Significance
Montane species may respond to global warming by shifting their distributions upslope in response to warming temperatures. Such shifts are well documented in the temperate zone, but the situation in the tropics, where species are predicted to be more sensitive to altered temperatures, remains unclear. We found strong upslope shifts in the bird species found on two independent New Guinean mountains originally surveyed in the 1960s. Moreover, we incorporated our results into a metaanalysis that suggests tropical montane species are responding more strongly to climate change than temperate-zone species. Though the magnitude of climate change is higher in the temperate zone, both overall biodiversity and distributional shifts due to warming temperatures are greater in the tropics.
Keywords: climate change, tropical biodiversity, tropical mountains
Abstract
Temperate-zone species have responded to warming temperatures by shifting their distributions poleward and upslope. Thermal tolerance data suggests that tropical species may respond to warming temperatures even more strongly than temperate-zone species, but this prediction has yet to be tested. We addressed this data gap by conducting resurveys to measure distributional responses to temperature increases in the elevational limits of the avifaunas of two geographically and faunally independent New Guinean mountains, Mt. Karimui and Karkar Island, 47 and 44 y after they were originally surveyed. Although species richness is roughly five times greater on mainland Mt. Karimui than oceanic Karkar Island, distributional shifts at both sites were similar: upslope shifts averaged 113 m (Mt. Karimui) and 152 m (Karkar Island) for upper limits and 95 m (Mt. Karimui) and 123 m (Karkar Island) for lower limits. We incorporated these results into a metaanalysis to compare distributional responses of tropical species with those of temperate-zone species, finding that average upslope shifts in tropical montane species match local temperature increases significantly more closely than in temperate-zone montane species. That tropical species appear to be strong responders has global conservation implications and provides empirical support to hitherto untested models that predict widespread extinctions in upper-elevation tropical endemics with small ranges.
Temperate species are responding to anthropogenic temperature increases by rapidly shifting geographic distributions to track their climatic niche (1–3). These shifts appear to be increasing in pace—a recent metaanalysis concluded that species are shifting their distributions poleward and upslope much faster than previously estimated (1, 2). Range shifts are less studied in tropical regions however (1, 4, 5), despite being home to the vast majority of biodiversity (6). Notwithstanding strong latitudinal bias in empirical studies, climate change-driven range shifts are predicted to cause widespread extinctions in both temperate and tropical species within the next century (7–10).
With scarce empirical data, models of tropical species’ response to temperature increases predict a wide range of responses (11). At one extreme, tropical species may be relatively unaffected, as the magnitude of temperature increases is relatively low in the tropics (12). Alternately, vulnerability to warming temperatures could be highest in the tropics if tropical species are physiologically specialized to narrow thermal niches (13–18). Such thermal specialization has been documented in tropical ectotherms (16, 17), but it is unclear whether similar patterns may apply to tropical endotherms, whose distributional shifts in response to warming may result from indirect rather than direct impacts of temperature increases (5).
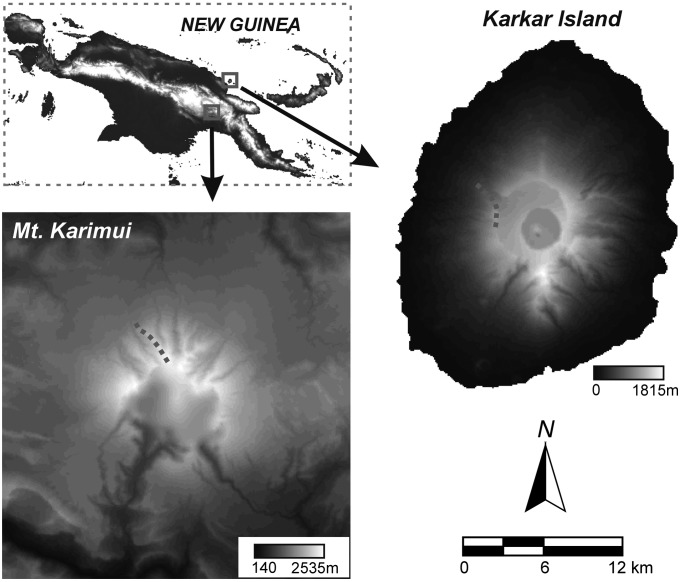
We resurveyed geographically and faunally independent elevational gradients in New Guinea nearly a half-century after they were first surveyed. The original transect surveys were conducted by J. Diamond to determine bird species’ elevational limits on Mt. Karimui (July–August 1965) (19) and Karkar Island (May 1969) (20). These environments differ significantly: Mt. Karimui is located in New Guinea’s biodiverse Central Ranges and harbors a diverse resident avifauna of ca. 250 resident landbirds (19), whereas Karkar Island is a small oceanic island off New Guinea’s north coast with a depauperate flora and fauna (ca. 50 resident landbirds) dominated by highly dispersive taxa (20) (Fig. 1).
