Abstract
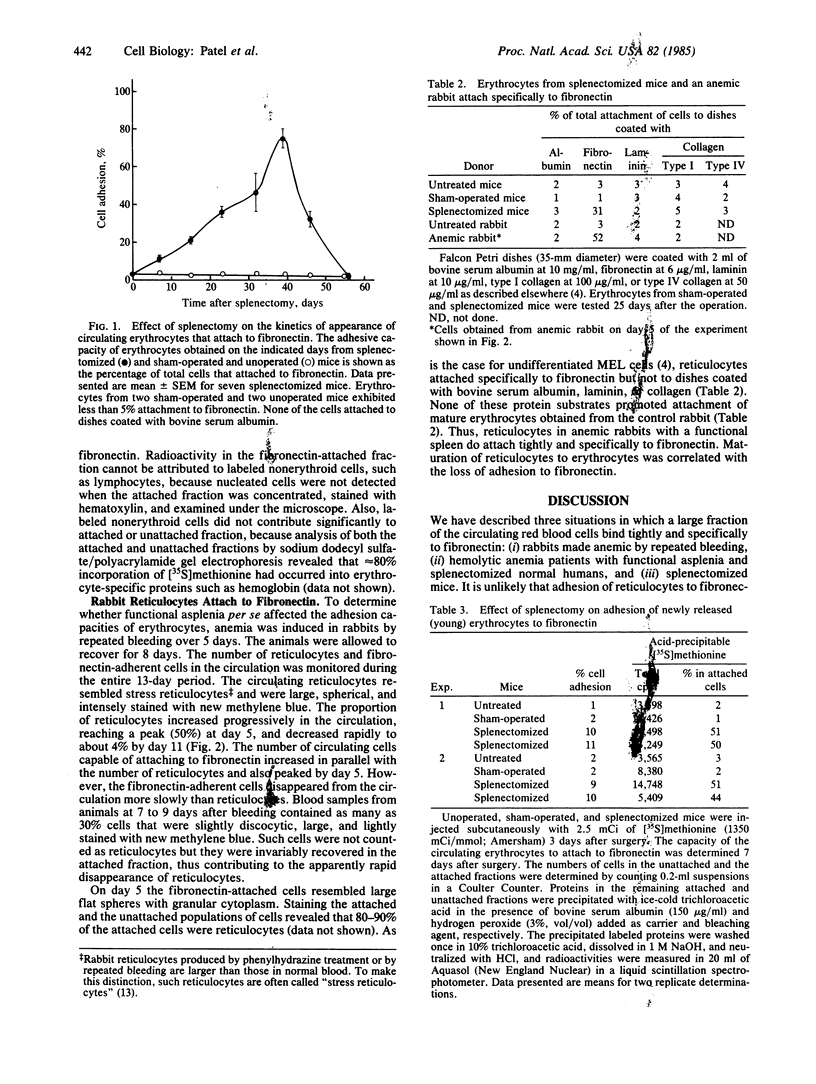
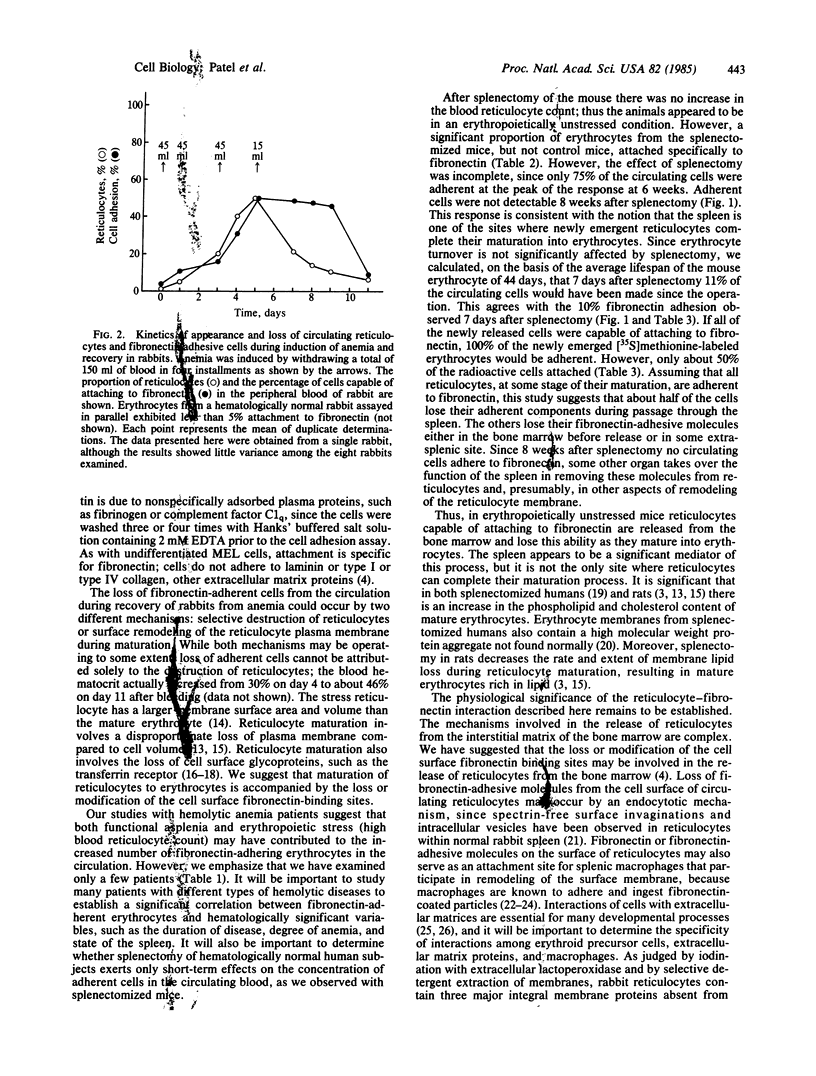
We describe three situations in which a large fraction of circulating red blood cells attach tightly and specifically to fibronectin: (i) rabbits made anemic by repeated bleeding, (ii) patients with hemolytic anemia and functional asplenia and splenectomized normal humans, and (iii) splenectomized mice. Upon induction of anemia in rabbits, the proportion of circulating red blood cells capable of specifically attaching to fibronectin-coated plastic increased in parallel with the number of reticulocytes. Fibronectin-adherent red cells were barely detectable when the rabbit had recovered from the anemia. Attachment of reticulocytes to fibronectin was specific; cells did not attach to dishes coated with albumin, laminin, or collagen. None of these proteins promoted the attachment of normal erythrocytes. About 75% of the erythrocytes from splenectomized mice (but not control mice) also attached specifically to fibronectin 40 days after surgery. The effect of splenectomy was incomplete and transient; adherent cells were not detectable 8 weeks after splenectomy. As judged by labeling studies with [35S]methionine, newly emergent reticulocytes preferentially attached to fibronectin. We suggest that about half of the reticulocytes in erythropoietically unstressed mice lose their ability to attach to fibronectin, possibly due to loss of fibronectin-adhesive components, during passage through the spleen. The others lose their ability to interact with fibronectin before release, in the bone marrow, or in some extrasplenic site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Come S. E., Shohet S. B., Robinson S. H. Surface remodeling vs. whole-cell hemolysis of reticulocytes produced with erythroid stimulation or iron deficiency anemia. Blood. 1974 Dec;44(6):817–830. [PubMed] [Google Scholar]
- Come S. E., Shohet S. B., Robinson S. H. Surface remodelling of reticulocytes produced in response to erythroid stress. Nat New Biol. 1972 Apr 5;236(66):157–158. doi: 10.1038/newbio236157a0. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Jandl J. H. The role of membrane lipids in the survival of red cells in hereditary spherocytosis. J Clin Invest. 1969 Apr;48(4):736–744. doi: 10.1172/JCI106031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., Jilek F., Adelmann B. C., Hörmann H. Similarity of antigelatin factor and cold insoluble globulin. Biochim Biophys Acta. 1978 Mar 28;533(1):227–237. doi: 10.1016/0005-2795(78)90566-4. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Ganzoni A., Hillman R. S., Finch C. A. Maturation of the macroreticulocyte. Br J Haematol. 1969 Jan-Feb;16(1):119–135. doi: 10.1111/j.1365-2141.1969.