Germ-line mutations in the tumor suppressor gene BRCA1 increase the lifetime risk for breast cancer and ovarian cancer by up to ∼80% and ∼50%, respectively. Population-based studies also support a sex- and tissue-specific tumor suppressor function of BRCA1, but the mechanisms of this specificity are not fully understood. Somatic loss of the normal functioning allele in BRCA1 carriers is common in cancer development and additional somatic events, including mutations of PTEN and TP53, occur at high frequencies (detailed below). Research over the last two decades has described the role of the ubiquitously expressed BRCA1 in maintaining genomic stability through its function in DNA repair and cell cycle checkpoint control, in addition to its activity as a transcriptional regulator. In PNAS, Gorrini et al. shed light on the specificity of BRCA1’s tumor suppressor function in breast tissue. The authors identify an estrogen-induced pathway that promotes the survival of BRCA1-deficient mammary epithelial cells (MECs), as well as Brca1-deficient mammary tumor cells from oxidative stress-induced cell death (1).
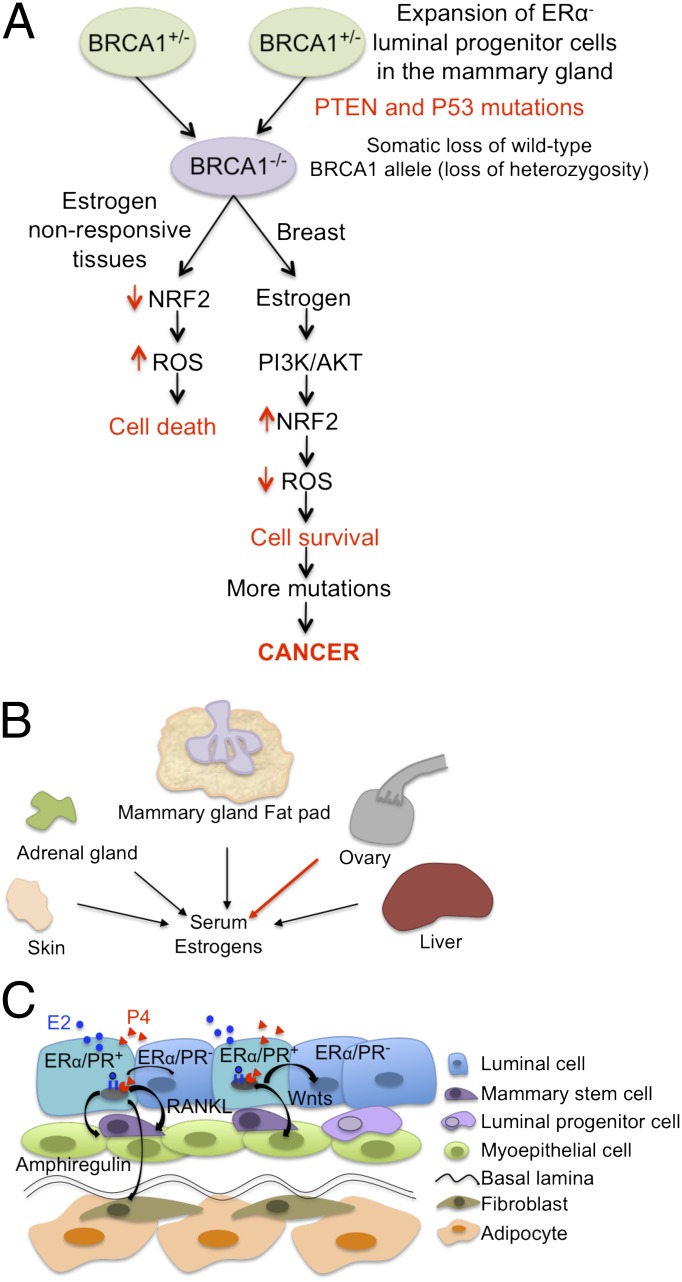
Oxidative stress plays important roles in cancer development and treatment (2). The NRF2-mediated antioxidant response pathway is the primary cellular defense against the cytotoxic effects of oxidative stress. Previously, Gorrini et al. showed how BRCA1 regulated the oxidative stress response through interaction with the NRF2 transcription factor to promote NRF2 stability and activation (3). Loss of BRCA1 resulted in defective NRF2 activation and reduced expression of NRF2-regulated antioxidant enzymes, leading to accumulation of reactive oxygen species (ROS) and cell death. Gorrini et al. now show that estrogen induces NRF2-regulated antioxidant genes in various cell types, likely through multiple pathways. They demonstrate that PI3 kinase activity is required, as treatment with a small molecular inhibitor of PI3K compromised expression of NRF2-regulated antioxidant genes. It is well established that PTEN mutations occur frequently in BRCA1-associated breast tumors (4, 5), prompting investigation into whether this pathway is involved in NRF2 regulation by estrogen. The researchers’ experiments with a PI3 kinase inhibitor and Pten-deficient mammary tumor cells indeed established a link between estrogen and the PTEN/PI3K pathway in the up-regulation of NRF2 (Fig. 1A). Importantly, estrogen was found to reduce cell death caused by BRCA1 knockdown. It was shown previously that Cre-mediated deletion of Brca1 in mouse mammary glands resulted in widespread apoptosis of MECs (6, 7). Similarly, Brca1 exon 11-deficient MECs failed to expand when transplanted into female mice. Gorrini et al. show that pregnancy, which dramatically up-regulates estrogen and progesterone with subsequent induction of prolactin (8), promotes proliferation of these cells. To confirm the importance of estrogen in Brca1-associated tumor development, Gorrini et al. injected Brca1/p53-deficient mammary tumor cells into the fat pads of female and male mice. Tumor cells grew poorly in the male mice as expected. Implantation of an estrogen-releasing pellet into the recipient mice further promoted tumor cell growth in the female mice. Estrogen also stimulated proliferation of the tumor cells in the male mice, resulting in dramatic tumor growth. Thus, the presence of elevated serum estrogen was sufficient to overcome the sex specificity of Brca1-associated tumorigenesis in this experimental setting.
