Abstract
Purpose
Proton pump inhibitors (PPIs) and corticosteroids are commonly prescribed drugs; however, each has been associated with fracture and community acquired pneumonia. How physicians select patients for co-therapy may have implications for potential additive or synergistic toxicities.
Methods
We conducted a retrospective cohort study of 13,749 incident corticosteroid users with no prior PPI exposure using the HealthCore Integrated Research DatabaseSM. We used logistic regression to evaluate the association between PPI initiation in the first 30 days of steroid therapy and corticosteroid dose, clinical risk factors including co-morbid diseases, and medication use including prescription nonsteroidal anti-inflammatory drugs (NSAIDs).
Results
1,050 (7.6%) patients filled a new PPI prescription within 30 days of starting corticosteroids. PPI use was associated with the number of baseline co-morbid conditions (OR 1.21 for each additional condition, CI 1.13–1.28), recent hospitalization (OR 4.71, CI 4.02–5.52), prednisone dose above 40mg/day (OR 1.87, CI1.45–2.41), history of gastroesophageal reflux or gastric ulcer disease (OR 1.54, CI 1.24– 1.91), renal insufficiency (OR 2.06, CI 1.73–2.46), and liver disease (OR 1.82, CI 1.45–2.28). Concomitant use of prescription NSAIDs was also associated with PPI use (OR 1.89, CI 1.32–2.70); however, the total use of PPIs in this group was low (6.3%, CI 4.4–8.2%).
Conclusions
Overall, PPI therapy among corticosteroid users was uncommon, even among those with risk factors for gastrointestinal toxicity. PPI use was significantly more common among patients who had recently been hospitalized, had a greater burden of co-morbid illness, or were receiving high daily doses of corticosteroids.
Keywords: proton pump inhibitors, corticosteroids, gastroprotection, adverse events
Introduction
Proton pump inhibitors (PPIs) are widely prescribed in the United States for the management of gastroesophageal reflux disease (GERD) as well as for prophylaxis against gastrointestinal ulcers. Although generally considered safe, a growing body of observational data suggests that PPIs may be associated with significant toxicities, including an increased risk of fracture1–7 and community acquired pneumonia.8–13 Although not all studies have supported these conclusions,14–16 there is sufficient evidence of potential risk to warrant further exploration into ways in which these drugs can be selectively prescribed to maximize their potential benefits while minimizing potential harms.
Chronic corticosteroid users represent a patient population for whom the balance between risks and benefits of PPI therapy may be especially salient. First, existing data suggest that corticosteroid users are more likely to be given a PPI than other patients.4, 17 Second, while corticosteroid users with risk factors for gastrointestinal bleeding including concomitant NSAID use are likely to benefit from PPI therapy,18 the role of corticosteroids in the development of significant GI toxicity in patients without additional risk factors is controversial,19–23 and the benefits of acid suppression in this group have not been established. Finally, chronic corticosteroid use has been associated with some of the same toxicities as PPI use, most notably fracture and infection.24
To date, no studies have defined whether the combination of PPI’s and corticosteroids results in an increased risk of toxicity compared to corticosteroids alone. However, the potential for additive or synergistic toxicity and the uncertain benefits of acid suppression in the majority of corticosteroid users suggest that physicians should be selective in the use of PPI’s in this population. We therefore designed this study to define risk factors that are associated with the use of PPIs among a cohort of new corticosteroid users, specifically focusing on those baseline factors that may increase the risk of gastrointestinal toxicity and therefore justify the use of a PPI, and those factors that may increase the risk of fracture and community acquired pneumonia.
Methods
We used data from the HealthCore Integrated Research DatabaseSM, an insurance claims database that includes diagnostic, procedural and prescription drug information for patients in 14 states in the United States, to conduct a retrospective cohort study of new chronic corticosteroid users. We examined records from January 1, 2002 through July 31, 2009 to identify all patients in the database with an incident prescription or prescriptions for corticosteroids with a total days supply of at least 60 consecutive days. The date of the first corticosteroid prescription fill meeting this criterion was used as the index date. We required included patients to have at least one year of enrollment in the database without corticosteroid exposure prior to the index date and at least 90 days of follow up in the database after the index date. We excluded those patients who had received a PPI in the twelve months prior to the index date. We also excluded patients who had a diagnosis of a hematologic malignancy that might be treated with corticosteroids or who had a diagnostic or procedure code for cancer chemotherapy prior to the index date. The study was reviewed and approved by the Institutional Review Board at the University of Pennsylvania.
Outcome definition
The primary outcome was the use of a proton pump inhibitor in conjunction with corticosteroid therapy. This outcome was defined using pharmacy claims data as a prescription fill for a PPI within 30 days of the index date.
Risk factor definitions
Patient age at the index date, the geographic region (Northeast, Midwest, West, and Southeast) in which the patient was enrolled, and the patient’s gender were obtained from the health plan enrollment files. We used ICD-9-CM codes (see appendix for complete codes) to identify medical claims recorded within one year prior to the index date for the following conditions identified as possible risk factors for falls, fracture or community acquired pneumonia25, 26: rheumatoid arthritis, asthma or chronic obstructive pulmonary disease, cough, disorders resulting in immunodeficiency, congestive heart failure, dementia, gait imbalance or a history of falls, coronary artery disease, cerebrovascular disease, peripheral vascular disease, chronic renal insufficiency, chronic liver disease, endocrine disorders affecting bone metabolism, intestinal malabsorption, alcohol abuse, tobacco use, osteoporosis, seizures or syncope, and diabetes. The following potential risk factors for non-fracture complications of corticosteroid therapy including gastrointestinal complications were also included: obesity, inflammatory bowel disease, and a history of GERD or peptic ulcer disease. In addition to evaluating each of these conditions separately, we calculated the number of distinct co-morbid illnesses other than GERD or peptic ulcer disease recorded in the year prior to the index date as a measure of overall burden of co-morbid illness.
