogenesis of atherosclerosis by promoting recruitment of leukocytes to sites of inflammation in blood vessel walls (36). Raised MCP-1 concentration predicts increased risk of future coronary heart disease (37). Taken together, these findings indicate that the combination of shorter telomeres and raised TA is associated with a profile of disrupted biological regulation that is manifest in delays in the restitution of healthy cardiovascular and inflammatory function following stress.
In addition, we found that the short TL/high TA phenotype was associated with blunted reactivity to acute stress in diastolic BP, heart rate, and cortisol in men. Although heightened stress reactivity is thought to predict future cardiovascular disease (38), reduced responsivity may be another marker of allostatic load and may reflect a failure to mount effective physiological defense responses. Thus, reduced cardiovascular and cortisol responsivity has been linked with increased risk of obesity, symptoms of depression, and poor self-rated health (39), and it is also associated with chronic adversity (40).
An alternative explanation of reduced stress responsivity in the short TL/high TA group is that these individuals were less stressed by the laboratory protocol, so it elicited smaller physiological responses. However, subjective stress ratings increased to the same extent across the telomere groups, so this hypothesis is unlikely to be the explanation of the pattern of results.
The association of telomere phenotypes with social support, optimism, and hostility extends previous work that has focused on psychosocial factors and TL alone. Our findings indicate that although shorter leukocyte telomeres may be related to low social support and low optimism (18, 20); the deficits are augmented when shorter TL is coupled with heightened TA. Low social support, low optimism, and raised hostility are all thought to be relevant to future impaired physical health (27, 41, 42).
We also observed an association between early life adversity and the combination of shorter telomeres and raised TA, as indicated by the greater likelihood of experiencing the death of one’s mother in childhood. Although this result is consistent with previous evidence linking shorter telomeres with childhood adversity (13), the finding should be interpreted with caution because accounts of childhood adversity were retrospective and the numbers reporting maternal loss were small.
The association between psychosocial risk factors, impaired biological responses, and the combination of shorter TL with raised TA was restricted to men in our analyses. The numbers of men and women in the short TL/high TA groups were similar, but there was no indication of comparable trends in physiological responses (Tables S1 and S2). Studies relating shorter telomeres to life stress have involved both men and women (10, 11, 14, 18, 20). Previous research relating telomeres to acute physiological stress responses have been carried out with female but not male samples (15, 21, 24), although associations between health risk and the combination of shorter telomeres and raised TA have been reported in both men and women (16, 25). The explanation for the sex difference in response profiles in our study is not clear. Hormonal processes are unlikely to be directly responsible, because women in this study were postmenopausal. However, estrogen is thought to induce telomerase (43) and might provide protection of telomeres, antagonizing the effect of increased allostatic load on telomere attrition during earlier stages of life. Additionally, testosterone increases susceptibility to oxidative stress (44) and reduces immunocompetence (45). Sex differences in body size and antioxidant capacity (46, 47) may be relevant but could not be evaluated in this investigation.
The combination of high TA with short TL could be indicative of increased cellular turnover in the bone marrow compartment taking place as a result of immune overstimulation. In addition, this combination may reflect the inability of the organism to reactivate telomerase to an extent high enough that it can counteract a more pronounced erosion of telomeres under conditions of heightened stress. Whether this phenotype might also represent an early marker of replicative immunosenescence remains to be determined.
A number of factors must be taken into account in interpreting our findings. First, the sample was restricted to a healthy older population (average age of 63 y), so it is not known if the links between mental stress, psychosocial factors, and TL/TA would be the same in a younger age range or among individuals with preexisting health problems. Additionally, our study investigated acute mental stress, and the associations with responses to chronic real-life stress were not tested. The study was cross-sectional, so we do not know the temporal relationship between telomere function, altered physiological stress responsivity, and reduced psychosocial resources. Nevertheless, the addition of TA to measures of TL is an advance in understanding the cellular substrate of stress-related psychosocial and biological processes, because it indicates a dynamic interplay between social environmental exposures and the mechanisms that support chromosome integrity. The relationship between telomere function and physiological indicators of allostatic load may place people with short telomeres and high TA at increased health risk. Elevated allostatic load predicts future mortality and functional decline at older ages (48, 49). Our study suggests that these processes could be mediated through accelerated cellular aging.
Materials and Methods
Participants.
