Significance
MicroRNAs (miRNAs) are small, noncoding RNAs regulating gene expression. The aberrant expression of miRNAs is commonly associated with cancer. miRNAs can be packaged in exosomes/microvesicles secreted by the cells and involved in cell-to-cell signaling and communication; tumor-secreted miRNAs promote tumor spread and growth in the surrounding microenvironment. Apoptosis is reported to take place in wasting muscle in cancer cachexia, a debilitating syndrome associated with multiple types of cancer, although the mechanism remains elusive. This study shows that tumor-secreted microvesicles contain an elevated expression of miR-21 and induce myoblast apoptosis in cancer cachexia via a Toll-like receptor 7-c-Jun N-terminal kinase-dependent pathway.
Keywords: TLRs, MVs, JNK, muscle stem cells
Abstract
MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression and, in cancers, are often packaged within secreted microvesicles. The cachexia syndrome is a debilitating state of cancer that predominantly results from the loss of skeletal muscle mass, which is in part associated with apoptosis. How tumors promote apoptosis in distally located skeletal muscles has not been explored. Using both tumor cell lines and patient samples, we show that tumor-derived microvesicles induce apoptosis of skeletal muscle cells. This proapoptotic activity is mediated by a microRNA cargo, miR-21, which signals through the Toll-like 7 receptor (TLR7) on murine myoblasts to promote cell death. Furthermore, tumor microvesicles and miR-21 require c-Jun N-terminal kinase activity to regulate this apoptotic response. Together, these results describe a unique pathway by which tumor cells promote muscle loss, which might provide a great insight into elucidating the causes and treatment options of cancer cachexia.
MicroRNAs (miRNAs) are a family of small, noncoding RNA molecules, 19–24 nucleotides in length, that are evolutionarily conserved and tissue-specific (1, 2). These noncoding RNAs function by regulating gene expression through mRNA degradation or the inhibition of protein translation (1, 2) and are dysregulated in all cancers (3). Interestingly, miRNAs have also recently been discovered extracellularly, contained in body fluids such as serum, plasma, urine, milk, and spinal fluid (4–7). These circulating miRNAs are embedded in microvesicles (MVs)/exosomes, which are small, membrane-derived particles, usually 30 nm to 1 μm in size (8). Although the mechanism of extracellular formation and secretion is not well-defined, evidence indicates that such vesicles possess the capability to “communicate” with neighboring or distal cells by fusing with the plasma membrane and subsequently delivering their cargo, consisting of various molecules that include proteins, mRNAs, and miRNAs (9). Moreover, transported miRNAs are capable of targeting mRNAs in recipient cells (9, 10).
MVs and exosomes are secreted from various cell types (9), and their miRNA content is associated with regulating cellular processes involved in cell communication, angiogenesis, and extracellular matrix remodeling (9–11). Recently, we revealed that tumor-secreted miRNAs contributed to tumorigenesis by acting in a paracrine manner to signal to target cells. Specifically, miR-21 and miR-29a were packaged in exosomes from lung cancer cells that signaled to the Toll-like receptor 8 (TLR8) in human macrophages (homologous to TLR7 in mice) to trigger a proinflammatory response that facilitated tumor development (12).
Cachexia is a syndrome characterized by weight loss resulting from a reduction of lean body mass and fat mass that accompanies many types of chronic diseases, including cancer (13). The weight loss in cachexia is not caused by malnutrition or starvation but, rather, by inflammatory changes associated with the presence of the tumor and the production of cytokines. Patients with advanced lung cancer and pancreatic cancer, as well as other gastrointestinal malignancies, most often suffer from the cachexia syndrome that promotes asthenia, physical weakness, and mental fatigue. Patients with cachexia are more susceptible to dose-limiting chemotoxicity (13), and the degree of weight loss is positively correlated with mortality (14, 15).