Fig. 1.
Map of resurvey sites in Papua New Guinea. The elevational transects recently revisited by the authors are marked by dashed lines (Mt. Karimui: 1,130–2,520 m; Karkar Island: 800–1,600 m). Mt. Karimui is an extinct volcano in the southern Central Ranges of New Guinea, whereas Karkar Island is an oceanic island located 10 miles from the New Guinean mainland. These elevational gradients were originally surveyed by Diamond in the 1960s [Mt. Karimui: 1965 (19); Karkar Island: 1969 (20)], and remain covered in primary forest.
We used elevational limits measured during historical transects and modern resurveys to investigate New Guinean montane birds’ response to warming temperatures. We predicted that species have moved upslope relative to historical range limits. Given that tropical species are hypothesized to be especially sensitive to temperature increases (either directly or via indirect ecological interactions), we additionally predicted that the magnitude of upslope shifts would closely match predicted shifts based on local temperature increases. We simultaneously tested two additional hypotheses, investigating whether upslope shifts at the leading range margin outpaced upslope shifts at the trailing range edge (21), and whether species’ dietary preferences influenced upslope shifts (22, 23). We then used our data in conjunction with recent tropical resurveys to test the tropical-species-are-strong-responders hypothesis, predicting that upslope shifts measured in tropical resurveys match predicted upslope shifts significantly more closely than for temperate-zone resurveys.
Results and Discussion
Land-use changes along the elevational gradients studied on Mt. Karimui and Karkar Island have been minimal: climate change is the only major environmental change that has occurred since Diamond’s historical transects (Methods) (24). Long-term temperature data from our study sites does not exist, so we used global climate models to estimate the magnitude of warming in the 0.5° × 0.5° grids containing our study sites (25), following the methodology of similar resurveys (1, 4, 5). These models estimated an increase in annual mean temperature of 0.39 °C and 0.46 °C between historical transect and modern resurvey for the grid cells containing Mt. Karimui and Karkar Island, respectively (25). Average temperature declines linearly with elevation on tropical mountains, and we measured the lapse rate on Mt. Karimui as 0.51 °C per 100 m. Applying this lapse rate to estimated temperature increases predicted elevational shifts of 76 m for Mt. Karimui and 90 m for Karkar Island.
We resurveyed identical transect locations at the same time of year (Mt. Karimui: June–July 2012; Karkar Island: April 2013), and with similar survey effort to Diamond’s historical surveys (Methods). We also conducted a second resurvey of Mt. Karimui in October–November 2012 to test whether seasonal variation affected avian elevational distributions. Although separated by only a few months, these two modern resurveys represent distinct seasons for birds in the New Guinea highlands; June–July is the peak of the dry season and after the end of the main breeding season, whereas October–November approaches the peak of the rainy season and is the beginning of the breeding season (19). There was no systematic bias in elevational limits measured on Mt. Karimui in October–November 2012 compared with limits measured in June–July 2012 for either upper limits (n = 96; 19 ± 163 m; Wilcoxon signed-rank V = 1,196, P = 0.23; all statistics presented are mean ± SD) or lower limits (n = 36; 13 ± 148 m; Wilcoxon signed-rank V = 316, P = 0.79). We therefore present the results of analyses using June–July data in this paper, as this resurvey closely matches the seasonality of the historical survey conducted in July–August.
