Abstract
AIM
To describe the baseline characteristics, racial/ethnic differences, and geographic differences among participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) study.
METHODS
The SPS3 trial enrolled patients with a symptomatic small subcortical stroke (lacunar stroke) within the prior 6 months and an eligible lesion on MRI, who were randomized, in a factorial design, to antiplatelet therapy (aspirin 325 mg daily plus clopidogrel 75 mg daily vs. aspirin 325 mg daily plus placebo) and to one of two levels of systolic blood pressure targets (“intensive” (<130 mmHg) vs. “usual” (130–149 mmHg)).
RESULTS
Among the 3020 participants recruited from 81 clinical sites in 8 countries, the mean age was 63 years, 63% were men, 75% had a history of hypertension, and 37% were diabetic. Fifty-one percent were White, 30% Hispanic, and 16% Black. Black participants were younger (mean age 58 years vs. 64 years, p<0.001) and more often had hypertension (95% vs. 89%, p<0.001) than White participants. Hispanic and Black participants more often had diabetes than White participants (42%, 40% vs. 32% respectively, both p<0.001). Tobacco smoking at the time of qualifying stroke was much more frequent among Spanish participants (32%) than those from North America (22%) or Latin America (8%) (p<0.001); systolic blood pressure at study entry was 5 mmHg lower among Spanish vs. North American participants (p<0.01).
CONCLUSIONS
The SPS3 cohort is the largest MRI-defined series of patients with S3. Among the racially/ethnically diverse SPS3 participants, there are important differences in patient features and vascular risk factors could influence prognosis for recurrent stroke and response to interventions.
Clinical Trial Registration Information
The SPS3 study is registered on www.clinicaltrials.gov (NCT00059306).
BACKGROUND
Small subcortical strokes (S3), also known as lacunar strokes, are a common stroke subtype, comprising about 25% of ischemic strokes.[1] While short-term prognosis is more favorable than in other stroke sub-types, stroke recurrence ranges between 4% and 11% per year, and S3 is the most common stroke subtype associated with vascular cognitive impairment and vascular dementia.[2–3] S3 are particularly frequent in Hispanic Americans,[4–5] who may have a worse prognosis.[6]
The rationale for the Secondary Prevention of Small Subcortical Strokes (SPS3) study has been published in detail elsewhere.[7] In brief, the optimal antiplatelet strategy is lacking for secondary stroke prevention in patients with S3, most due to small vessel disease. The efficacy of aspirin and clopidogrel compared with aspirin alone for prevention of vascular events has been demonstrated[8–10] and prompted evaluation in those specifically with S3.
Hypertension is a highly prevalent and significant risk factor for stroke in general and particularly for S3.[11–12] Treatment of hypertension has also been associated with reduced cognitive decline.[13] Relatively little is known, however, about the optimal target levels of blood pressure control for secondary stroke prevention in well-defined ischemic stroke subtypes.
The SPS3 study was designed to rigorously test in parallel whether combination antiplatelet therapy consisting of aspirin plus clopidogrel is superior to aspirin alone, and whether “intensive” blood pressure lowering is superior to “usual” blood pressure management, for reducing stroke recurrence (the primary endpoint), cognitive decline, and major vascular events. A secondary aim was to compare absolute differences in benefit of these interventions on outcomes between Hispanic and non-Hispanic White participants. In this report, the demographic and clinical features of participants included in SPS3 are described, focusing on differences according to race/ethnicity.
METHODS
Design and Sample
SPS3 is a randomized, multicenter clinical trial, sponsored by the U.S. NINDS/NIH. Details of the study design have been published elsewhere with pertinent aspects summarized here.[7] Patients were eligible if they had a clinical lacunar stroke syndrome or subcortical transient ischemic attack (TIA) in the 6 months prior to enrollment with MRI confirmation, were without clinical and radiological evidence of cortical involvement, and without surgically-amenable ipsilateral carotid artery disease and major-risk cardioembolic sources. Subcortical TIAs required positive MRI diffusion imaging to be eligible. Exclusion criteria included radiological or clinical evidence of a prior cortical/retinal stroke/TIA, previous intracranial hemorrhage or hemorrhagic infarct, disabling stroke, impaired renal function, high risk of bleeding, or cognitive impairment. Patients were randomized, in a 2-by-2 factorial design, to two interventions: (i) antiplatelet therapy: aspirin (325 mg/day) vs. aspirin (325 mg/day) plus clopidogrel (75 mg/day) (double blind, placebo control); and (ii) two target levels of systolic blood pressure control, “usual” (130–149 mmHg) vs. “intensive” (<130 mmHg). The SPS3 study was approved by the institutional review boards of all participating centers and all patients provided written informed consent.
Procedures
Medical history prior to the qualifying stroke was collected from all participants at study entry. A history of hypertension was defined by at least one of the following criteria: i) consistent recording of hypertension in medical records ≥ 1 year; ii) medical record or self-report of use of at least one antihypertensive medication and/or adjustment to achieve blood pressure control; iii) recording in medical records of elevation of blood pressure sustained ≥ 3 months. A history of diabetes was defined as chronic elevation of the fasting serum glucose exceeding 120 mg/dl or chronic requirement for hypoglycemic medications. Appendix 1 provides detailed study definitions for all vascular risk factors.