Cancer cachexia emaciates not only adipose tissue but also skeletal muscle, which constitutes 40% of total body weight in humans (16). Loss of skeletal muscle in cachexia originates from a decrease in protein synthesis as well as an increase in protein degradation resulting from an altered metabolism in response to a progressing tumor (17). Recently, we reported on the role of the deregulation of muscle stem cells as a contributing factor in the regulation of tumor-induced muscle wasting. In both tumor-bearing mice and patients with pancreatic cancer and weight loss, we found that the transcription factor, Pax7, which controls the self-renewal of muscle stem cells, was persistently expressed. This sustained expression of Pax7 caused committed stem cells to be impaired in their differentiation program, resulting in their inability to fuse with damaged myofibers, which in turn enhanced muscle atrophy (18). These results showed that events in the muscle microenvironment are important in tumor-induced muscle wasting. In addition to events on muscle stem cells, apoptosis has also been associated with cancer cachexia and proposed to regulate skeletal muscle loss in various cachexia conditions (19–22), but exactly which populations of cells undergo cell death is not clear, nor is the mechanism causing cell death well-understood.
In this article, we demonstrate that lung cancer- and pancreatic cancer-derived MVs containing miRNAs induce apoptosis of skeletal muscle cells. In particular, miR-21 secreted through MVs activates TLR7 receptor on murine myoblasts and promotes apoptosis through c-Jun N-terminal kinase (JNK) activity. These findings describe a mechanism to better understand cancer cachexia and could suggest future strategies for its treatment.
Results and Discussion
Lung and Pancreatic Tumor-Derived MVs Induce Cell Death on Murine Myoblasts.
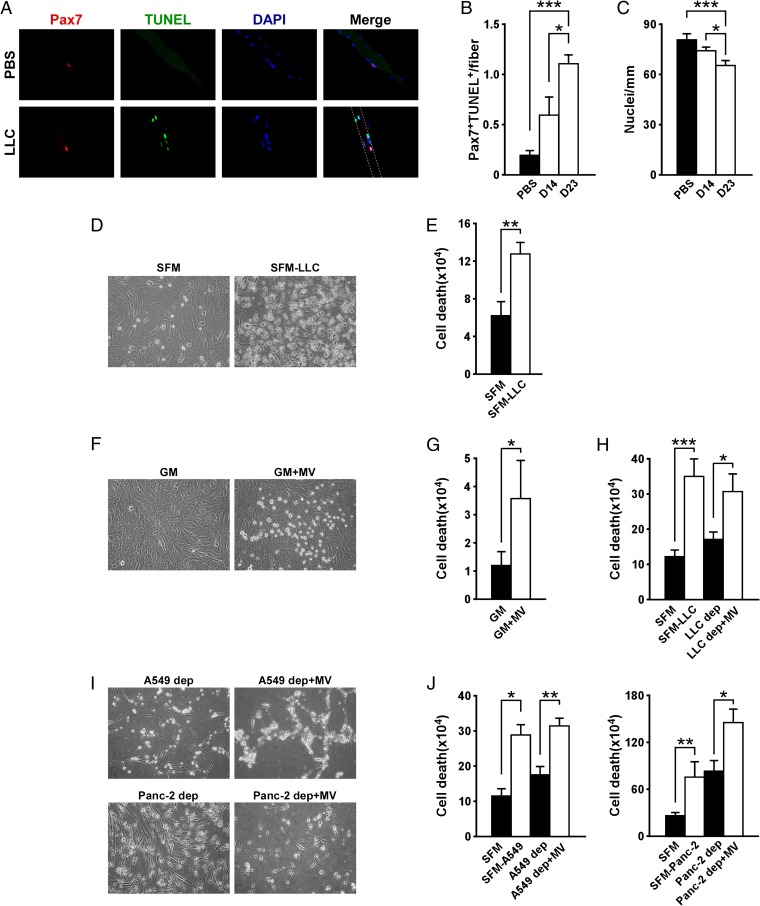
When we examined the role of muscle progenitors in cachexia, we observed a considerably higher number of apoptotic cells associated with muscle cells from Lewis lung carcinoma (LLC) tumor-bearing mice, which suffered from severe cachexia, compared with those from tumor-free mice (Fig. S1 A and B). Such results are in line with reports suggesting that cachexia is associated with apoptosis of skeletal muscle myonuclei (17, 23, 24). Interestingly, costaining with TUNEL and the muscle stem cell marker, Pax7, revealed a significant increase of apoptotic muscle stem cells (Fig. 1 A and B). This increase in apoptotic stem cells correlated with an overall decrease in the number of muscle nuclei (Fig. 1C).