The data analyzed in this report come from the Heart Scan Study, a subsample of the Whitehall II epidemiological cohort recruited between 2006 and 2008 to investigate socioeconomic and psychosocial factors, physiological stress responsivity, and subclinical coronary artery disease (29). We recruited a subsample of 543 men and women of white European origin with no history or objective signs of coronary heart disease and no previous diagnosis or treatment for hypertension, diabetes, inflammatory diseases, or allergies. Civil service employment grade was used as an indicator of SES, and recruitment was stratified to include a range of employment grades. Sampling of leukocyte telomeres was delayed with respect to the start of the study, which, together with some assay and transportation difficulties, led to leukocyte TL being measured in 434 participants and TA being measured in 416 participants. Of these participants, complete datasets were obtained on 333 (165 men and 168 women) aged 54–76 y, which form the basis of this analysis. All participants gave full informed consent, and ethical approval was obtained from the University College London Hospital Committee on the Ethics of Human Research.
Laboratory Stress Testing.
We tested participants individually in a light- and temperature-controlled laboratory. Scheduling demands required that sessions be held both in the morning and in the afternoon. Over the 7 d before testing, participants were required not to take anti-inflammatory or antihistamine medications and were rescheduled if they reported colds or other infections on the day of testing. They were instructed to refrain from drinking caffeinated beverages or smoking for at least 2 h before testing and to avoid vigorous exercise and alcohol from the previous evening. At the start of the session, we measured height, weight, and waist and hip circumference using standardized techniques, and BMI was computed. BP, heart rate, and heart rate variability were monitored continuously using a Finometer (Finapres Medical Systems), and a venous cannula was inserted for the collection of blood samples, after which the participant rested for 30 min. Systolic and diastolic BP and heart rate were monitored over the last 5 min of this period to provide baseline values, after which the baseline blood sample was drawn and saliva was collected for the analysis of cortisol. Two 5-min behavioral tasks were then administered in random order. One was a computerized version of the Stroop color-word interference task, which involved successive presentation of target color words (e.g., red, blue) printed in another color (31). There were four names of colors printed in incongruous colors at the bottom of the computer screen. Participants were requested to press the computer key that corresponded to the position at the bottom of the screen of the name of the color in which the target word was printed. To ensure sustained demands, the rate of presentation of stimuli was adjusted to the performance of the participant. The second task was mirror tracing, which involved tracing with a metal stylus a star that could only be seen in a mirror image. Each time the stylus came off the star, a mistake was registered and a loud beep was emitted by the apparatus (Lafayette Instruments Corp.). Participants were told that the average person could complete five circuits of the star in the available time. These tasks were selected because they have been shown to stimulate similar appraisals of involvement and engagement from participants across the social gradient (31). Cardiovascular activity was monitored throughout tasks, and a second blood and saliva sample was taken immediately after tasks. Monitoring of poststress recovery patterns of cardiovascular activity took place 40–45 min and 70–75 min following tasks, and additional blood samples were drawn at 45 min. We also obtained ratings of subjective stress at the same time points using a seven-point scale (from 1 = no stress to 7 = maximum stress).
Psychological Measures.
Social support was assessed using the seven-item Enhancing Recovery in Coronary Heart Disease Social Support Instrument, which assesses structural instrumental and emotional support (50), and optimism was measured using the LOT (51). We assessed cynical hostility with 10 items from the Cook Medley hostility scale and depressive symptoms with the Center for Epidemiologic Studies Depression Scale. The Cronbach α was satisfactory for the different measures (range: 0.77–0.87).
Early Life Adversity.
We asked respondents whether they had experienced the death of a parent or sibling before they were 16 y of age; whether they had been separated from their parents for at least 12 mo in childhood; and whether they had lived in a family in which one member had severe mental illness (including depression), drug problems, or chronic illness. Death of a parent (mother of 23 participants, father of 27 participants) was the most common acute event, so it was analyzed together with a combined index of death of a parent or sibling, separation, and living with chronic adversity.
Biological Measures.