Bird species significantly shifted their upper limits upslope on both Mt. Karimui (113 ± 197 m; n = 123; t122 = 6.30, P < 0.001, Table S1) and Karkar Island (153 ± 184 m; n = 22; t21 = 3.90, P < 0.001, Table S2) (Fig. 2). Upslope shifts also occurred at species’ lower limits on Mt. Karimui (95 ± 190 m; n = 53 species; t52 = 3.63, P < 0.001) (Fig. 2). Few species are restricted to montane elevations on Karkar Island. Consequently, upslope shifts in species’ lower limits at this site were not statistically significant (123 ± 200 m; n = 5 species; t4 = 1.38, P = 0.24), and we do not further consider shifts at lower limits on Karkar Island. Upslope shifts significantly outnumbered downslope shifts at both upper limits (Mt. Karimui: 87 upslope, 36 downslope, P < 0.001; Karkar Island: 17 upslope, 5 downslope, P = 0.017) and lower limits (Mt. Karimui: 39 upslope, 14 downslope, P < 0.001, Fig. 2). Because species on Mt. Karimui moved upslope at both upper and lower limits, the total elevational extent of species’ elevational distributions did not change (n = 41; paired t test: t40 = −0.82, P = 0.42). Finally, average upslope shifts exceeded predicted shifts based on estimated warming in all cases, although we caution that global temperature models may not precisely estimate local temperature increases and note the wide variation in measured upslope shifts.
Fig. 2.

Changes in species’ elevational limits for Mt. Karimui upper elevational limits (A), Mt. Karimui lower elevational limits (B), and Karkar Island upper elevational limits (C). Changes in species’ elevational limits between historical and modern resurveys are plotted against historical elevational limits measured in the 1960s (19, 20). Points on the solid zero-change lines represent species with unchanged elevational limits.
Upper limit range shifts were greater than lower limit range shifts at both study sites, but this difference was not significant (Mt. Karimui, n = 41; paired t test: t40 = 0.82, P = 0.42). Variation in species’ responses was weakly influenced by diet on Karkar Island (upper limit: F5 = 2.6, P = 0.066) but not on Mt. Karimui (upper limit: F4 = 0.64, P = 0.63; lower limit: F3 = 1.53, P = 0.22). Specifically, upslope shifts in Karkar Island species’ upper limits were larger for omnivores (n = 3: 330 ± 289 m) and frugivores (n = 7: 242 ± 178 m) than for insectivores (n = 5: 107 ± 181 m) and nectivores (n = 3: 88 ± 60 m).
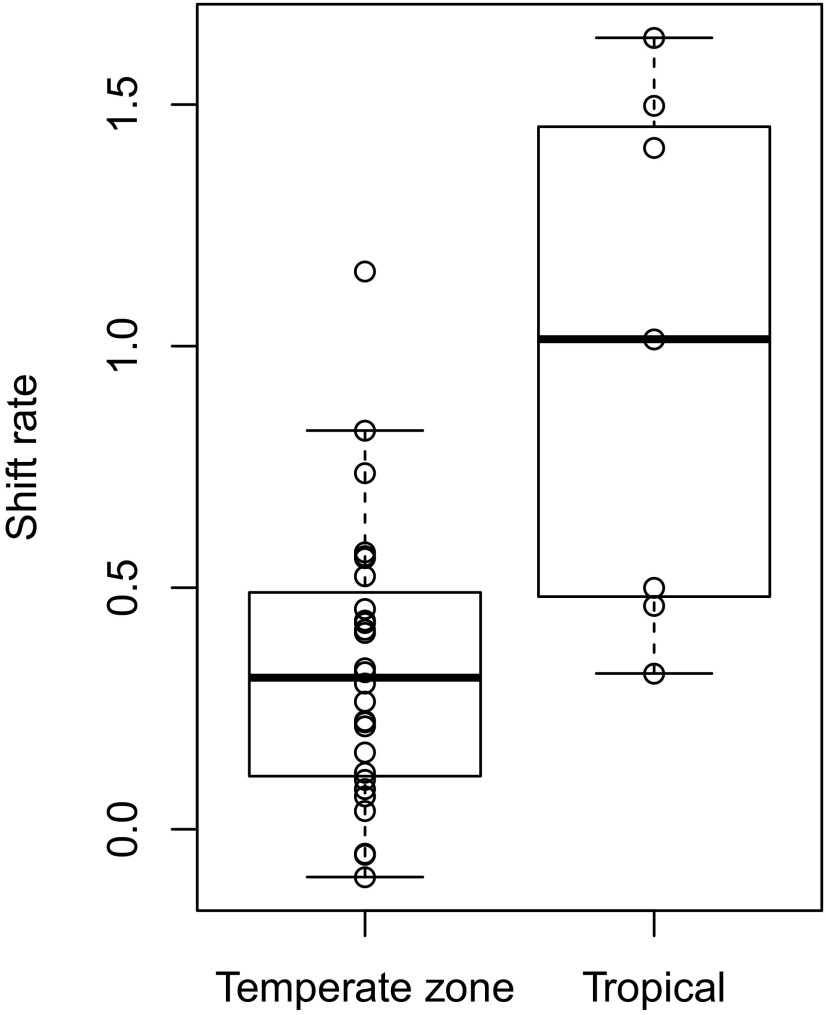
A recent metaanalysis of 31 resurveys in a variety of taxa found that elevational shifts averaged less than half of predicted shifts based on local temperature increases (1). That analysis, however, contained only two resurveys from tropical regions, and therefore did not compare upslope shifts between tropical and temperate species. Our two resurveys, in conjunction with recent tropical resurveys across a variety of taxonomic groups (5, 26, 27), provide sufficient tropical data points to permit such a statistical comparison (Table S3). We used shift rate (observed shift/expected shift given local temperature increase) to summarize upslope shifts, where lag times in upslope shifts are represented by shift rates less than 1. Tropical species’ upslope shifts closely matched predicted shifts, with shift rates significantly greater than temperate-zone species (tropics: 0.97 ± 0.55, n = 7; temperate zone: 0.33 ± 0.29, n = 28; t5.6 = −3.1, P = 0.021) (Fig. 3). Although the number of tropical resurveys conducted is still small, this result supports the hypothesis that tropical montane species are more sensitive to changes in annual mean temperature than temperate-zone montane species.