Fig. 1.
(A) Model for NRF2 regulation in BRCA1-associated tumorigenesis. In breast tissue, estrogen induces NRF2 activation through the PI3K/AKT pathway in BRCA1-deficient cells and protects them from ROS-induced cell death. (B) Major estrogen sources in humans and mice. Red arrow indicates major tissue source of estrogen in premenopausal women. (C) Paracrine signaling in the mammary gland. In response to estrogen and progesterone, paracrine mediators promote the proliferation and differentiation of stem/progenitor cells, as well as ERα-/PR-negative luminal epithelial cells.
BRCA1 and 53BP1 play key roles in DNA double-strand break repair pathway choice (9). BRCA1 binds to satellite DNA and ubiquitinates histone 2A at satellite repeats, resulting in heterochromatin formation and transcriptional repression (10). These findings highlight BRCA1’s roles in genomic integrity protection and transcriptional regulation but provides insufficient explanation for its tissue-specific tumor suppression function. The ovarian steroid hormones estrogen and progesterone, acting through their respective receptors, are essential for breast development and are linked to breast carcinogenesis (8). BRCA1 is known to impact ovarian steroid hormone function in multiple ways, including repression of estrogen synthesis (11), modulation of estrogen receptor α (ERα) and progesterone receptor (PR) activity, and regulation of PR stability (7).
Estrogens are synthesized from androgens by the granulosa cells in the ovary. When ovarian function declines with menopause, serum estrogens and local estrogens are generated from the conversion of androgens by the aromatase enzyme in various tissues (Fig. 1B). In postmenopausal women, the decline of ovarian function leads to significant reductions in both serum estrogen and progesterone. However, the fat pad that surrounds the mammary tissue continues to provide estrogen locally (Fig. 1B). Thus, there are estrogens available for the initiation of the PI3K/Akt/NRF2 signaling pathways demonstrated by Gorrini et al. (1). Interestingly, BRCA1 represses expression of aromatase (11). Aromatase levels are elevated and serum estrogens are increased not only in mice carrying homozygous mutations of Brca1 in granulosa cells, but also in heterozygous mutants. The heterozygous mutant mice are likely to mimic human carriers in terms of aromatase activity in the granulosa cells (12). Intriguingly, an increase in serum estradiol and progesterone levels in postmenopausal BRCA1/BRCA2 carriers was reported recently (13). The increased steroid hormone levels are likely to promote proliferation of breast epithelial cells. Although the mechanisms leading to the increase in circulating estrogen and progesterone are not completely clear, particularly the dramatic elevation in serum progesterone, these findings may offer some explanation for the increased breast and ovarian cancer risk in these women.