Blood samples were collected in EDTA tubes and centrifuged immediately at 1300 × g for 10 min at room temperature. Plasma was removed from the tube, aliquoted into 0.5-mL portions, and stored at 80 °C until analysis. Plasma IL-6 was assayed using a Quantikine1 high sensitivity two-site ELISA from R&D Systems. The sensitivity of the assay ranged from 0.016 to 0.110 pg/mL, and the intra- and interassay coefficients of variation (CVs) were 7.3% and 7.7%, respectively. MCP-1 was assayed in duplicate using fluorescent-labeled capture antibody beads from Millipore Corporation (Milliplex Human Cytokine/Chemokine kit), and concentrations were determined with Luminex technology using a Bio-Plex array reader (Bio-Rad). The limit of detection was 1.2 pg/mL, and the mean intra- and interassay CVs were 6.1% and 12%, respectively. Saliva samples were obtained for the assessment of cortisol. The samples were collected using Salivettes (Sarstedt), and cortisol levels were assessed using a time-resolved immunoassay with fluorescence detection at the University of Dresden. The intra- and interassay CVs were less than 8%.
Telomere Assays.
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMCs) in a QIAcube workstation with the QIAamp DNA Blood Mini Kit (Qiagen) according to instructions of the manufacturer and stored in 10 mmol/L Tris⋅hydrochloric acid and 0.5 mmol/L EDTA (pH 9.0) at −20 °C. Relative mean TL was measured in triplicate by a monochrome multiplex quantitative real-time PCR assay with a Bio-Rad CFX96 Real-Time PCR Detection System, as previously described (22). Reactions containing serial dilutions of a reference DNA standard were included in each PCR plate to generate the telomere (T) and beta-globin gene (S) standard curves required for quantitation, and relative mean TL, expressed as a T/S ratio, was derived.
Leukocyte TA was measured by the Telomerase Repeat Amplification Protocol with a commercial assay (TRAPeze, Telomerase Detection Kit; Upstate/CHEMICON) as described previously (16). As positive control standards, 293T human cancer cells were used, and TA was expressed as the equivalent of the number of 293T cells per 10,000 PBMCs.
Data Reduction and Statistical Analysis.
BP, heart rate, and heart rate variability were averaged into five 5-min periods: baseline, color-word interference task, mirror tracing task, 40–45 min poststress, and 70–75 min poststress. Heart rate variability was quantified in a time domain measure as the rms of successive differences, and it was log-transformed before analysis. Cardiovascular activity was analyzed with repeated measures ANOVA with TL/TA group and sex as between-person factors and trial (baseline, combined task trials, and 45- and 75-min recovery trials) as within-person factors. We quantified cardiovascular reactions to stress by computing difference scores between task trials and baseline, and carrying out analysis of covariance with age, sex, SES, BMI, and smoking status as covariates. Stress recovery was quantified by calculating difference scores between task values and 45- and 75-min trials, so more effective recovery was reflected in larger difference scores. We measured cortisol stress responses as differences between baseline and posttask saliva samples, and we measured IL-6 and MCP-1 responses as differences between baseline and posttask blood samples. Values of IL-6 and MCP-1 were log-transformed, and analyzed with age, sex, SES, BMI, and smoking status as covariates, and cortisol responses were additionally adjusted for the time of day at which stress testing took place. Differences between TL/TA groups in social support, optimism, hostility, and depressive symptoms were also analyzed with analysis of covariance, whereas logistic regression was used to analyze early life adversity. Sex was included as a factor in all initial analyses, but interactions between telomere group and sex were observed, and we subsequently found that differences between TL/TA groups were present only in men. The results for women are therefore not presented, except in the case of early life adversity, where the sexes were combined because exposure rates were low.
Supplementary Material
Acknowledgments
We thank Lena Brydon, Lee Butcher, Katie O’Donnell, Romano Endrighi, Yoichi Chida, Nadine Messerli-Burgy, Andrew Wawrzyniak, and Bev Murray for their contributions to data collection. This research was funded by the British Heart Foundation (Grant RG/10/005/28296), the Medical Research Council (Grant G0601647), and the Bernard and Barbro Fund (E.H.B.).
Footnotes
Conflict of interest statement: J.L. and E.H.B. are cofounders of Telome Health, a diagnostic company measuring telomere biology. All other authors report no biomedical financial interests or potential conflicts of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322145111/-/DCSupplemental.