Prior to entry, patients were screened for cognitive dysfunction with the Folstein Mini Mental Status Examination (MMSE),[14] and a detailed cognitive assessment was performed. Patients were entered into the trial only if their MMSE did not fall more than two standard deviations below the mean for age and education. All patients underwent a standardized neurological exam,[15] functional recovery was assessed using the modified Rankin Scale,[16–17] Barthel Index,[18] and Edinburgh Stroke Outcome Questions.[19]
Classification of Race/Ethnicity
Race and ethnicity were determined primarily by self-report, using the two Census 2000 questions regarding race and Hispanic/Latino ethnicity. Based on a recommended combined format, the following categories were utilized to collect the racial/ethnic data: American Indian or Alaskan Native; Asian or Pacific Islander; Black, not of Hispanic origin; Hispanic; and White, not of Hispanic origin.[20] The first two categories (and ‘other race’ category) were collapsed due to very small numbers. All participants declaring themselves as Hispanic were classified as Hispanic, including participants from Latin America. All participants declaring themselves as White (and not Hispanic) were classified as non-Hispanic White (hereafter referred to as White). Participants from Spain were included in this category.[21] Similarly, participants self-reporting their race as Black or African American (and not Hispanic) were classified as non-Hispanic Black (hereafter referred to as Black). There were 22 participants (18 from North America and 4 from Latin America) who self-identified as Hispanic and Black and these were classified as Black.
Data Analyses
Frequencies (percentages), means (standard deviations) / medians (interquartile ranges) are presented for categorical and continuous variables, respectively. Analyses examined differences by ethnic/racial sub-groups. Specific comparisons were made between Hispanic and White sub-groups and Black and White sub-groups using Chi-square tests, Student t-tests, and Wilcoxon rank sum tests as appropriate. All tests of significance were 2-sided. Significant differences at the 0.01 and 0.001 levels of significance are noted. Differences were also examined by geographic region. Although Mexico is part of both North America and Latin America, for comparisons undertaken here, Mexico was included with Latin America. SAS version 9.2 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
RESULTS
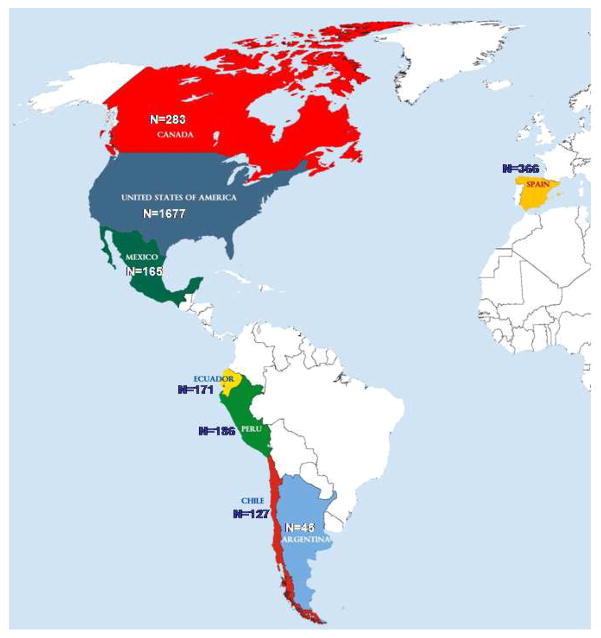
A total of 3020 patients with a recent symptomatic S3, from 81 clinical centers in USA, Canada, Mexico, Ecuador, Peru, Chile, Argentina, and Spain (Figure 1), were recruited between May 2003 and April 2011 and randomized into one of the four treatment groups. Detailed information on randomizations by clinical site can be found in Appendix 2. Average time between qualifying stroke and randomization was 76 ± 47 days. Demographic and clinical characteristics for the overall cohort and by race/ethnic subgroup are presented in Tables 1 and 2, with findings from the overall cohort and differences by race/ethnicity highlighted in the text below.
Figure 1.
Randomization by Country
Table 1.
Demographic and clinical characteristics of study participants
| Overall (n=3020) | Non- Hispanic White (n=1538) | Hispanic (n=916) | Non-Hispanic Black1 (n=492) | |
|---|---|---|---|---|
|
| ||||
| Mean age, years | 63 ± 11 | 64 ± 10.8 | 64 ± 10.7 | 58 ± 9.5** |
|
| ||||
| Male, % | 63 | 65 | 63 | 54** |
|
| ||||
| Lifestyle behaviors prior to qualifying stroke | ||||
| Smoking status, % | ** | * | ||
| Never | 40 | 35 | 50 | 32 |
| Former | 40 | 42 | 40 | 37 |
| Current | 20 | 23 | 10 | 31 |
| Alcohol use (≥ 7 drinks per week), % | 13 | 17 | 9** | 9** |
| Exercise, mean times per week | 3.1 ± 3 | 4 ± 3 | 2 ± 2.8** | 2 ± 2.7** |
|
| ||||
| Medical history prior to qualifying event, % | ||||
| Hypertension | 75 | 70 | 76** | 87** |
| Hyperlipidemia | 49 | 52 | 38** | 58 |
| Diabetes | 37 | 32 | 42** | 40** |
| Parental history of stroke | 30 | 31 | 25** | 33 |
| Ischemic heart disease | 11 | 13 | 6** | 12 |
| Prior symptomatic small subcortical stroke | 10 | 9 | 12 | 13* |
| Prior subcortical TIA | 5 | 6 | 4 | 5 |
| Intermittent claudication/peripheral vascular disease | 3 | 4 | 1** | 4 |
| Heart failure | 0 | 0 | 0 | 1 |
|
| ||||
| Antithrombotic medication used regularly in 7 days prior to qualifying event, % | 31 | 37 | 22** | 32 |
| Aspirin used regularly in 7 days prior to qualifying event, % | 28 | 33 | 21** | 26* |
|
| ||||
| Qualifying event, % | ||||
| Subcortical stroke | 97 | 96 | 98 | 98 |
| Subcortical TIA with DWI-positive corresponding lesion | 3 | 4 | 2 | 2 |
|
| ||||
| Clinical syndrome of qualifying event, % | ** | * | ||
| Pure motor hemiparesis | 33 | 30 | 40 | 31 |
| Sensorimotor stroke | 31 | 29 | 33 | 34 |
| Pure sensory stroke | 10 | 12 | 7 | 10 |
| Ataxic hemiparesis | 9 | 11 | 5 | 13 |
| Dysarthria clumsy-hand syndrome | 6 | 8 | 3 | 5 |
| Other | 11 | 11 | 12 | 6 |
Data are presented as percentages or mean ± SD
significantly different from Non-Hispanic White population at p ≤0.01
significantly different from Non-Hispanic White population at p ≤0.001
includes 22 patients who also reported Hispanic ethnicity
Table 2.