Fig. 1.
Tumor-derived MVs induce myoblast cell death in cancer cachexia. (A) Immunofluorescence performed on single myofibers isolated from in vivo xenograft LLC mouse models. As control, myofibers derived from PBS-injected mice were used. Pax7 is shown in red, TUNEL is shown in green, nuclei staining is in blue, and their colocalization is shown in the Merge panels. Staining was performed 14 and 23 d (D14 and D23, respectively) after tumor injection. (B) TUNEL quantitation related to immunofluorescence shown in A. (C) Determination of nuclei number per single myofiber. (D and E) Picture of C2C12 cells incubated with LLC-conditioned medium for 4 h (D). Serum-free medium (SFM) was used as a negative control. Cell death was determined with Trypan blue dye staining (E). (F–H) Picture of C2C12 cells incubated with LLC-derived MVs (F). Growth medium was used as negative control. Cell death was assessed with Trypan blue dye staining (G). The same assay was also performed on C2C12 cells incubated with MV-depleted medium (LLC dep) and LLC-derived MVs (LLC dep + MV) (H). (I and J) Induction of C2C12 cell death by A549- and Panc-2-derived MVs. Data are combined from at least three independent experiments. Results are presented as average ± SD. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
To determine the regulation of this apoptotic response, we tested the effects of proinflammatory cytokines, given their connection to cachexia (15). Incubation with individual cytokines or a mixture containing TNFα, IL-1β, IL-6, and IFNγ had little or no effect on the viability of proliferating murine C2C12 myoblasts (Fig. S1C). Similar results were observed with primary myoblasts (Fig. S1D). However, use of conditioned media from cultured LLC cells promoted apoptosis of myoblasts within 24 h after the incubation compared with use of medium alone (Fig. 1 D and E). This suggested that factors secreted from tumor cells possessed a cell death activity on myoblasts. Because this activity did not seem to derive from inflammatory cytokines, we tested the effects of LLC-prepared MVs. Indeed, these MVs readily induced cell death when added to C2C12 myoblasts (Fig. 1 F and G). Importantly, this killing activity was reduced by 50.9% (P < 0.01) when LLC-conditioned medium was depleted of MVs but was restored to 88.2% when MVs were reconstituted in conditioned medium that had been previously depleted of the same vesicles (Fig. 1H). This suggests that the cell killing effect derives specifically from MVs. MV-mediated cell death was not unique to mouse LLC cells, as a similar response was observed when Pax7+ muscle cells were exposed to either conditioned media or MVs isolated from a human lung cell line, A549, as well as three human pancreatic cancer cell lines: PC1, Panc-2, and MIA-PACA (Fig. 1 I and J and Fig. S1 F and G), which represents two cancer types that are commonly associated with cachexia. In contrast, cell death was not recapitulated with MVs derived from established human breast cancer cell lines (Fig. S1 E–G), which represents a cancer type that is less prone to inducing cachexia (25).
MVs Derived from Pancreatic Cancer Patient Sera Induce Cell Death.
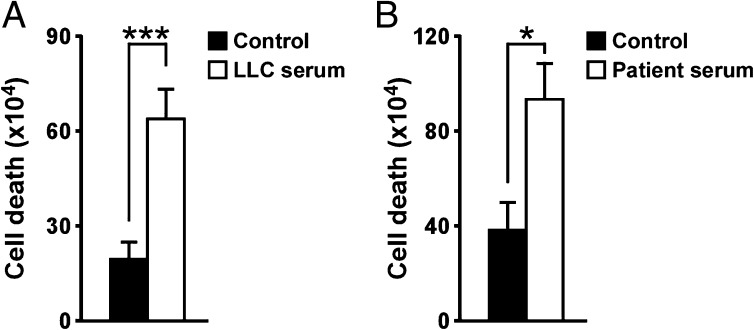
To substantiate our in vitro results, we next injected LLC cells into wild-type mice to induce muscle wasting, and MVs were prepared from cachectic serum and subsequently incubated with proliferative myoblasts. Compared with serum from healthy mice, MVs from cachectic mice significantly enhanced the cell death of myoblasts (Fig. 2A and Fig. S2A). Importantly, a similar cell-killing activity was observed when MVs were prepared from serum from patients diagnosed with pancreatic adenocarcinoma (Fig. 2B and Fig. S2B). Taken together, these data strongly support that circulating MVs in the cachectic serum are responsible for inducing apoptosis of muscle progenitor cells.