Several studies demonstrate that BRCA1 exerts transcriptional repression on ERα through estrogen-independent and -dependent mechanisms in cell lines and in Brca1 mouse models (7). In addition to modulation of ERα activities, PR is stabilized in Brca1-deficient MECs, in part due to reduced GSK-3 activity and GSK-3–mediated phosphorylation of PR (14). Expression of PR is subject to transcriptional regulation by ERα. Thus, as noted above, BRCA1 affects ovarian steroid hormone signaling not only by altering hormone levels, but also through regulation of receptor expression and stability.
BRCA1 influences mammary cell fate determination as demonstrated by in vitro studies, fine analyses of mouse models, and characterization of various epithelial cell populations in breast tissue from BRCA1 mutation carriers. Earlier studies showed that depletion of BRCA1 impairs differentiation of mammary epithelial cells in vitro (15). Interestingly, BRCA1 is required for the differentiation of ERα-negative stem/progenitor cells, and loss of heterozygosity of BRCA1 is found in lobules that consist of entirely ERα- and PR-negative undifferentiated cells in women with germ-line BRCA1 mutations (16). The effects of BRCA1 on cell fate are found not only in cells harboring homozygous mutations of BRCA1 but also in cells carrying one WT allele. Several reports show an increase of luminal progenitor cells and suggest that these are likely to be the cells of origin of BRCA1-associated breast cancer in BRCA1 carriers (17). Importantly, Cre-mediated deletion of Brca1 in the luminal progenitor cells, but not in the basal stem cells, leads to tumors that phenocopy human BRCA1-associated breast cancers. Mechanistically, BRCA1 haploinsufficiency biases luminal cells toward a basal-like fate through aberrant expression of SLUG, a zinc finger-containing transcription factor involved in epithelial-to-mesenchymal transformation that is likely to have a negative impact on epithelial differentiation (18). In addition to cell-intrinsic actions, ERα and PR also act in a paracrine fashion to promote cell proliferation and differentiation of ERα-/PR-negative stem/progenitor cells and luminal epithelial cells (Fig. 1C). Amphiregulin, FGF, Wnts, and Rankl were shown to mediate the paracrine actions of ERα and PR (8). Recent elegant experiments using transgenic mouse models and antibody blockade demonstrated how steroid hormones influence the expansion and differentiation of steroid hormone receptor-negative mammary progenitor cells (19). With BRCA1’s regulatory roles in estrogen biosynthesis, activation of ERα, and the signal pathway described by Gorrini et al. (1), a scenario emerges in which an increase in the serum and/or local estrogen concentrations (Fig. 1B) accompanied by the presence of paracrine factors (Fig. 1C) would favor the expansion of progenitor cells and survival of BRCA1-deficient epithelial cells, leading to tumor development.
Based on single-cell profiling and computational identification of evolution paths in BRCA1-associated breast cancer, PTEN loss and TP53 mutation is predicted to precede loss of the WT BRCA1 in basal-type and luminal-type breast cancers, respectively (5), highlighting multiple pathways for overcoming apoptosis in BRCA1-deficient cells and the heterogeneity of BRCA1-associated breast cancers (Fig. 1A). As loss of heterozygosity of tumor suppressor genes occurs during the initiation and progression phases of tumor development, it would be interesting to learn whether there is a haploinsufficiency phenotype in the PI3K/AKT pathway described by Gorrini et al. Of course, many other intriguing questions remain. What roles do the other estrogen receptors play? Do cell cycle regulators interface with this survival pathway? What paracrine mediators are involved?