Fig. 3.
Shift rates of temperate-zone and tropical montane organisms in resurveys documenting distributional responses to temperature increases. Shift rates represent the average upslope shift (m) documented in a resurvey compared with the predicted upslope shift (m) given local temperature increase. A shift rate of 1 signifies observed shifts that match predicted shifts. Boxplots with median and quartile values for temperate-zone and tropical shift rates are overlain on points summarizing shift rates of temperate-zone (n = 28) and tropical (n = 7) resurveys of a variety of taxonomic groups (Table S3).
Comparing single resurveys to historical data may be confounded by seasonal variation in elevational distributions (5). However, modern elevational limits were not systematically biased upslope or downslope between dry and wet seasons on Mt. Karimui, suggesting the upslope shifts we report are robust to seasonal variation in elevational limits. A second problem affecting resurveys is accounting for differences in survey methodology (28). Comparison of modern and historical (19, 20) databases and methods (Methods) suggests that our methodologies and survey efforts for both Mt. Karimui and Karkar Island were similar, and our data support this contention. In particular, if modern and historical surveys differed in observer ability or effort, the survey with the greater effort should tend to yield broader elevational ranges merely owing to increased sample sizes. Instead, we found that species generally shifted upslope at both upper and lower range limits, as predicted for responses to warming temperatures. Lastly, readily-detected species did not demonstrate stronger upslope shifts: the magnitude of estimated distributional shifts was unrelated to relative detectability at both upper (F1,136 = 2.40, P = 0.12, adjusted r2 = 0.010) and lower limits (F1,52 = 1.70, P = 0.20, adjusted r2 = 0.013) (Methods).
Substantial variation existed among species in observed upslope shifts (Fig. 2), typical for such resurveys (1, 22). This variation may arise from idiosyncratic, species-specific responses to either temperature itself or altered resource availability, habitat structure, competitive dynamics, disease prevalence, and/or predation associated with increased temperature at given elevations. Ecological traits associated with dietary breadth have been hypothesized to explain variation in species’ responses (5, 22). We found no correlation between diet and range shifts on Mt. Karimui. However, upslope shifts were largest in omnivores and frugivores in the depauperate avifauna of Karkar Island, providing weak support for the hypothesis that dietary breadth (omnivores) and patchiness of resource supply (frugivores) may predispose species to stronger distributional responses to increases in mean annual temperature. We conclude that the mechanisms driving upslope shifts in New Guinean birds remain largely uncertain and merit further experimental investigation.