Fig. 2.
Serum-derived MVs induce cell death. (A) Trypan blue assay performed on C2C12 cells incubated for 24 h with MVs isolated from xenograft B6 mouse model-derived serum. As control, MVs isolated from normal mouse serum were used. (B) The same assay was performed on primary myoblasts incubated for 20 h with MVs isolated from cachectic patient sera. As control, MVs isolated from normal patient sera were used. Results are presented as average ± SEM. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
MV-Induced Apoptosis of Murine Myoblasts Is Mediated by TLR7 Receptor.
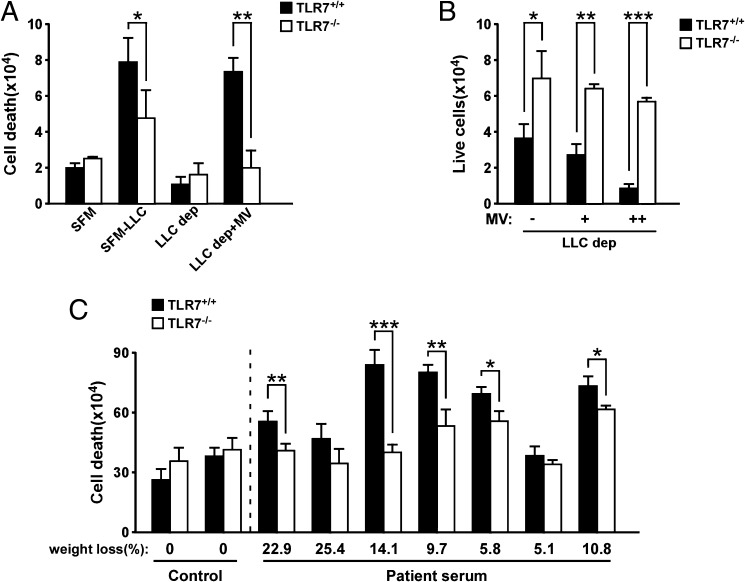
Recently, we showed that TLR signaling is required for extracellular vesicles to mediate a proinflammatory metastatic state. Specifically, vesicle-containing miRNAs signal through TLRs on macrophages to regulate the secretion of proinflammatory cytokines, which in turn promotes expansion of epithelial tumor cells (12). Because TLR7 is expressed on immature myoblasts and associated with myoblast turnover in inflammatory myopathies (26), we asked whether TLR signaling was involved in mediating MV-induced myoblast cell death. We therefore isolated primary myoblasts from TLR7+/+ or TLR7−/− mice and then incubated cells with LLC-conditioned medium. Compared with TLR7+/+ myoblasts, cell death was significantly reduced in TLR7−/− cells, suggesting that TLR7 is required for the killing effect. Significantly, depletion of MVs from LLC-conditioned media reduced cell death to control levels in both TLR7+/+ and TLR7−/− myoblasts, and cell death was restored to 92.1% when TLR7+/+, but not TLR7−/−, myoblasts were reconstituted with MVs from conditioned media originally depleted of MVs (Fig. 3 A and B and Fig. S3A). Furthermore, we tested whether TLR7 was involved in myoblast cell death induced by MVs prepared from cachectic cancer patient sera. Impressively, a similar protection from cell death was observed in primary TLR7−/− myoblasts exposed to MVs isolated from 5 of 7 pancreatic cancer patients (Fig. 3C and Fig. S3D), confirming our previous results.
Fig. 3.
