Abstract
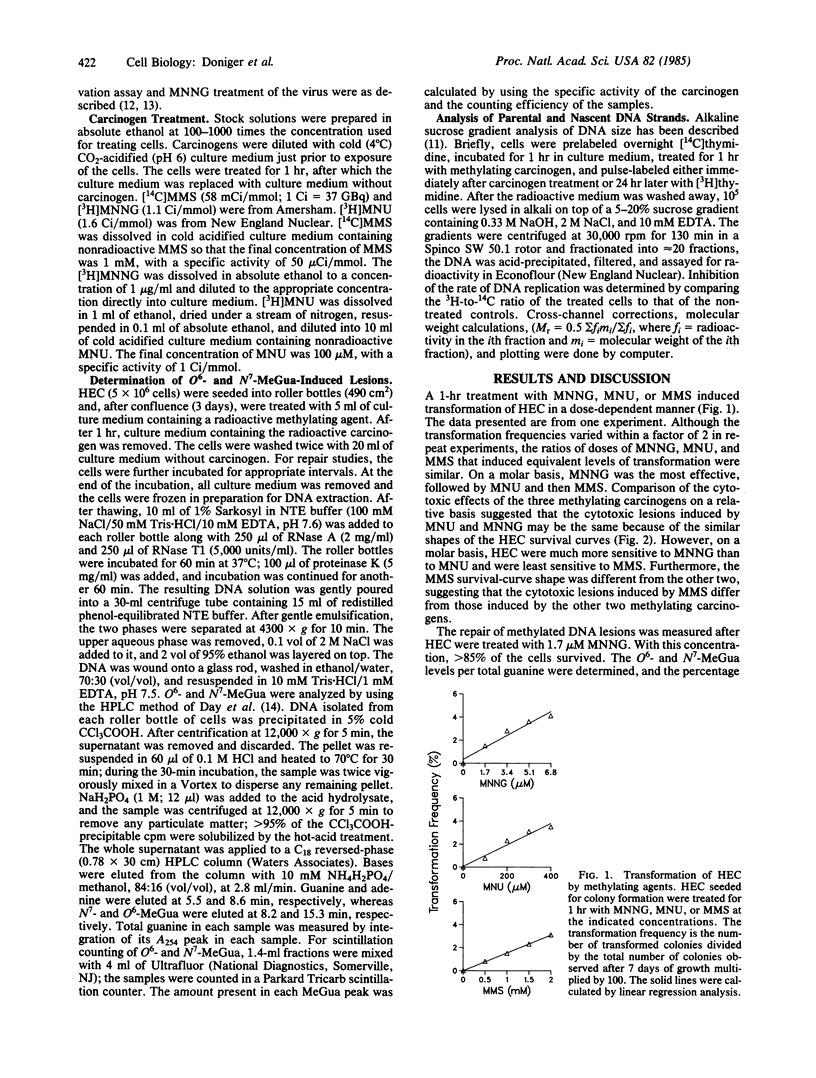
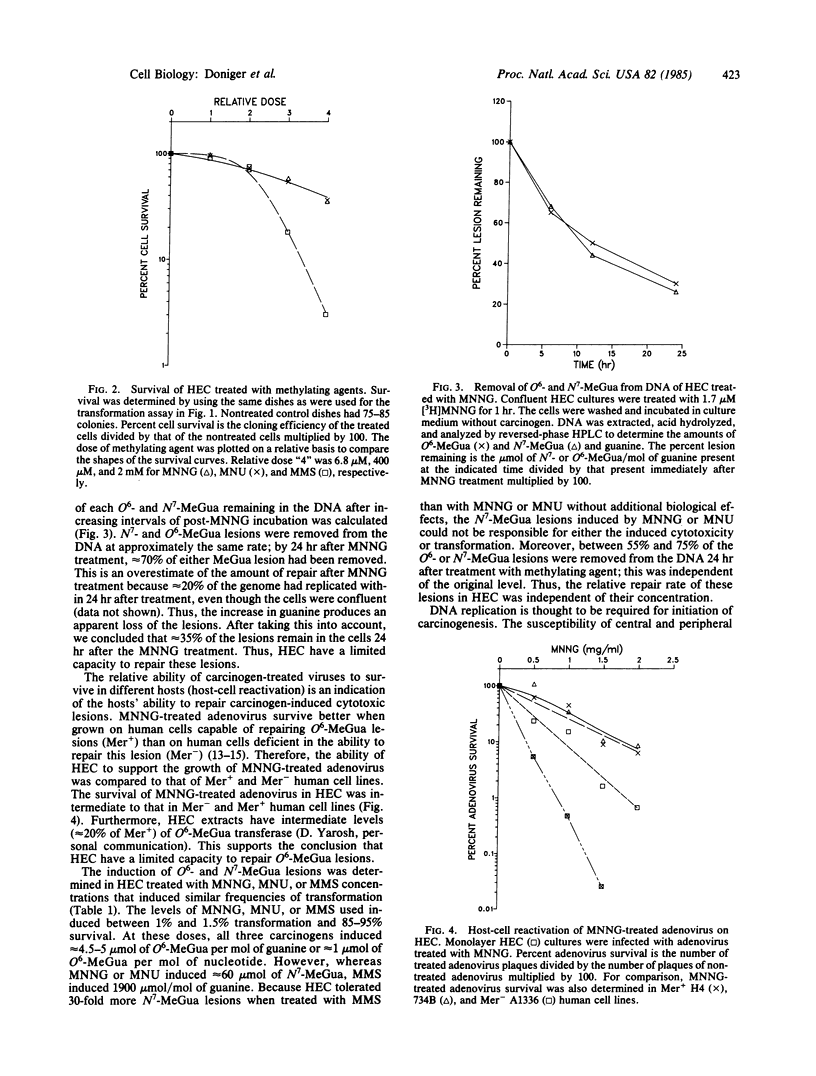
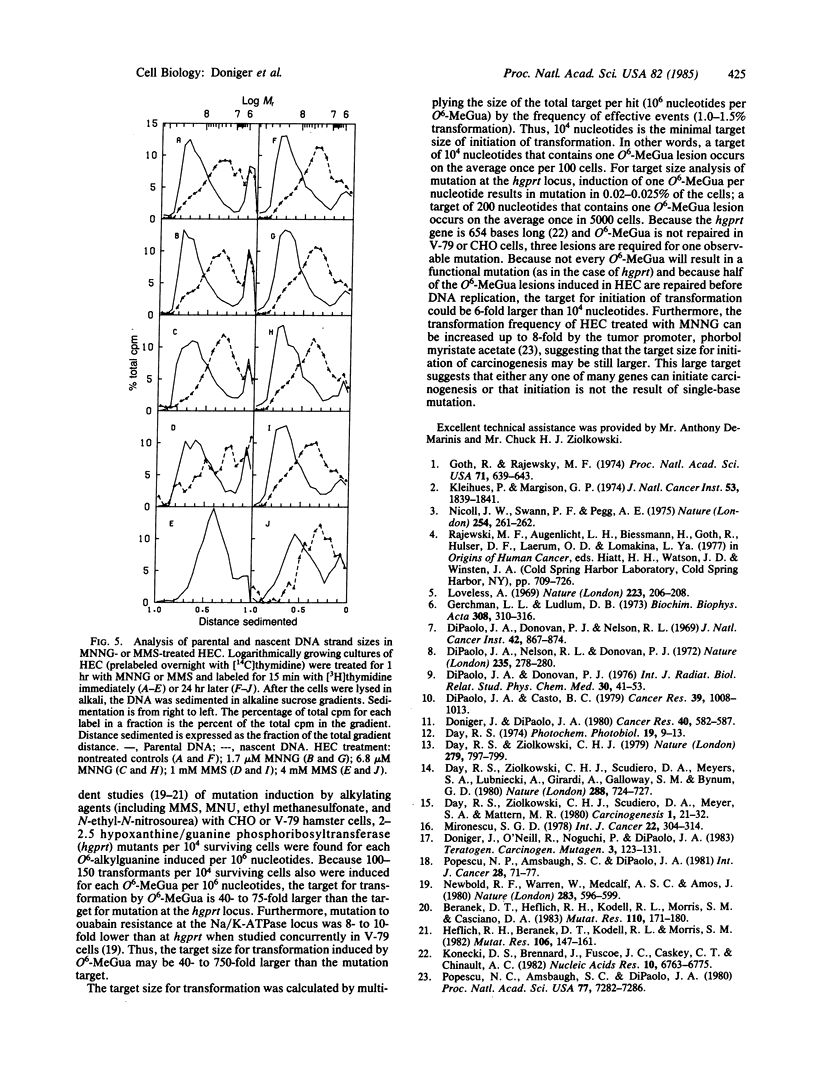
Induction of transformation, cell lethality, and DNA lesions were quantitatively compared in Syrian hamster embryo cells (HEC) treated with three different methylating agents: N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), N-methyl-N-nitrosourea (MNU), or methyl methanesulfonate (MMS). Each induced transformation in a dose-dependent manner. On a molar basis, MNNG was approximately equal to 100- and 500-fold more effective than MNU and MMS, respectively. For each carcinogen the induction and repair of O6- and N7-methylguanine (O6- and N7-MeGua) relative to total guanine content was compared. At concentrations that induced equivalent transformation frequencies, the induction of O6-MeGua was the same for all three carcinogens, but N7-MeGua induction was 30-fold higher with MMS than with MNNG or MNU. The capacity to repair methylation lesions in HEC is limited because only between 50% and 70% of both O6- and N7-MeGua lesions were removed from the DNA within 24 hr after treatment, independent of methylating carcinogen. No consistent effect on either the rate of DNA replication or the size distribution of nascent strands correlated with O6-MeGua induction. These data support the hypothesis that O6-MeGua is the critical lesion for initiation of carcinogenesis by methylating agents. The frequency of transformation relative to O6-MeGua induction is 40- to 750-fold more than that of mutation. Based on the quantitative data for induction of O6-MeGua and transformation, the target size for initiation of carcinogenesis was calculated as a minimum of 10(4) nucleotides. This suggests that one of many genes can initiate carcinogenesis or that initiation is not the result of a single base mutation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beranek D. T., Heflich R. H., Kodell R. L., Morris S. M., Casciano D. A. Correlation between specific DNA-methylation products and mutation induction at the HGPRT locus in Chinese hamster ovary cells. Mutat Res. 1983 Jun-Jul;110(1):171–180. doi: 10.1016/0027-5107(83)90026-x. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd Cellular reactivation of ultraviolet-irradiated human adenovirus 2 in normal and xeroderma pigmentosum fibroblasts. Photochem Photobiol. 1974 Jan;19(1):9–13. doi: 10.1111/j.1751-1097.1974.tb06467.x. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H. Human brain tumour cell strains with deficient host-cell reactivation of N-methyl-N'-nitro-N-nitrosoguanidine-damaged adenovirus 5. Nature. 1979 Jun 28;279(5716):797–799. doi: 10.1038/279797a0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Casto B. C. Quantitative studies of in vitro morphological transformation of Syrian hamster cells by inorganic metal salts. Cancer Res. 1979 Mar;39(3):1008–1013. [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P. J. In vitro morphologic transformation of Syrian hamster cells by U.V.-irradiation is enhanced by X-irridation and unaffected by chemical carcinogens. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Jul;30(1):41–53. doi: 10.1080/09553007614550791. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P., Nelson R. Quantitative studies of in vitro transformation by chemical carcinogens. J Natl Cancer Inst. 1969 May;42(5):867–874. [PubMed] [Google Scholar]
- DiPaolo J. A., Nelson R. L., Donovan P. J. In vitro transformation of Syrian hamster embryo cells by diverse chemical carcinogens. Nature. 1972 Feb 4;235(5336):278–280. doi: 10.1038/235278a0. [DOI] [PubMed] [Google Scholar]
- Doniger J., DiPaolo J. A. Excision and postreplication DNA repair capacities, enhanced transformation, and survival of Syrian hamster embryo cells irradiated by ultraviolet light. Cancer Res. 1980 Mar;40(3):582–587. [PubMed] [Google Scholar]