New Guinean birds have responded to a half-century of warming temperatures by rapidly shifting their distributions upslope. Temperatures in New Guinea are predicted to increase 2.5 °C by 2100 (24). Extrapolating from documented shifts suggests New Guinean birds will respond to rising temperatures by shifting as much as 500 m further upslope by 2100. These shifts are likely to cause at least four local mountaintop extinctions on Mt. Karimui and two on Karkar Island. For example, the lower elevational limit of the montane White-winged Robin (Penoethello sigillatus) on Mt. Karimui has moved upslope over 100 m since 1965. This species is now found only above 2,330 m on the steep slopes near Mt. Karimui’s summit (at 2,520 m), whereas Mountain Gerygone (Gerygone cinerea), Crested Satinbird (Cnemophilus macgregorii), and Crested Berrypecker (Paramythia montium) are currently restricted to the immediate vicinity of Mt. Karimui’s summit. A further ∼1-°C temperature increase would likely lead to the extirpation of all four species on Mt. Karimui, although populations will persist on taller mountains elsewhere in New Guinea.
Resurveys to document distributional responses to temperature increase have now been performed in enough different regions (4, 5, 26, 27) to support the hypothesis that tropical montane species—compared with temperate species—are disproportionately sensitive to warming temperatures. Tropical species’ sensitivity to temperature may be a consequence of the relative constancy of thermal environments in tropical environments (13, 14), although it remains unclear whether tropical endotherms’ sensitivity to temperature results from indirect ecological interactions or the direct impact of increased temperature (5, 19).
This finding has global conservation implications, as elevational gradients on tropical mountains harbor the most spectacular concentrations of biodiversity of any terrestrial environment (29). In particular, our findings provide empirical support for models that predict widespread extinctions of tropical birds due to temperature increases (8, 10), with global extinctions especially likely in tropical species endemic to single mountains or small mountain ranges (10, 18). Further, we emphasize that species' upslope shifts on Mt. Karimui have not resulted in expanded elevational distributions. Instead, species’ entire elevational distributions are shifting upwards. Because less land area typically exists at higher elevations (15), upslope shifts in response to warming temperatures will inexorably cause reductions in species’ populations, increasing the probability that these diminished populations will go extinct (30).
Conservation of tropical montane biotas in the face of warming temperatures clearly will require protection of entire elevational gradients (31). Whereas species appear likely to shift their distributions upslope, intact elevational gradients will accommodate the large majority of such upslope shifts. We urge that conserving intact tropical elevational gradients become a global goal, and suggest that synergies between biodiversity conservation and the ecosystem services provided by forested tropical mountains (e.g., watershed conservation) provide useful frameworks for translating this scientific recommendation into political reality.
Methods
Resurveys.
Our resurveys closely followed Diamond’s original methodology (19, 20) and were conducted blind, without prior knowledge of species’ elevational limits measured in Diamond’s historical surveys. We used a barometric altimeter (Garmin GPS 62S; accuracy within forest ∼ ± 5–8 m in horizontal position) to measure elevation, averaging readings taken on multiple days. Readings taken at the same locality on different days typically varied to a relatively minor degree (e.g., the SD for readings measured on 16 different days at our second camp was 8.6 m). Like Diamond (19, 20), we calibrated our altimeter for Mt. Karimui at the Karimui airstrip (1,112 m) and on Karkar Island at sea level.
We recorded species’ elevational limits as the most extreme elevational observation of a given species. However, we followed Diamond (19, 20) in discounting single observations of common species well above or below their typical elevational distribution. We considered single observations more than 300 m in elevation above or below the next most extreme records to represent outlier observations. Such outlier observations were rare, with only four examples from Mt. Karimui and none from Karkar Island. Including all records, including outlier observations, does not change any results of this study. Finally, we recorded elevational limits only for species where limits fell within the elevational expanse surveyed at our study sites.
Mt. Karimui.
Mt. Karimui is an extinct volcano in New Guinea’s Central Ranges, in Chimbu Province (Fig. 1). In July–August 1965, Diamond surveyed the avifauna of Mt. Karimui’s northwestern ridge from ca. 1,100 m to the summit at 2,520 m (19). We conducted a modern resurvey in June–July 2012, studying the same ridgeline at the same time of year. Mt. Karimui was entirely covered in primary forest in 1965, and land-use changes in the intervening decades have been minimal. Small-scale forest clearance for subsistence agriculture is limited to elevations below 1,275 m, with the exception of a small (ca. 1 ha) patch atop the ridge’s summit cleared during recent construction of a cellular phone tower. We avoided sampling in the vicinity of subsistence gardens by surveying lower elevations (from 1,130 m to 1,330 m) on an undisturbed forested ridge 0.5 km to the east. We then surveyed Diamond’s exact transect on Mt. Karimui’s northwest ridge from 1,330 m to the summit at 2,520 m.