TLR7 is required for tumor-derived MV-induced cell death. (A) Trypan blue dye staining performed on primary myoblasts isolated from TLR7+/+ and TLR7−/− B6 mice and incubated with LLC-derived MVs for 48 h. As control, myoblasts incubated with serum-free medium (SFM), LLC-conditioned medium (SFM-LLC), and LLC MV-depleted medium (LLC dep) were used. (B) After 5 d of incubation with MVs, live cell number was determined by using a cell counter. MVs were resuspended in MV-depleted medium. “+” or “++” indicate a low or high amount of MVs being used to treat myoblasts. (C) Primary myoblasts isolated from TLR7+/+ and TLR7−/− B6 mice were incubated with MVs isolated from control serum of healthy donors (n = 2) or cachectic serum (n = 7) of pancreatic cancer patients with cachexia. Twenty hours after treatment, trypan blue dye staining was performed to assess for myoblast cell death. Results are presented as average ± SEM. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
MV Cargo miR-21 Promotes Apoptosis Through JNK Activation.
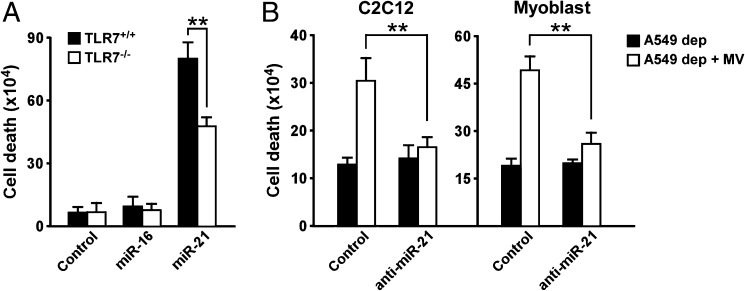
Next, we asked what cargo is contained in MVs that signaled through TLR7 to mediate myoblast cell death. Because miR-21 is frequently elevated in multiple types of cancer and was reported as a possible modulator of muscle wasting in intensive care patients (27), and because miR-21 directly binds to TLR7 (12), we tested whether this miR contributed to TLR7-mediated cell death of progenitor myoblasts. We first evaluated miR-21 expression levels within MVs derived from cancer cell lines. Results showed that miR-21 expression was elevated in MVs secreted by lung and pancreatic cancer cell lines that induced myoblast cell death compared with MVs from breast cancer cell lines that have little effect on the muscle cell viability (Fig. S4A). Moreover, the exogenous addition of miR-21 induced pronounced cell death in TLR7+/+ primary myoblasts, but this response was significantly blunted in TLR7−/− cells (Fig. 4A). In comparison controls, Dotap treatment alone or overexpression of miR-16 did not recapitulate this same cell death response. Finally, MVs prepared from LLC cells that had been depleted of miR-21 strongly reduced MV-mediated killing of C2C12 myoblasts, as well as primary TLR7+/+ myoblasts (Fig. 4B). To investigate what signaling pathways MVs and miR-21 activated to mediate cell killing of myoblasts, we treated cells with MVs or miR-21 in the absence or presence of pharmacological inhibitors for JNK, ERK1/2, p38α/β, and NF-κB, which have all been implicated in regulating apoptosis (28, 29). Inhibitors of JNK and p38 were effective at significantly reducing myoblast apoptosis in the presence of MVs or miR-21; interestingly, inhibition of NF-κB accentuated cell killing (Fig. S5A). To substantiate the relevance of JNK and p38, the activation of these pathways was tested in the presence of MVs and miR-21. Results showed that JNK and c-JUN were transiently induced in proliferating myoblasts under MVs and miR-21 exposure, whereas no significant change was seen with p38 activity (Fig. S5B). These data support that MVs containing miR-21 signal through TLR7 downstream to JNK to promote cell death of muscle myoblasts. The increase in cell death observed by inhibition of NF-κB is consistent with the notion from previous findings (29) that this occurs through activation of JNK.
Fig. 4.
MiR-21 promotes cell death on primary myoblasts. (A) TLR7+/+ and TLR7−/− primary myoblasts were treated with Dotap formulations of miR-16 and miR-21 for 24 h, and then cell viability was assessed with a cell counter. As negative control, cells were incubated with Dotap alone. (B) Determination of live primary myoblasts after incubation for 24 h with MVs isolated from A549 cells previously transfected with LNA-antinegative control and LNA-anti-miR-21. Experiments in A and B were performed in quadruplicate. Results are presented as average ± SEM. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Apoptosis has been associated with cancer cachexia. However, it is not clear so far as to the signals inducing cell death and the type of cells undergoing apoptosis. Our results indicate that circulating MVs secreted by mouse and human cancer cells cause apoptosis of muscle cells, and that this phenomenon is dependent on TLR7 (mouse) or TLR8 (human). Importantly, cancer cell lines that are usually associated with cancer cachexia, such as lung cancer and pancreatic cancer cell lines, were able to induce myoblast cell death, but not breast cancer cell lines. These results suggest the specificity of MV-mediated cell death in the context of muscle wasting associated with cancer cachexia.