Selected clinical characteristics measured at trial entry
| Overall (n=3020) | Non-Hispanic White (n=1538) | Hispanic (n=916) | Non-Hispanic Black1 (n=492) | |
|---|---|---|---|---|
|
| ||||
| Functional recovery2 | ||||
| Barthel Index ≥ 95, % | 80 | 86 | 68** | 83 |
| Rankin score of 0–1, % | 67 | 71 | 65** | 55** |
|
| ||||
| Mean body mass index | 29.1 ± 6.8 | 29 ± 5.8 | 28 ± 5.7* | 31 ± 10.6** |
|
| ||||
| Mean systolic blood pressure (mmHg) | 143 ± 19 | 141 ± 17.2 | 144 ± 20** | 147 ± 19.9** |
| Mean diastolic blood pressure (mmHg) | 78 ± 11 | 77 ± 9.9 | 79 ± 11** | 82 ± 11.6** |
|
| ||||
| Medications at time of trial entry | ||||
| Anti-hypertensive medications, % | 85 | 83 | 84 | 92** |
| Angiotensin-converting enzyme inhibitor, % | 52 | 49 | 55* | 58** |
| Diuretics, % | 36 | 36 | 26** | 56** |
| Calcium channel blockers, % | 26 | 23 | 24 | 38** |
| Beta blockers, % | 25 | 25 | 17** | 37** |
| Angiotensin-receptor blocker, % | 16 | 17 | 18 | 9** |
| Lipid-lowering medications, % | 73 | 76 | 65** | 76 |
| Statins, % | 69 | 72 | 61** | 72 |
| Hypoglycemic medications, % | 33 | 28 | 39** | 37** |
|
| ||||
| Selected laboratory values | ||||
| Glucose (mg/dl) | 125 ± 55.1 | 124 ± 50.3 | 125 ± 59.2 | 130 ± 61.2 |
| Glycosylated hemoglobin (%) (diabetics only) |
8.3 ± 2.2 | 7.7 ± 2.2 | 8.6 ± 2.1** | 9.1 ± 2.3** |
| Total cholesterol (mg/dl) | 187 ± 49.9 | 187 ± 49.2 | 183 ± 48.8 | 196 ± 53.0** |
| LDL cholesterol (mg/dl) | 108 ± 45.7 | 106 ± 47.4 | 105 ± 42.8 | 120 ± 43.4** |
| HDL cholesterol (mg/dl) | 45 ± 21.3 | 46 ± 24.8 | 43 ± 15.8** | 47 ± 18.2 |
| Triglycerides (mg/dl) | 164 ± 119.2 | 162 ± 120.2 | 180 ± 129.5** | 139 ± 84.3** |
Data are presented as percentages, mean ± SD, or median (interquartile range)
significantly different from Non-Hispanic White population at p ≤0.01
significantly different from Non-Hispanic White population at p ≤0.001
includes 22 patients who also reported Hispanic ethnicity
Barthel Index ranges from 0 to 100 with higher scores indicating better function; Rankin Score ranges from 0 to 6 with lower scores indicating better function
LDL (low-density lipoprotein); HDL (high-density lipoprotein);
SPS3 Cohort
The mean age was 63 ± 11 years and 63% of the cohort was male (Table 1). The most prevalent risk factors were hypertension (75%), hyperlipidemia (49%), and diabetes (37%). Thirty percent reported a parental history of stroke, 10% a prior stroke, and 5% a TIA. About one-third of the cohort reported taking antithrombotic medication regularly in the 7 days prior to the qualifying event, with 28% reporting aspirin specifically.
The qualifying event for almost all participants was a stroke (97%), with pure motor hemiparesis the most common presenting syndrome (33%), followed by sensorimotor strokes (31%), with only 6% of the cohort presenting with a dysarthria clumsy-hand syndrome. Site investigators determined the localization based on clinical and radiological data. They localized 24% of lesions to the corona radiata/centrum semiovale, 28% to the basal ganglia/internal capsule, 22% to the thalamus and 26% to the brainstem or cerebellum (data not shown).
Table 2 provides information on selected clinical characteristics at the time of trial entry (approximately 2.5 months after their stroke). Two-thirds of the overall sample showed good functional recovery from the qualifying stroke at study enrollment, as indicated by a modified Rankin Scale score of 0 or 1. It is noteworthy that the mean body mass index (BMI) was 29 (± 6.8), very close to the accepted diagnostic criterion of obesity (30 kg/m2). The mean systolic and diastolic blood pressures of the participants at baseline were 143 ± 19 mmHg and 78 ± 11 mmHg, respectively. A high percentage of patients reported taking anti-hypertensive medications (85%) and lipid-lowering medications (73%) at trial entry. Of note, glucose was elevated in the overall cohort (125 mg/dl ± 55.1) and glycosylated hemoglobin was elevated in the sub-group of diabetics (8.3% ± 2.2).
Racial/Ethnic Comparisons
Fifty-one percent of the sample was classified as non-Hispanic White, 30% as Hispanic, and 16% as Non-Hispanic Black (Figure 2). There were 74 participants (3%) who reported their race as American Indian/Alaskan Native, Asian/Pacific Islander or ‘other’. Because of the small numbers and heterogeneity of the ‘other’ group, these data are not presented.
Figure 2.