Diamond used extensive mist netting, specimen collection, audial censuses, and ad libitum observations to determine species’ distributional limits, spending a total of 33 field days on Mt. Karimui (19). We attempted to replicate this field effort, completing extensive mist netting, point counts, and collecting ad libitum observations in 38 field days. The mist-netting field studies were approved by the Institutional Animal Care and Use Committee of Cornell University. We used flagging tape to mark elevational zones every 25 m of elevation, providing a consistent basis for measuring elevation. We then conducted mist-net censuses from 1,130 m to 2,420 m with consistent effort across elevation, opening mist nets at each elevational zone for two mornings (from 0600 hours to 1230 hours). Difficult terrain led to reduced mist-net effort in the short (canopy <15 m), heavily mossed ridgeline forest above 2,250 m and entirely prevented mist netting above 2,400 m. This reduction in mist-net effort above 2,250 m likely increased the probability of overlooking species present near the ridge’s summit. Because the preponderance of species’ range borders near the summit were upper limits (not lower limits), these potential omissions likely reduced our ability to detect upslope shifts in the upper limits of montane birds. Hence, this methodological bias constrained our ability to document upslope shifts of montane species and is conservative with respect to the hypothesis that warming temperatures have led to upslope shifts.
Point counts were conducted by one observer (B.G.F.) with good working knowledge of New Guinean bird vocalizations. A total of 40 point counts were conducted along the elevational gradient (from 1,130 m to 2,520 m). Points were separated by at least 150 m, with each point count site visited on three mornings (from 0630 hours to 1200 hours) for 5 min per count. A second set of point counts was completed in our second Mt. Karimui resurvey in October–November 2012. This independent Mt. Karimui resurvey covered elevations from 1,330 m to 2,520 m, and used point count data and ad libitum observations compiled during 34 d of fieldwork. Point counts were completed at 30 sites from 1,330 m to 2,520 m in this second Mt. Karimui resurvey following the methodology described above.
Karkar Island.
Our second resurvey took place on Karkar Island, Madang Province, an oceanic island located 10 miles off the coast of northern New Guinea (Fig. 1). An active volcano, Karkar has forested slopes that rise to a steep-sided caldera at 1,300–1,400 m in the island’s center. The island’s high point, at ca. 1,800 m, is Mt. Kanagioi, at the southern rim of the caldera. Diamond surveyed Karkar Island’s upland (ca. >400 m) avifauna in May 1969 (20). We conducted our resurvey of Karkar Island’s montane avifauna in April 2013, at the same time of year and visiting the same locations as Diamond’s original transect. However, because plantation agriculture currently extends to around 600 m on Karkar Island, we restricted our resurvey to undisturbed montane forest from 800 m to 1,600 m. Diamond used mist netting, audial censuses, and shotgun-based collecting to document elevational limits of species on the northwest side of the island, spending 9 field days in Karkar’s montane forests (20). We expended similar field effort, gathering distributional data over 10 d of mist netting, conducting point counts (35 sites, methods described above), and collecting ad libitum observations.
Statistical Analysis.
We used parametric and nonparametric t tests and sign tests to evaluate upslope shifts. One-sample t tests tested the significance of upslope shifts at both upper and lower limits for both Mt. Karimui and Karkar Island. Sign tests compared the number of upslope to downslope shifts at both upper and lower limits. Two-sample t tests compared the magnitude of upslope shifts between study sites. Because changes in elevational limits measured on Mt. Karimui in June–July and October–November were not normally distributed, we used Wilcoxon signed-rank tests to test for systematic biases in the seasonality of elevational limits. We used paired t tests for two comparisons of Mt. Karimui species, limiting our database to species where both upper and lower limits were measured (n = 41 species). First, we tested whether the magnitude of upslope shift differed between upper and lower limits. Second, we tested whether species expanded their elevational breadth between Diamond’s original transect and our resurvey.