We further found that miR-21 levels secreted into the MVs were elevated in those cell lines that induced muscle cell death. One of the pancreatic cell lines, PC1, induced the strongest effect of cell death (Fig. S1 F and G) and contained the highest levels of miR-21 in the MV cargo. Inhibition of miR-21, a ligand of TLR7 and TLR8, inhibited the induction of apoptosis of the muscle cells. Thus, on the basis of these findings, we predict that MVs secreted by cancer cells overexpressing miR-21, similar to those derived from pancreatic and lung malignancies (12, 30), fuse with muscle cells and induce apoptosis by activating TLR7/8 (12).
One interesting observation is that cancer cell lines that induce muscle cell death also seem to secrete more MVs (Table S1), which contributes to the phenomenon of a higher level of miR21 expression. Future studies will investigate whether a higher number of MVs is associated with the deregulation of other miRNAs or signaling pathways, as blocking TLR7 does not completely rescue miR21-induced cell death (Fig. S4A).
Taken together, our results provide insights into therapeutic avenues for cachexia, possibly by inhibiting MV secretion, inhibiting fusion of MV with muscle cells, or blocking the binding of miR-21 to TLR7/8, which are currently under investigation.
Methods
Cell Culture.
All cell lines were purchased from American Type Culture Collection unless indicated otherwise. Human cancer cell lines A549, H460, AsPC-1, Panc-2, and MDA-MB-231 and murine LLC cells were cultured in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% (vol/vol) FBS and maintained using standard conditions. C2C12, MCF7, mouse embryonic fibroblasts, and MIA PaCa-2 were cultured in DMEM (Sigma-Aldrich) supplemented with 10% (vol/vol) FBS.
MV Isolation.
For all experiments, MVs were isolated from 250 × 106 cells cultured in serum-free medium for 48 h. Serum-free conditioned media were then collected and harvested at 300 g for 10 min to eliminate large cells. The supernatant was then recovered, and successive centrifugations at increasing speed were performed: one at 2,000 × g for 20 min to eliminate dead cells, then one at 10,000 × g for 30 min to remove cell debris, and finally one ultracentrifuge at 100,000 × g for 70 min to pellet MVs. The resulting pellet was then washed in PBS and ultracentrifuged again at the same speed. The obtained pellet was finally resuspended in 1 mL serum-free medium and used for treatment. MVs isolated from patient and mouse sera were isolated through ultracentrifugation, as described earlier.
MV Treatment.
C2C12 immortalized myoblasts or primary myoblasts isolated from mice were treated with MVs at the indicated points. The cell number of myoblasts was counted on a hemocytometer.
siRNA Transfection.
For transfection of A549 cells with Exiqon Negative Control A or miRCURY, LNA inhibitor hsa-miR-21 Lipofectamine 2000 (Invitrogen) was used, following the manufacturer’s instructions.
Single Fiber Isolation.
Single myofibers were prepared from gastrocnemius muscles according to previously established protocol (31). Isolated single myofibers were fixed with 2% (vol/vol) formaldehyde.
Myoblast Isolation and Culture.
Primary myoblasts were isolated as previously described (18) and were preplated twice using noncoated tissue culture dishes and then cultured either on a matrigel-coated 96-well plate for Trypan blue and 3-(4,5-dimethylthiazol-2-yl)-2,5-dipheniltetrazolium bromide (MTS) assay reading at the indicated points or on a matrigel-coated 12-well plate for protein and RNA analysis.
Immunofluorescence and TUNEL Staining.
Immunofluorescence staining and Western blotting were performed as previously described (18). TUNEL staining was performed using an In Situ Cell Death Detection Kit (Roche), following the manufacturer’s protocol. Quantitation was performed from 50 myofibers per muscle per animal.