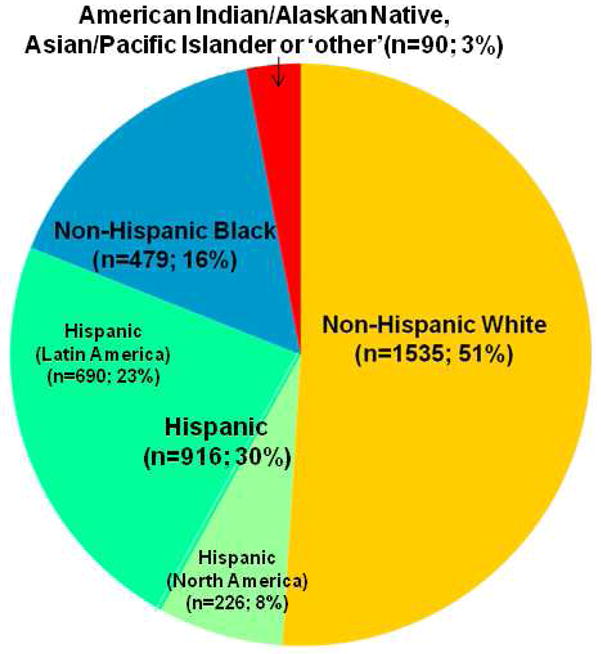
Racial/Ethnic Distribution
The average age of Black participants at trial entry was 58 years, significantly younger compared with the average age of 64 years for both Hispanic and White participants (both p<0.001) (Table 1). There were significant differences by race/ethnicity in lifestyle behaviors including smoking status, alcohol use, and regular exercise. Compared with Whites, both Hispanic and Black participants were significantly more likely to have a history (prior to the qualifying stroke) of hypertension (70% vs. 76% and 87% respectively; both p< 0.001) and diabetes (32% vs. 42% and 40% respectively; both p<0.001). In contrast, Whites were significantly more likely to have a history of ischemic heart disease compared with Hispanics (13% vs. 6%; p<0.001). Black participants were significantly more likely to report a prior symptomatic S3 compared with Whites (13% vs. 9%, p<0.01) but significantly less likely to report regular aspirin in the 7 days prior to the qualifying event compared with White participants (26% vs. 33%; p<0.01). Hispanic participants were also less likely to report regular aspirin use compared with Whites (21% vs. 33%; p<0.001).
Seventy-one percent of White participants scored 0 to 1 on the modified Rankin Scale at study enrollment (Table 2). In contrast, this same level of recovery was shown by only 65% of Hispanic and 55% of Black participants (both p<0.001). Compared with White participants (141 ± 17.2 mm Hg), average systolic blood pressure was significantly higher among Hispanic and Black participants (144 ± 20 mm Hg and 147 ± 19.9 mm Hg respectively; both p<0.001). Black participants were more likely to be using anti-hypertensive medications (92%) compared with White participants (83%) (p<0.001) at study entry and there were racial/ethnic differences by anti-hypertensive class. Hispanic participants were less likely to be taking lipid-lowering medications (65%) at study entry compared with White participants (76%) (p<0.001). Compared with Whites (7.7% ± 2.2), glycosylated hemoglobin was even higher for Hispanic and Black participants (8.6% ± 2.1 and 9.1% ± 2.3 respectively; both p<0.001).
Geographic Comparisons
Of the 3020 participants in SPS3, 65% were from North America (United States and Canada), 23% from Latin America (Mexico, Ecuador, Peru, Chile, and Argentina), and 12% from Spain (Table 3). Although the majority of Hispanic participants were from Latin American sites, participants from North America showed diversity with 40% of participants classified as race/ethnicity other than non-Hispanic White. Participants from North America were significantly younger at stroke onset than those from Latin America and Spain (62, 66, and 65 years respectively; p <0.01) and less likely to be male (60%, 65%, 75% respectively; p <0.01). Tobacco smoking at the time of qualifying stroke was much more frequent among Spanish participants (32%) than among those from North American (22%) or Latin American (8%) sites (p<0.01). There were significant differences in vascular risk factors by geographic region. Participants from Spain were significantly less likely to report a history of hypertension compared to North and Latin America (60%, 78%, 76% respectively; p <0.01) and entry systolic blood pressure was 5 mmHg lower among Spanish vs. North American participants (p<0.01). Participants from North America were significantly more likely to report a history of hyperlipidemia and ischemic heart disease compared with participants from Latin America and Spain (p< 0.01). Participants from North America were significantly more likely to report regular aspirin use in the 7 days before the qualifying stroke (37%) compared with participants from Latin America (20%) and Spain (21%) (p<0.01). There were also significant differences by geographic region in the use of both antihypertensive and lipid-lowering medications at trial entry (p<0.01). There were significant differences in mean body mass index (BMI) by geographic region, with the highest mean BMI found in North America (30 ± 7.5), followed by Spain (28 ± 4), and Latin America (27 ± 5).
Table 3.