To consider the possibility that estimated changes in elevational distributions were influenced by species’ relative abundance during our modern resurvey, we compared species’ detectability to estimated shifts at both upper and lower elevational limits. Specifically, we used linear regression models to test the influence of species’ relative detectability (summed number of times a species was detected on point counts and captured in mist nets) on upper and lower limit distributional shifts. We also investigated whether diet impacted species’ upslope movement. First, we used reference material (19, 32) to classify species into five dietary guilds: carnivores, frugivores, insectivores, omnivores, and nectivores. We then used ANOVAs to test for differences in species’ upslope movements between foraging guilds, testing both range margins on Mt. Karimui and upper limits on Karkar Island.
Finally, we estimated change in average temperature for our study sites using the 0.5° × 0.5° grid cells containing our study sites within the Climatic Research Unit (CRU) time series 3.2 database (25). Specifically, we compared mean annual temperatures for the decade before the historical transect (Mt. Karimui: 1955–1964; Karkar Island: 1959–1968) to the decade before our modern resurveys (2002–2011). We measured the lapse rate on Mt. Karimui by placing temperature loggers along the elevational gradient and regressing mean daily temperature against elevation.
Tropical vs. Temperate-Zone Resurveys.
We analyzed the rate of upslope shifts between temperate and tropical regions by summarizing observed and expected shifts from 35 resurveys. Specifically, we added novel data (our two resurveys and three further recent tropical resurveys) (5, 26, 27) to the database originally presented in table S1b by Chen et al. (1) (Table S3). We followed Chen et al.’s methodology (1) in adding recent resurveys measuring distributional responses to warming temperatures. For example, we did not treat upslope shifts at leading and trailing range margins measured on Mt. Karimui and Karkar Island as independent. Instead, we included each site as a single resurvey, summarizing upslope shifts using weighted means based on the number of species in each group (upper and lower limit shifts) at each study site (Table S3). Additionally, for novel resurveys, we used observed and expected elevational shifts reported in the original studies. We note the following caveats to our metaanalysis: published tropical resurveys are still few, diverse in taxa studied (e.g., trees, plants, birds, lizards, and moths), and vary in scope [e.g., elevational shifts in one study (27) were presented at the genus, rather than species, level].
To investigate hypothesized differences between responses in tropical and temperate biotas, we first categorized resurveys as either tropical (occurring between the Tropics of Cancer and Capricorn) or temperate (occurring pole-ward of the tropical zone) (Table S3). We categorized the recent resurvey of montane Taiwanese plants (26) as tropical, as Taiwan is located almost exactly on the Tropic of Cancer. We then calculated shift rates (observed shift/predicted shift based on temperature increase) for each resurvey (Table S3), and compared shift rates between tropical and temperate-zone species using a two-sample t test.
Supplementary Material
Acknowledgments
We thank numerous local landowners for facilitating our research, particularly J. Anuabo, L. Gande, S. Banu, J. Buga Tane, W. Paro, and D. Goma from Karimui. N. Martin, Dr. T. Ihle, Dame C. Kidu, Sir P. Barter, P. Toko, P. Navratil, and B. and V. Middleton provided invaluable support. Comments from J. Diamond, K. Feeley, J. Fitzpatrick, S. Freeman, W. Hochachka, M. Tingley, and one anonymous reviewer greatly improved this manuscript. F. La Sorte assisted in extracting climate data from the CRU database, and G. Becker helped prepare Fig. 1. Fieldwork was supported by grants from the Athena Fund of the Cornell Laboratory of Ornithology and the Explorer’s Club. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant 2011083591 (to B.G.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318190111/-/DCSupplemental.