References
- 1.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 2.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Codd V, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427. doi: 10.1038/ng.2528. 427e1–427e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 7.Epel ES, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany, NY Online) 2009;1(1):81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick AL, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, et al. Short leukocyte telomere length predicts risk of diabetes in american indians: The strong heart family study. Diabetes. 2014;63(1):354–362. doi: 10.2337/db13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks CG, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18(2):551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikgren M, et al. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71(4):294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Epel ES, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: An overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon NM, et al. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Tomiyama AJ, et al. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav. 2012;106(1):40–45. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brydon L, et al. Hostility and cellular aging in men from the Whitehall II cohort. Biol Psychiatry. 2012;71(9):767–773. doi: 10.1016/j.biopsych.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huzen J, et al. Telomere length and psychological well-being in patients with chronic heart failure. Age Ageing. 2010;39(2):223–227. doi: 10.1093/ageing/afp256. [DOI] [PubMed] [Google Scholar]
- 18.O’Donovan A, et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23(4):446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaffer JA, et al. Depressive symptoms are not associated with leukocyte telomere length: Findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PLoS ONE. 2012;7(10):e48318. doi: 10.1371/journal.pone.0048318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll JE, Diez Roux AV, Fitzpatrick AL, Seeman T. Low social support is associated with shorter leukocyte telomere length in late life: Multi-ethnic study of atherosclerosis. Psychosom Med. 2013;75(2):171–177. doi: 10.1097/PSY.0b013e31828233bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donovan A, et al. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun. 2012;26(4):573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steptoe A, et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 2011;25(7):1292–1298. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Uchino BN, et al. Social relationships and health: Is feeling positive, negative, or both (ambivalent) about your social ties related to telomeres? Health Psychol. 2012;31(6):789–796. doi: 10.1037/a0026836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epel ES, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24(4):531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epel ES, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke CH, et al. Telomerase, telomere length, and coronary artery calcium in black and white men in the CARDIA study. Atherosclerosis. 2012;220(2):506–512. doi: 10.1016/j.atherosclerosis.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: A meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53(11):936–946. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 29.Hamer M, O’Donnell K, Lahiri A, Steptoe A. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. Eur Heart J. 2010;31(4):424–429. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steptoe A, et al. Stress responsivity and socioeconomic status: A mechanism for increased cardiovascular disease risk? Eur Heart J. 2002;23(22):1757–1763. doi: 10.1053/euhj.2001.3233. [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Marmot M. Psychosocial, hemostatic, and inflammatory correlates of delayed poststress blood pressure recovery. Psychosom Med. 2006;68(4):531–537. doi: 10.1097/01.psy.0000227751.82103.65. [DOI] [PubMed] [Google Scholar]
- 33.Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens. 2005;23(3):529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- 34.Steptoe A, Wardle J. Cardiovascular stress responsivity, body mass and abdominal adiposity. Int J Obes (Lond) 2005;29(11):1329–1337. doi: 10.1038/sj.ijo.0803011. [DOI] [PubMed] [Google Scholar]
- 35.Hillebrand S, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. doi: 10.1093/europace/eus341. [DOI] [PubMed] [Google Scholar]
- 36.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 37.Herder C, et al. Chemokines and incident coronary heart disease: Results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Arterioscler Thromb Vasc Biol. 2006;26(9):2147–2152. doi: 10.1161/01.ATV.0000235691.84430.86. [DOI] [PubMed] [Google Scholar]
- 38.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 39.de Rooij SR. Blunted cardiovascular and cortisol reactivity to acute psychological stress: A summary of results from the Dutch Famine Birth Cohort Study. Int J Psychophysiol. 2013;90(1):21–27. doi: 10.1016/j.ijpsycho.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: Studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry. 2012;71(4):344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giltay EJ, Geleijnse JM, Zitman FG, Hoekstra T, Schouten EG. Dispositional optimism and all-cause and cardiovascular mortality in a prospective cohort of elderly dutch men and women. Arch Gen Psychiatry. 2004;61(11):1126–1135. doi: 10.1001/archpsyc.61.11.1126. [DOI] [PubMed] [Google Scholar]
- 42.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kyo S, et al. Estrogen activates telomerase. Cancer Res. 1999;59(23):5917–5921. [PubMed] [Google Scholar]
- 44.Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G. Testosterone and oxidative stress: The oxidation handicap hypothesis. Proc Biol Sci. 2007;274(1611):819–825. doi: 10.1098/rspb.2006.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17(5):527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- 46.Borrás C, et al. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34(5):546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 47.Vassalle C, Maffei S, Boni C, Zucchelli GC. Gender-related differences in oxidative stress levels among elderly patients with coronary artery disease. Fertil Steril. 2008;89(3):608–613. doi: 10.1016/j.fertnstert.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 48.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell PH, et al. A short social support measure for patients recovering from myocardial infarction: The ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23(6):398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.