Selected demographic and clinical characteristics stratified by geographic region
| North America1 (n=1960) | Latin America1 (n=694) | Spain (n=366) | |
|---|---|---|---|
|
| |||
| Race/Ethnicity, n (%)* | |||
| Non-Hispanic White | 1172 (59.8) | 0 | 366 (100) |
| Hispanic | 226 (11.5) | 690 (99.4) | 0 |
| Non-Hispanic Black2 | 488 (24.9) | 4 (0.6) | 0 |
| Other3 | 74 (3.8) | 0 | 0 |
|
| |||
| Mean age, years* | 62 ± 11 | 66 ± 11 | 65 ± 11 |
|
| |||
| Male, %* | 60 | 65 | 75 |
|
| |||
| Lifestyle behaviors prior to qualifying stroke | |||
| Smoking status, %* | |||
| Never | 36 | 52 | 33 |
| Former | 42 | 38 | 35 |
| Current | 22 | 9 | 32 |
| Alcohol use (≥ 7 drinks per week), %* | 13 | 8 | 22 |
| Exercise, mean times per week* | 3 ± 3 | 2 ± 3 | 5 ± 3 |
|
| |||
| Medical history prior to qualifying stroke, % | |||
| Hypertension* | 78 | 76 | 60 |
| Hyperlipidemia* | 57 | 33 | 37 |
| Diabetes | 36 | 38 | 36 |
| Parental history of stroke* | 32 | 23 | 28 |
| Ischemic heart disease* | 14 | 4 | 5 |
| Prior symptomatic small subcortical stroke | 10 | 11 | 8 |
| Prior subcortical TIA | 6 | 4 | 6 |
| Intermittent claudication/peripheral vascular disease* | 4 | 1 | 5 |
| Heart failure | 1 | 0 | 0 |
|
| |||
| Antithrombotic medication used regularly in 7 days before qualifying stroke, %* | 37 | 20 | 21 |
|
| |||
| Rankin score of 0–1, % | 67 | 64 | 67 |
|
| |||
| Barthel Index ≥ 95, %* | 85 | 64 | 84 |
|
| |||
| Mean body mass index* | 30 ± 7.5 | 27 ± 5 | 28 ± 4 |
|
| |||
| Mean systolic blood pressure (mmHg)* | 143 ± 19 | 144 ± 21 | 139 ± 16 |
| Mean diastolic blood pressure (mmHg) | 78 ± 11 | 79 ± 12 | 78 ± 9 |
|
| |||
| Anti-hypertensive medications, %* | 87 | 84 | 75 |
| Lipid-lowering medications, %* | 77 | 62 | 72 |
| Hypoglycemic medications, % | 33 | 34 | 34 |
Data are presented as percentages, mean ± SD, or median (interquartile range)
difference among groups significant at p <0.01
North America (United States and Canada); Latin America (Mexico, Ecuador, Peru, Chile, and Argentina)
includes 22 patients (18 in North America and 4 in Latin America) who also reported Hispanic ethnicity
Other includes those who reported their race as American Indian/Alaskan Native, Asian/Pacific Islander, or ‘other’
DISCUSSION
In this large, racially and geographically diverse cohort with recent subcortical ischemic strokes, significant differences in age, sex, and vascular risk factors between Whites, Blacks and Hispanics emerged. These differences are likely to impact the prognoses for recurrent stroke and cognitive impairment and perhaps the response to blood pressure control and antiplatelet therapies. For example, blood pressure control may offer larger relative benefits to Blacks, who have the highest prevalence of hypertension, compared with Hispanics, who have the highest frequency of diabetes. These hypotheses will be addressed using outcome data involving stroke, cognition and death accumulated during the anticipated 4-year mean follow-up of the cohort.
The differences in stroke risk factors among White, Black, and Hispanic participants reported here are consistent with findings from previous studies and may reflect the ethnic disparities in stroke care.[21] Important to note is the 6 year difference in age of onset of stroke with an average age of 58 years (SD=9.5) for Black participants compared with 64 years for White (SD=10.8) and Hispanic (SD=10.7) participants. This younger age, coupled with the level of functional recovery at entry to trial with only 55% of Blacks reporting normal or near normal status in comparison to 65% and 71% for Hispanic and White participants respectively, underscores the important effects of stroke for Blacks on disability-free years. Furthermore, Black participants were more likely to have a history of prior subcortical stroke but yet less likely to report regular aspirin use in the 7 days before the index stroke. Hispanic participants were similarly less likely to report regular use of antiplatelet medication prior to the index stroke. Morgenstern and colleagues reported that only about two-thirds of Mexican Americans were taking antiplatelet medications for secondary stroke prevention in the Brain Attack Surveillance in Corpus Christi (BASIC) Project.[22] Consistent with previous reports, Black participants were significantly more likely to be hypertensive, with a higher blood pressure at trial entry.[23–25] Similar to findings from the BASIC Project,[26] Hispanics had a significantly higher prevalence of diabetes (42%) compared with Whites (32%), while White participants (13%) had a significantly higher prevalence of ischemic heart disease than Hispanic participants (6%). A comparison of the proportion of diabetes in Latin American participants (38%) compared with the proportion in Hispanics overall (42%) suggests that this higher prevalence of diabetes in Hispanics may be driven by a high prevalence in North American Hispanics.
SPS3 was designed to address several important clinical and scientific questions relevant to a common stroke subtype, lacunar infarct. The inclusion criteria were stringent to ensure that small vessel disease was the most likely underlying mechanism of the lacunar infarct. The exclusion criteria were carefully considered to enhance generalizability of the trial findings to the millions of patients with this common vascular disorder. Nonetheless, clinical trials suffer from problems with external validity and SPS3 is no exception. To examine if the SPS3 sample is representative of the larger population of patients with lacunar stroke, we compared baseline characteristics from SPS3 participants with available characteristics from published series of both hospital and population-based patients with lacunar stroke, selected because patients in the series underwent neuro-imaging (Table 4). These samples are diverse in the country of origin and also in the time they were studied with publications dating from 1989 to 2011. The average age at trial entry for SPS3 participants was 63 years, falling within the range seen in this series, and consistent with reports of a younger average age for those with lacunar stroke compared to other stroke sub-types.[27–28] There is considerable variability in the prevalence of vascular risk factors across the series. For example, the reported prevalence of hypertension ranged from a low of 41% in the Finnish cohort[29] to a high 88% in the cohort from Germany,[30] with the majority reporting at least 60% prevalence and SPS3 reporting 75%. SPS3 was among those studies reporting the highest prevalence of diabetes. Differences in prevalence may relate to the inconsistencies in how the vascular risk factor was defined as well as the source of the cohort. SPS3 is the only clinical trial in the series. Despite the strict criteria imposed on patient inclusion in a clinical trial, the baseline characteristics of the SPS3 cohort fall within the range reported in these series and provide support that the results from SPS3 will be applicable to the overall population of patients with lacunar stroke.
Table 4.