References
- 1.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333(6045):1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37(1):637–669. [Google Scholar]
- 3.Moritz C, et al. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322(5899):261–264. doi: 10.1126/science.1163428. [DOI] [PubMed] [Google Scholar]
- 4.Chen IC, et al. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc Natl Acad Sci USA. 2009;106(5):1479–1483. doi: 10.1073/pnas.0809320106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forero-Medina G, Terborgh J, Socolar SJ, Pimm SL. Elevational ranges of birds on a tropical montane gradient lag behind warming temperatures. PLoS ONE. 2011;6(12):e28535. doi: 10.1371/journal.pone.0028535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feeley KJ, Silman MR. The data void in modeling current and future distributions of tropical species. Glob Change Biol. 2011;17(1):626–630. [Google Scholar]
- 7.Maclean IMD, Wilson RJ. Recent ecological responses to climate change support predictions of high extinction risk. Proc Natl Acad Sci USA. 2011;108(30):12337–12342. doi: 10.1073/pnas.1017352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Sorte FA, Jetz W. Projected range contractions of montane biodiversity under global warming. Proc R Soc B. 2010;277(1699):3401–3410. doi: 10.1098/rspb.2010.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427(6970):145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 10.Sekercioglu CH, Schneider SH, Fay JP, Loarie SR. Climate change, elevational range shifts, and bird extinctions. Conserv Biol. 2008;22(1):140–150. doi: 10.1111/j.1523-1739.2007.00852.x. [DOI] [PubMed] [Google Scholar]
- 11.Feeley KJ, Rehm EM, Machovina B. The responses of tropical forest species to global climate change: Acclimate, adapt, migrate, or go extinct? Frontiers of Biogeography. 2012;4(2):69–84. [Google Scholar]
- 12.Corlett RT. Impacts of warming on tropical lowland rainforests. Trends Ecol Evol. 2011;26(11):606–613. doi: 10.1016/j.tree.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. Are mountain passes higher in the tropics? Janzen’s hypothesis revisited. Integr Comp Biol. 2006;46(1):5–17. doi: 10.1093/icb/icj003. [DOI] [PubMed] [Google Scholar]
- 14.Janzen DH. Why mountain passes are higher in the tropics. Am Nat. 1967;101:233–249. [Google Scholar]
- 15.Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322(5899):258–261. doi: 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- 16.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105(18):6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinervo B, et al. Erosion of lizard diversity by climate change and altered thermal niches. Science. 2010;328(5980):894–899. doi: 10.1126/science.1184695. [DOI] [PubMed] [Google Scholar]
- 18.Pounds JA, Fogden MPL, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398(6728):611–615. [Google Scholar]
- 19.Diamond JM. Avifauna of the Eastern Highlands of New Guinea. Cambridge, MA: Nuttall Ornithological Club; 1972. p. vii. [Google Scholar]
- 20.Diamond JM, Lecroy M. Birds of Karkar and Bagabag Islands, New Guinea. Bull Am Mus Nat Hist. 1979;164:467–532. [Google Scholar]
- 21.Chen I. Asymmetric boundary shifts of tropical montane Lepidoptera over four decades of climate warming. Glob Ecol Biogeogr. 2011;20(1):34–45. [Google Scholar]
- 22.Angert AL, et al. Do species’ traits predict recent shifts at expanding range edges? Ecol Lett. 2011;14(7):677–689. doi: 10.1111/j.1461-0248.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 23.Tingley MW, Koo MS, Moritz C, Rush AC, Beissinger SR. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Glob Change Biol. 2012;18(11):3279–3290. [Google Scholar]
- 24.Solomon S, et al., editors. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 25.Harris I, Jones P, Osborn T, Lister D. Updated high-resolution grids of monthly climatic observations–the CRU TS3. 10 Dataset. Int J Climatol. 2013 in press. [Google Scholar]
- 26.Jump AS, Huang TJ, Chou CH. Rapid altitudinal migration of mountain plants in Taiwan and its implications for high altitude biodiversity. Ecography. 2012;35(3):204–210. [Google Scholar]
- 27.Feeley KJ, et al. Upslope migration of Andean trees. J Biogeogr. 2011;38(4):783–791. [Google Scholar]
- 28.Tingley MW, Beissinger SR. Detecting range shifts from historical species occurrences: New perspectives on old data. Trends Ecol Evol. 2009;24(11):625–633. doi: 10.1016/j.tree.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 30.Lande R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am Nat. 1993;142(6):911–927. doi: 10.1086/285580. [DOI] [PubMed] [Google Scholar]
- 31.Laurance WF, et al. Global warming, elevational ranges and the vulnerability of tropical biota. Biol Conserv. 2011;144(1):548–557. [Google Scholar]
- 32.Beehler BM, Pratt TK, Zimmerman DA. 1986. Birds of New Guinea (Princeton Univ Press, Princeton) p xiii, 255 leaves of plates, 293.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.