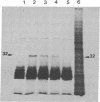
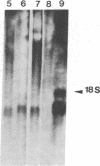
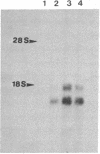
Abstract
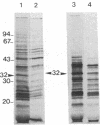
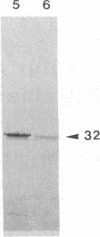
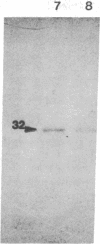
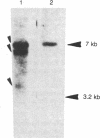
The uncoupling protein (UCP) of mammalian brown fat is a specialized and unique component responsible for energy dissipation as heat. Translation and immunoprecipitation from sucrose-fractionated mRNA indicated that the mRNA of UCP sedimented at 14-16 S. A recombinant cDNA library prepared from mRNA of thermoactive brown fat enriched for UCP mRNA has been constructed and cloned in Escherichia coli. Recombinant plasmids were screened by differential colony hybridization to a cDNA probe complementary to poly(A)+ RNA isolated from thermogenic or from weakly thermogenic brown fat. Several differentially hybridizing plasmids were shown to contain UCP cDNA sequences by their ability to select a mRNA coding for an in vitro translation product that was immunoprecipitable with antibodies against UCP. Blot hybridization of brown fat mRNA to a 32P-labeled UCP cDNA probe revealed two major species of mRNA (15S and 18S). As compared to non-thermogenic tissue, a strikingly increased hybridization to the probe was observed with brown fat mRNA from thermoactive tissue. Moreover, hybridization was observed with RNA of brown adipose tissue from rat, hamster, or mouse but not with RNA from rat or mouse liver.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Ainsworth A. T., Ellis R. D., Piercy V., Thody V. E., Thurlby P. L., Wilson C., Wilson S., Young P. Treatment of obesity with thermogenic beta-adrenoceptor agonists: studies on BRL 26830A in rodents. Int J Obes. 1984;8 (Suppl 1):1–11. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillaud F., Ricquier D., Mory G., Thibault J. Increased level of mRNA for the uncoupling protein in brown adipose tissue of rats during thermogenesis induced by cold exposure or norepinephrine infusion. J Biol Chem. 1984 Sep 25;259(18):11583–11586. [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. The biochemistry of an inefficient tissue: brown adipose tissue. Essays Biochem. 1985;20:110–164. [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Freeman K. B., Chien S. M., Litchfield D., Patel H. V. Synthesis in vitro of rat brown adipose tissue 32 000 Mr protein. FEBS Lett. 1983 Jul 25;158(2):325–330. doi: 10.1016/0014-5793(83)80606-1. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mory G., Bouillaud F., Combes-George M., Ricquier D. Noradrenaline controls the concentration of the uncoupling protein in brown adipose tissue. FEBS Lett. 1984 Jan 30;166(2):393–396. doi: 10.1016/0014-5793(84)80120-9. [DOI] [PubMed] [Google Scholar]
- Mory G., Ricquier D., Nechad M., Hemon P. Impairment of trophic response of brown fat to cold in guanethidine-treated rats. Am J Physiol. 1982 Mar;242(3):C159–C165. doi: 10.1152/ajpcell.1982.242.3.C159. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Peeters B. L., Mous J. M., Rombauts W. A., Heyns W. J. Androgen-induced messenger RNA in rat ventral prostate. Translation, partial purification, and preliminary characterization of the mRNAs encoding the components of prostatic binding protein. J Biol Chem. 1980 Jul 25;255(14):7017–7023. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricquier D., Mory G., Nechad M., Combes-George M., Thibault J. Development and activation of brown fat in rats with pheochromocytoma PC 12 tumors. Am J Physiol. 1983 Sep;245(3):C172–C177. doi: 10.1152/ajpcell.1983.245.3.C172. [DOI] [PubMed] [Google Scholar]
- Ricquier D., Thibault J., Bouillaud F., Kuster Y. Molecular approach to thermogenesis in brown adipose tissue. Cell-free translation of mRNA and characterization of the mitochondrial uncoupling protein. J Biol Chem. 1983 Jun 10;258(11):6675–6677. [PubMed] [Google Scholar]
- Rougeon F., Mach B. Stepwise biosynthesis in vitro of globin genes from globin mRNA by DNA polymerase of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3418–3422. doi: 10.1073/pnas.73.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfest C. W., Kwiatkowski R. W., Dottin R. P. Molecular cloning of a DNA sequence complementary to creatine kinase M mRNA from chickens. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4997–5000. doi: 10.1073/pnas.79.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]