Lacunar stroke series
| Study | Country | Sample size | Mean age | Male, % | Imaging, % | Vascular Risk Factors, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| CT | MRI | HTN | DM | IHD | PVD | TIA | Stroke | HPL | |||||
| Norrving,[31] | Sweden | 61 | 58 | 67 | 100 | 53 | 8 | 8 | NR | NR | NR | 16 | |
| Tegeler,[32] | USA | 55 | 61±10 | 71 | 100 | 60 | 35 | NR | NR | 7 | 24 | NR | |
| Landi,[33] | Italy | 88 | 66±12 | 63 | 100 | 65 | 19 | 11 | NR | 23 | NR | NR | |
| Toni,[34] | Italy | 170 | 67±10 | 65 | 100 | 56 | 16 | 22 | NR | 23 | NR | NR | |
| Salgado,[35] | Portugal | 145 | 65±11 | 64 | 100 | 72 | 25 | 5 | 8 | 18 | NR | 28 | |
| Yip,[36] | Taiwan | 195 | 66±9 | 54 | 100 | 85 | 36 | 14 | NR | NR | 24 | 18 | |
| Marti-Vilalta,[37] | Spain | 399 | 67±11 | NR | 100 | 76 | 28 | 22 | 10 | 9 | NR | 12 | |
| Awada,[38] | Saudi | 248 | 59 | 68 | 100 | 57 | 56 | 14 | NR | NR | NR | NR | |
| Hajat,[39] | UK | 282 | 72 | 48 | NR | 66 | 21 | 26 | NR | NR | 20 | NR | |
| Yokota,[40] | Japan | 556 | 66±11 | 70 | 100 | 75 | 27 | 9 | NR | 15 | NR | 32 | |
| Soda,[41] | Japan | 198 | 70±10 | 66 | 100 | 69 | 37 | NR | NR | 24 | 39 | ||
| Wessels,[30] | Germany | 63 | 63 | 57 | 100 | 88 | 30 | NR | NR | NR | 30 | 38 | |
| Bejot,[42] | France | 89 | 74 | 42 | 100 | 73 | 19 | NR | NR | 11 | NR | 61 | |
| Turin,[43] | Japan | 751 | 70±0.7 | 55 | 100 | 57 | 21 | NR | NR | 6 | NR | 19 | |
| Wardlaw,[44] | UK | 67 | 64.5 | 75 | 100 | 52 | NR | 12 | 3 | 8 | 3 | NR | |
| Melkas,[29] | Finland | 63 | 73±7 | 50 | 100 | 41 | 24 | 32 | 16 | NR | 14 | NR | |
| SPS3,[7] | NA, LA, Spain | 3020 | 63±11 | 63 | 100* | 75 | 37 | 7 | 3 | 5 | 10 | 49 | |
Data are presented as mean ± SD or percentage
HTN (hypertension), DM (diabetes mellitus), IHD (ischemic heart disease), PVD (peripheral vascular disease), TIA (transient ischemic attack), HPL (hyperlipidemia), NR (not reported), NA (North America), LA (Latin America)
6 patients (0.1%) were unable to undergo MRI related to medical reasons and were entered into the study with CT scan that demonstrated the corresponding lesion
There is no generally accepted or consistent way that ethnicity is defined across studies[21] and this has been a challenging issue in this study, particularly with the inclusion of sites from Spain and Latin America. We made several assumptions in our classification including that participants from South America were Hispanic and that those participants from Spain were not Hispanic. Even within each racial/ethnic group, there is considerable heterogeneity relative to culture and environment which limits conclusions regarding any differences between racial/ethnic groups. Furthermore, the results presented here, highlighting race/ethnic differences, were undertaken for the purpose of describing fully the clinical features of the SPS3 cohort. As they were unadjusted and are cross-sectional, we are unable to draw conclusions regarding the reasons for the differences by race/ethnicity. These unadjusted findings do emphasize the greater prevalence of stroke risk factors in Blacks and Hispanics and also the greater impact on functional recovery at approximately 3 months after the index stroke.
In conclusion, examination of the baseline characteristics in the SPS3 cohort compared with other published lacunar stroke series demonstrates that the cohort is representative of lacunar stroke patients in general. This finding will enhance generalizability of the SPS3 trial results to the millions of patients around the world with this common disorder. Due to its multi-racial cohort, the results of SPS3 should further the understanding of racial/ethnic differences in stroke risk and prevention.
Acknowledgments
Sources of Funding
This study is funded by the National Institute of Neurological Disorders and Stroke (NINDS # 2 U01 NS38529-04A1). Clopidogrel and matching placebo were donated by Sanofi-Aventis, USA and Bristol-Myers Squibb, USA.