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Gasko O., Danon D. Endocytosis and exocytosis in membrane remodelling during reticulocyte maturation. Br J Haematol. 1974 Dec;28(4):463–470. doi: 10.1111/j.1365-2141.1974.tb06665.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M. Isolation and partial characterization of a high molecular weight red cell membrane protein complex normally removed by the spleen. Blood. 1977 Oct;50(4):625–641. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983 Jul;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. Loss of adhesion of murine erythroleukemia cells to fibronectin during erythroid differentiation. Science. 1984 Jun 1;224(4652):996–998. doi: 10.1126/science.6585955. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Spencer R. P., Cornelius E. A. Functional asplenia in sickle-cell anemia. N Engl J Med. 1969 Oct 23;281(17):923–926. doi: 10.1056/NEJM196910232811703. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G. Two active sites with different characteristics in fibronectin. FEBS Lett. 1979 Jan 15;97(2):221–224. doi: 10.1016/0014-5793(79)80088-5. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Weber P., Kaplan J. E. Physiologic role of cold-insoluble globulin in systemic host defense: implications of its characterization as the opsonic alpha 2-surface-binding glycoprotein. Ann N Y Acad Sci. 1978 Jun 20;312:43–55. doi: 10.1111/j.1749-6632.1978.tb16792.x. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Cooper R. A. Maturation of macroreticulocyte membranes in vivo. J Lab Clin Med. 1972 Feb;79(2):215–227. [PubMed] [Google Scholar]
- Weiss R. E., Reddi A. H. Appearance of fibronectin during the differentiation of cartilage, bone, and bone marrow. J Cell Biol. 1981 Mar;88(3):630–636. doi: 10.1083/jcb.88.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig S. E., Tokuyasu K. T., Singer S. J. Member-associated changes during erythropoiesis. On the mechanism of maturation of reticulocytes to erythrocytes. J Supramol Struct Cell Biochem. 1981;17(2):163–181. doi: 10.1002/jsscb.380170207. [DOI] [PubMed] [Google Scholar]
- Zweig S., Singer S. J. Concanavalin A-induced endocytosis in rabbit reticulocytes, and its decrease with reticulocyte maturation. J Cell Biol. 1979 Feb;80(2):487–491. doi: 10.1083/jcb.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bockxmeer F. M., Morgan E. H. Transferrin receptors during rabbit reticulocyte maturation. Biochim Biophys Acta. 1979 Apr 18;584(1):76–83. doi: 10.1016/0304-4165(79)90237-x. [DOI] [PubMed] [Google Scholar]
- van de Water L., 3rd, Schroeder S., Crenshaw E. B., 3rd, Hynes R. O. Phagocytosis of gelatin-latex particles by a murine macrophage line is dependent on fibronectin and heparin. J Cell Biol. 1981 Jul;90(1):32–39. doi: 10.1083/jcb.90.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]