Trypan Blue Staining.
Trypan blue dye was purchased from Gibco (15250-061), and staining was performed following manufacturer’s protocol. Dead cells are blue in color under the microscope and were counted using a hemocytometer.
Mice.
Cachexia in the LLC model was induced as previously described (18). TLR7−/− mice were obtained from Jackson Laboratory. Wild-type C57B6 male mice at the same age and weight were used as TLR7+/+ controls. All genotypes were determined by PCR, using tail DNA. All procedures used in this study complied with federal guidelines and the institutional policies of the Ohio State University Animal Care and Use Committee.
Statistics.
All quantitative data are represented as mean or mean ± SEM. Analysis was performed between different groups, using a two-tailed Student t test. Statistical significance was set at a P value of 0.05 as significant and a value of 0.01 as highly significant.
Supplementary Material
Acknowledgments
We thank L. Rizzotto and C. Deighan for technical support, and Dr. T. Williams for providing us with pancreatic cell lines. This work was supported by National Institutes of Health Grants CA180057 (to D.C.G.) and U01 CA152758 (to C.M.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402714111/-/DCSupplemental.
References
- 1.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 3.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allegra A, et al. Circulating microRNAs: New biomarkers in diagnosis, prognosis and treatment of cancer (review) Int J Oncol. 2012;41(6):1897–1912. doi: 10.3892/ijo.2012.1647. [DOI] [PubMed] [Google Scholar]
- 5.Hanke M, et al. A robust methodology to study urine microRNA as tumor marker: MicroRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2010;28(6):655–661. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1(1):7. doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandrov PN, et al. microRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF) Int J Biochem Mol Biol. 2012;3(4):365–373. [PMC free article] [PubMed] [Google Scholar]
- 8.Camussi G, et al. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1(1):98–110. [PMC free article] [PubMed] [Google Scholar]
- 9.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 10.Montecalvo A, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellana D, et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: A role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69(3):785–793. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 12.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Tisdale MJ. Cancer cachexia. Br J Cancer. 1991;63(3):337–342. doi: 10.1038/bjc.1991.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Sandri M, Carraro U. Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol. 1999;31(12):1373–1390. doi: 10.1016/s1357-2725(99)00063-1. [DOI] [PubMed] [Google Scholar]
- 18.He WA, et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest. 2013;123(11):4821–4835. doi: 10.1172/JCI68523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argilés JM, López-Soriano FJ, Busquets S. Apoptosis signalling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions. Int J Biochem Cell Biol. 2008;40(9):1674–1678. doi: 10.1016/j.biocel.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13(3):225–229. doi: 10.1097/mco.0b013e32833862df. [DOI] [PubMed] [Google Scholar]
- 21.Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest. 2008;118(4):1450–1457. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell PO, Pavlath GK. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol. 2004;287(6):C1753–C1762. doi: 10.1152/ajpcell.00292.2004. [DOI] [PubMed] [Google Scholar]
- 23.Busquets S, et al. Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr. 2007;26(5):614–618. doi: 10.1016/j.clnu.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Agustí AG, et al. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(4):485–489. doi: 10.1164/rccm.2108013. [DOI] [PubMed] [Google Scholar]
- 25.Rubin H. Cancer cachexia: Its correlations and causes. Proc Natl Acad Sci USA. 2003;100(9):5384–5389. doi: 10.1073/pnas.0931260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tournadre A, Lenief V, Miossec P. Expression of Toll-like receptor 3 and Toll-like receptor 7 in muscle is characteristic of inflammatory myopathy and is differentially regulated by Th1 and Th17 cytokines. Arthritis Rheum. 2010;62(7):2144–2151. doi: 10.1002/art.27465. [DOI] [PubMed] [Google Scholar]
- 27.Fredriksson K, et al. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS ONE. 2008;3(11):e3686. doi: 10.1371/journal.pone.0003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22(4):954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 29.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18(23):2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannetti E, et al. MicroRNA-21 in pancreatic cancer: Correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 31.Keire P, Shearer A, Shefer G, Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol. 2013;946:431–468. doi: 10.1007/978-1-62703-128-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.