Appendix 1. Operational definitions of vascular risk factors
| Hypertension | Present if any of the following criteria was met:
|
| Diabetes | Present if either of the following criteria was met:
|
| Ischemic Heart Disease | Includes history of definite myocardial infarction, definite/atypical angina, or revascularization procedure.Myocardial infarction definite if any of the following criteria was met:
|
| Heart Failure | Diagnosis based on a clinically convincing situation (complete medical record documentation if not currently active) which could include constellations of:
|
| Peripheral Vascular Disease | Present if either of the following criteria was met:
|
| Hyperlipidemia |
|
Appendix 2. Country and clinical site (number of enrolled patients)
USA, 50 sites (n=1,677): University of Texas Health Science Center at San Antonio, San Antonio, TX (108); Boston University, Boston, MA (92); Mayo Clinic Rochester, Rochester, MN (90); University of California San Diego, San Diego, CA (82); Mayo Clinic, Scottsdale, AZ (74); The Metro Health System, Cleveland, OH (70); Minneapolis Medical Research Foundation, Minneapolis, MN (68); University of Kentucky Medical Center, Lexington, KY (64); St. Louis University, St. Louis, MO (57); Wayne State University School of Medicine, Detroit, MI (57); The Methodist Hospital, Houston, TX (57); University of Arizona, Tucson, AZ (54); St. John’s Mercy Medical Center, St. Louis, MO (52); Henry Ford Hospital, Detroit, MI (51); Melbourne Internal Medical Associates, Melbourne, FL (50); Vanderbilt University Medical Center, Nashville, TN (46); Columbia University Medical Center, New York, NY (45); The Ohio State University Medical Center, Columbus, OH (41); University of South Alabama, Mobile, AL (39); Rochester General Hospital, Rochester, NY (34); University of Texas Southwestern, Dallas, TX (33); Medical College of Wisconsin, Milwaukee, WI (33); University of Rochester Medical Center, Rochester, NY (32); Oregon Health and Science University, Portland, OR (30); Wake Forest University School of Medicine, Winston-Salem, NC (29); University of Washington, Seattle, WA (27); Cooper Health System, Camden, NJ (26); St. Joseph’s Hospital and Medical Center, Phoenix, AZ (25); Marshfield Clinic Research Foundation, Marshfield, WI (24); Sutter Medical Center Sacramento, Sacramento, CA (22); Iowa Neurology Research, Inc., Des Moines, IA (21); University of Miami Miller School of Medicine, Miami, FL (17); John Hopkins Bayview Medical Center, Baltimore, MD (16); Case Western Reserve University, Cleveland, OH (16); Helen Hayes Hospital, Rensselaer, NY (15); Emory University, Atlanta, GA (13); Research Foundation of Sunny-Buffalo, NY (9); Cedars Sinai Medical Center, Los Angeles, CA (9); Florida Neurovascular Institute, Tampa, FL (9); North General Hospital, New York, NY (9); Mt. Sinai School of Medicine, New York, NY (9); Washington University School of Medicine at St. Louis, MO (5); The Regents of the University of California, Fresno, CA (4); Loyola University of Chicago, IL (4); Indiana University, Indianapolis, IN (2); University of Illinois at Chicago, IL (2); Colorado Neurological Institute, Englewood, CO (2); Spartanburg Regional Medical Center, Spartanburg, SC (1); Sunrise Hospital and Medical Center, Las Vegas, NV (1); Stanford University, Stanford, CA (1); CANADA, 8 sites (n=283): Centre Hospitalier affilié Universitaire de Québec, QC (48); Montreal General Hospital, McGill University, Montreal, QC (42); Capital District Health Authority, Halifax, NS (41); The Ottawa Hospital, Ottawa, ON (37); Jewish General Hospital, McGill University, Montreal, QC (37); Centre de Recherche, Greenfield Park, QC (36); University of Calgary, Calgary, AB (35); University of Alberta, Edmonton, AB (7); ECUADOR, 1 site (n=171): Hospital Clínica Kennedy, Guayaquil (171); CHILE, 2 sites (n= 127): Hospital Naval Almirante Nef, Viña del Mar (69); Hospital Clínico Universidad Católica de Chile, Santiago (58); MÉXICO, 4 sites (n= 165): Instituto Nacional de Neurología y Neurocirugía, México DF (92); Hospital Civil de Guadalajara, Jalisco (31); Instituto Nacional de Ciencias Médicas y Nutrición, México DF (26); Hospital de la Universidad Autónoma de Nuevo León, Monterrey, Nuevo León (16); ARGENTINA, 5 sites (n= 45): Centro Neurológico, Buenos Aires (14); Hospital Británico, Buenos Aires (12); Hospital Ramos Mejía, Buenos Aires (7); Instituto FLENI, Buenos Aires (8); Hospital Universitario Austral, Buenos Aires (4); PERÚ, 1 site (n=186): Fundación Cayetano Heredia, Lima (186); SPAIN, 10 sites (n= 366): Hospital Universitario de Bellvitge, Barcelona (66); Hospital del Mar, Barcelona (58); Hospital Universitario Sagrat Cor, Barcelona (54); Corporació Sanitaria Parc Taulí, Sabadell (51); Hospital Universtario Dr. Josep Trueta, Girona (45); Hospital Universitario German Trias i Pujol, Badalona (27); Hospital de la Santa Creu i Sant Pau, Barcelona (25); Hospital Universitario de Santiago de Compostela (23); Hospital La Paz, Madrid (15); Hospital General de Cataluña, Barcelona (2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolominsky-Rabas PL, Weber M, Gefeller O, et al. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 2.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2:238–245. doi: 10.1016/s1474-4422(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 3.Norrving B. Lacunar infarcts: no black holes in the brain are benign. Practical Neurology. 2008;8:222–228. doi: 10.1136/jnnp.2008.153601. [DOI] [PubMed] [Google Scholar]
- 4.Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 5.Worley KL, Lalonde DR, Kerr DR, et al. Survey of the causes of stroke among Mexican Americans in South Texas. Tex Med. 1998;94:62–67. [PubMed] [Google Scholar]
- 6.Benavente O, Hart R, Palacio S, et al. Stroke recurrence, cognitive impairment and white matter abnormalities are frequent in Hispanic Americans with lacunar stroke. Cerebrovasc Dis. 2002;13(suppl 3):87. (abstr) [Google Scholar]
- 7.Benavente OR, White CL, Pearce L, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6:164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 9.The Clopidogrel in Unstable Angina to Prrevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 10.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 11.Gueyffier F, Froment A, Gouton M. New meta-analysis of treatment trials of hypertension: improving the estimate of therapuetic benefit. J Hum Hypertens. 1996;10:1–8. [PubMed] [Google Scholar]
- 12.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 13.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Norris J. Comment on “Study design of stroke treatments”. Stroke. 1982;13:527–528. doi: 10.1161/01.str.13.4.527. [DOI] [PubMed] [Google Scholar]
- 16.Farrell B, Godwin J, Richards S, et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2:200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 18.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 19.Lindley RI, Waddell F, Livingstone M, et al. Can simple questions assess outcome after stroke? Cerebrovasc Dis. 1994;4:314–324. [Google Scholar]
- 20.Office of Management and Budget. [Accessed August 2011];Standards for classifcation of federal data on race and ethnicity. http://www.whitehouse.gov/omb/fedreg_race-ethnicity.