A significant number of BRCA1-associated breast cancers are classified as triple-negative (TNBCs), essentially lacking both ERα and PR expression as well as HER2 amplification. These BRCA1-mutated cancers constitute a distinct subset of the heterogeneous TNBCs. Clinical trials are ongoing addressing the utility of neoadjuvant cisplatin therapy and inhibitors of poly ADP ribose polymerase (PARP) that target the DNA repair defects found in BRCA1-mutated breast cancers (20). The prevalence of TNBCs among BRCA1 tumors leads one to ask whether, and how, the pathway identified by Gorrini et al. is involved in the development of TNBCs. Nonetheless, based on the well-established facts that oophorectomy reduces risk of breast cancer by 48% and tamoxifen treatments reduce the risk of contralateral breast cancer in BRCA1 carriers by 50%, it is clear that blocking ERα activity is effective in reducing breast cancer risk. The findings by Gorrini et al. support other strategies, such as PI3K inhibitors, for treating BRCA1-associated breast cancers.
Acknowledgments
We thank Yoon Kim for contributions to research in the E.Y.-H.P.L. laboratory, and Alex Ball and Allen Lee for discussion and editing. Research in E.Y.-H.P.L.’s laboratory is supported by National Cancer Institute Grants R01CA137102 and BCRF-35127.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4472.
References
- 1.Gorrini C, et al. Estrogen controls the survival of BRCA1-deficient cells via a PI3K–NRF2-regulated pathway. Proc Natl Acad Sci USA. 2014;111:4472–4477. doi: 10.1073/pnas.1324136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vurusaner B, Poli G, Basaga H. Tumor suppressor genes and ROS: Complex networks of interactions. Free Radic Biol Med. 2012;52(1):7–18. doi: 10.1016/j.freeradbiomed.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Gorrini C, et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J Exp Med. 2013;210(8):1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saal LH, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40(1):102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martins FC, et al. Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov. 2012;2(6):503–511. doi: 10.1158/2159-8290.CD-11-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3(3):389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 7.Dine J, Deng CX. Mouse models of BRCA1 and their application to breast cancer research. Cancer Metastasis Rev. 2013;32(1-2):25–37. doi: 10.1007/s10555-012-9403-7. [DOI] [PubMed] [Google Scholar]
- 8.Brisken C. Progesterone signalling in breast cancer: A neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13(6):385–396. doi: 10.1038/nrc3518. [DOI] [PubMed] [Google Scholar]
- 9.Daley JM, Sung P. 53BP1, BRCA1 and the choice between recombination and end joining at DNA double-strand breaks. Mol Cell Biol. 2014 doi: 10.1128/MCB.01639-13. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Q, et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477(7363):179–184. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y. BRCA1, hormone, and tissue-specific tumor suppression. Int J Biol Sci. 2009;5(1):20–27. doi: 10.7150/ijbs.5.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen HY, et al. Alterations in Brca1 expression in mouse ovarian granulosa cells have short-term and long-term consequences on estrogen-responsive organs. Lab Invest. 2012;92(6):802–811. doi: 10.1038/labinvest.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widschwendter M, et al. The sex hormone system in carriers of BRCA1/2 mutations: A case-control study. Lancet Oncol. 2013;14(12):1226–1232. doi: 10.1016/S1470-2045(13)70448-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, et al. Progesterone receptor A stability is mediated by glycogen synthase kinase-3β in the Brca1-deficient mammary gland. J Biol Chem. 2013;288(36):26265–26274. doi: 10.1074/jbc.M113.476556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuta S, et al. Depletion of BRCA1 impairs differentiation but enhances proliferation of mammary epithelial cells. Proc Natl Acad Sci USA. 2005;102(26):9176–9181. doi: 10.1073/pnas.0503793102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, et al. Basal-HER2 phenotype shows poorer survival than basal-like phenotype in hormone receptor-negative invasive breast cancers. Hum Pathol. 2008;39(2):167–174. doi: 10.1016/j.humpath.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Lindeman GJ, Visvader JE. Cell fate takes a slug in BRCA1-associated breast cancer. Breast Cancer Res. 2011;13(2):306. doi: 10.1186/bcr2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proia TA, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell. 2011;8(2):149–163. doi: 10.1016/j.stem.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HJ, et al. Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development. 2013;140(7):1397–1401. doi: 10.1242/dev.088948. [DOI] [PubMed] [Google Scholar]
- 20.Hirshfield KM, Ganesan S. Triple-negative breast cancer: Molecular subtypes and targeted therapy. Curr Opin Obstet Gynecol. 2014;26(1):34–40. doi: 10.1097/GCO.0000000000000038. [DOI] [PubMed] [Google Scholar]