- Doniger J., O'Neill R., Noguchi P., DiPaolo J. A. Initiation of carcinogenesis dependence upon MNNG-induced release of the G1 block of density-inhibited Syrian hamster cells. Teratog Carcinog Mutagen. 1983;3(2):123–131. doi: 10.1002/1520-6866(1990)3:2<123::aid-tcm1770030204>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflich R. H., Beranek D. T., Kodell R. L., Morris S. M. Induction of mutations and sister-chromatid exchanges in Chinese hamster ovary cells by ethylating agents. Mutat Res. 1982 Nov;106(1):147–161. doi: 10.1016/0027-5107(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Carcinogenicity of N-methyl-N-nitrosourea: possible role of excision repair of O6-methylguanine from DNA. J Natl Cancer Inst. 1974 Dec;53(6):1839–1841. [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mironescu S. G. Inhibition of morphological transformation induced with N-methyl-N'-nitro-N-nitrosoguanidine in cultures of hamster embryo cells by 5'-bromo-2'-deoxyuridine-photolysis. Int J Cancer. 1978 Sep 15;22(3):304–314. doi: 10.1002/ijc.2910220314. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Warren W., Medcalf A. S., Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980 Feb 7;283(5747):596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- Popescu N. C., Amsbaugh S. C., DiPaolo J. A. Enhancement of N-methyl-N'-nitro-N-nitrosoguanidine transformation of Syrian hamster cells by a phorbol diester is independent of sister chromatid exchanges and chromosome aberrations. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7282–7286. doi: 10.1073/pnas.77.12.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu N. C., Amsbaugh S. C., DiPaolo J. A. Relationship of carcinogen-induced sister chromatid exchange and neoplastic cell transformation. Int J Cancer. 1981 Jul 15;28(1):71–77. doi: 10.1002/ijc.2910280113. [DOI] [PubMed] [Google Scholar]