- 21.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: The American experience: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern LB, Smith MA, Lisabeth LD, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravata DM, Wells CK, Gulanski B, et al. Racial disparities in stroke risk factors: the impact of socioeconomic status. Stroke. 2005;36:1507–1511. doi: 10.1161/01.STR.0000170991.63594.b6. [DOI] [PubMed] [Google Scholar]
- 24.Cushman M, Cantrell RA, McClure LA, et al. Estimated 10-year stroke risk by region and race in the United States. Ann Neurol. 2008;64:507–513. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors. The Northern Manhattan Stroke Study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 26.Uchino K, Risser JMH, Smith MA, et al. Ischemic stroke subtypes among Mexican Americans and non-Hispanic whites: the BASIC Project. Neurology. 2004;63:574–576. doi: 10.1212/01.wnl.0000133212.99040.07. [DOI] [PubMed] [Google Scholar]
- 27.Arboix A, Marti-Vilalta JL. Lacunar stroke. Expert Rev Neurother. 2009;9:179–196. doi: 10.1586/14737175.9.2.179. [DOI] [PubMed] [Google Scholar]
- 28.You R, McNeil JJ, O’Malley HM, et al. Risk factors for lacunar infarction syndromes. Neurology. 1995;45:1483–1487. doi: 10.1212/wnl.45.8.1483. [DOI] [PubMed] [Google Scholar]
- 29.Melkas S, Putaala J, Oksala NKJ, et al. Small-vessel disease relates to poor poststroke survival in a 12-year follow-up. Neurology. 2011;76:734–739. doi: 10.1212/WNL.0b013e31820db666. [DOI] [PubMed] [Google Scholar]
- 30.Wessels T, Rottger C, Jauss M, et al. Identification of embolic stroke patterns by diffusion-weighted MRI in clinically defined lacunar stroke syndromes. Stroke. 2005;36:757–761. doi: 10.1161/01.STR.0000158908.48022.d7. [DOI] [PubMed] [Google Scholar]
- 31.Norrving B, Cronqvist S. Clinical and radiologic features of lacunar versus nonlacunar minor stroke. Stroke. 1989;20:59–64. doi: 10.1161/01.str.20.1.59. [DOI] [PubMed] [Google Scholar]
- 32.Tegeler CH, Shi F, Morgan T. Carotid stenosis in lacunar stroke. Stroke. 1991;22:1124–1128. doi: 10.1161/01.str.22.9.1124. [DOI] [PubMed] [Google Scholar]
- 33.Landi G, Cella E, Boccardi E, et al. Lacunar versus non-lacunar infarcts: pathogenetic and prognostic differences. J Neurol Neurosurg Psychiatry. 1992;55:441–445. doi: 10.1136/jnnp.55.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toni D, Fiorelli M, De Michele M, et al. Clinical and prognostic correlates of stroke subtype misdiagnosis within 12 hours from onset. Stroke. 1995;26:1837–1840. doi: 10.1161/01.str.26.10.1837. [DOI] [PubMed] [Google Scholar]
- 35.Salgado AV, Ferro JM, Gouveia-Oliveira A. Long-term prognosis of first-ever lacunar stroke: A hospital-based study. Stroke. 1996;27:661–666. doi: 10.1161/01.str.27.4.661. [DOI] [PubMed] [Google Scholar]
- 36.Yip PK, Jeng JS, Lee TK, et al. Subtypes of ischemic stroke. A hospital-based stroke registry in Taiwan (SCAN-IV) Stroke. 1997;28:2507–2512. doi: 10.1161/01.str.28.12.2507. [DOI] [PubMed] [Google Scholar]
- 37.Martí-Vilalta JL, Arboix A. The Barcelona Stroke Registry. Eur Neurol. 1999;41:135–142. doi: 10.1159/000008036. [DOI] [PubMed] [Google Scholar]
- 38.Awada A, Al Rajeh S. The Saudi Stroke Data Bank. Analysis of the first 1000 cases. Acta Neurol Scand. 1999;100:265–269. [PubMed] [Google Scholar]
- 39.Hajat C, Dundas R, Stewart JA, et al. Cerebrovascular risk factors and stroke subtypes: differences between ethnic groups. Stroke. 2001;32:37–42. doi: 10.1161/01.str.32.1.37. [DOI] [PubMed] [Google Scholar]
- 40.Yokota C, Minematsu K, Hasegawa Y, et al. Long-term prognosis, by stroke subtypes, after a first-ever stroke: a hospital-based study over a 20-year period. Cerebrovasc Dis. 2004;18:111–116. doi: 10.1159/000079258. [DOI] [PubMed] [Google Scholar]
- 41.Soda T, Nakayasu H, Maeda M, et al. Stroke recurrence within the first year following cerebral infarction--Tottori University Lacunar Infarction Prognosis Study (TULIPS) Acta Neurol Scand. 2004;110:343–349. doi: 10.1111/j.1600-0404.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 42.Bejot Y, Catteau A, Caillier M, et al. Trends in incidence, risk factors, and survival in symptomatic lacunar stroke in Dijon, France, from 1989 to 2006: a population-based study. Stroke. 2008;39:1945–1951. doi: 10.1161/STROKEAHA.107.510933. [DOI] [PubMed] [Google Scholar]
- 43.Turin TC, Kita Y, Rumana N, et al. Ischemic stroke subtypes in a Japanese population: Takashima Stroke Registry, 1988–2004. Stroke. 2010;41:1871–1876. doi: 10.1161/STROKEAHA.110.581033. [DOI] [PubMed] [Google Scholar]
- 44.Wardlaw JM, Doubal FN, Eadie E, et al. Little association between intracranial arterial stenosis and lacunar stroke. Cerebrovasc Dis. 2011;31:12–18. doi: 10.1159/000319773. [DOI] [PMC free article] [PubMed] [Google Scholar]