To capture the use of other prescription medications at the time of corticosteroid initiation, we used pharmacy records of filled prescriptions in the 90 days prior to, but not including, the index date. We recorded the use of bisphosphonates and calcium/vitamin d supplements; anticoagulants including warfarin, heparin and low molecular weight heparin; anti-platelet agents including aspirin, clopidogrel and ticlopidine; thyroid replacement hormone; cardiovascular medications including antihypertensives, rate controlling agents, diuretics and statins; and additional immunosuppressive agents including cyclophosphamide, azathioprine, and disease modifying anti-rheumatic drugs.
During the first 30 days of therapy beginning with the index date, we calculated the average daily dose of corticosteroids prescribed by first multiplying the number of pills dispensed by the strength of the pills prescribed, then dividing this product by the number of days’ supplied in the prescription. Prescriptions for corticosteroids other than prednisone were then converted into prednisone-equivalent doses.27 All corticosteroid daily doses were then divided into categories: less than 5 mg, 5–<10 mg, 10–<20 mg, 20–<30 mg, 30–<40 mg, 40–<50 mg, 50–<60 mg, 60–<70 mg, 70–<80 mg, and ≥80 mg. Because of the risk of gastrointestinal toxicity associated with the combined use of non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids28, we also recorded whether the patient filled a prescription for a NSAID in the 30 days after the index date.
To explore whether location of care or recent acute illness affects prescribing behavior, we recorded a history of hospital admission in the thirty days prior to corticosteroid initiation. Furthermore, as a marker of functional status and overall comorbidity, we recorded any nursing home admissions in the 12 months prior to corticosteroid initiation. Finally, as a measure of intensity of medical care, we recorded the number of physician visits29 and the number of distinct drugs prescribed30 in the year prior to the index date.
Analytic methods
The distribution of proposed risk factors were compared between the treatment groups using binomial tests of proportions for dichotomous risk factors and Student’s t-test or Wilcoxon rank-sum test for continuous variables. Unadjusted associations between each risk factor variable and PPI use were analyzed using separate logistic regression models for each exposure. To determine the conditional associations of each risk factor with the outcome, we then constructed multivariable logistic regression models that included all the risk factors. Separate models were used to assess corticosteroid dose expressed linearly and categorically in the dose categories defined earlier in the methods. Additionally, separate models were constructed in which specific co-morbid illnesses were removed and the number of co-morbidities was included first as a continuous variable then as a categorical variable defined as 0,1,2,3,4,or ≥5 co-morbid diseases. As a sensitivity analysis, we then repeated the analyses after excluding patients with an age less than 19 years. A p-value of 0.05 was used as a threshold for statistical significance in all analyses.
Results
We identified 13,749 incident corticosteroid users who met inclusion and exclusion criteria. Of these, only 1,050 (7.6%) received a prescription for a PPI within 30 days of initiating corticosteroid therapy. The median duration of PPI therapy was 6 months (IQR 3–13 months). A total of 4.6% of patients were prescribed NSAIDs within 30 days of corticosteroid initiation with a median duration of NSAID use of 8 months (IQR 4–18 months). Comparisons of the pre-index characteristics, co-morbid conditions, and medication use for each group are shown in Table 1. Users of PPIs were less likely to be female (60.0% vs. 52.4%, p<0.01), and proportions of comorbid conditions were higher in nearly every category among PPI users compared to the corticosteroid-alone group (see Table 1). PPI users were also much more likely to have been hospitalized prior to initiating corticosteroid therapy, and to have been admitted to a nursing home. Unadjusted analyses demonstrated a statistically significant association between PPI initiation and all the risk factor variables examined except: age; a history of dementia, inflammatory bowel disease, hypothyroidism, and osteoporosis; the frequency of physician visits; use of anti-platelet medications; and prior use of an H2 receptor antagonist (see Table 2).
Table 1.
Characteristics of patients treated with corticosteroids alone and corticosteroids plus a proton pump inhibitor
| Steroids alone (n=12,699 pts) |
Steroids + PPI (n=1,050 pts) |
P–value | |
|---|---|---|---|
| Age in yrs (continuous) | Mean 53.35 (SD 18.91) | Mean 53.14 (SD 19.38) | 0.74 |
| Gender (female) | 60.0% | 52.4% | <0.01 |
| Geographic region Midwest† Northeast Southeast West |
24.1% 18.1% 17.8% 40.1% |
20.9% 18.9% 16.6% 43.7% |
|
| Corticosteroid dose (continuous) | Median 10 mg (IQR 5–20) | Median 20 mg (IQR 6.7–45) | <0.01 |
| Corticosteroid dose category (mg) 0–<5† 5–<10 10–<20 20–<30 30–<40 40–<50 50–<60 60–<70 70–<79 ≥80 |
19.9% 19.3% 26.6 12.4% 6.1% 7.5% 1.2% 5.0% 0.2% 1.8% |
17.0% 10.2% 18.5% 12.9% 7.2% 9.8% 4.3% 13.5% 1.0% 5.7% |
<0.01 |
| Number of comorbidities | 1.02 | 1.66 | <0.01 |
| Number of comorbidities 0† 1 2 3 4 >5 |
40.2% 35.0% 14.2% 6.1% 2.7% 1.8% |
23.9% 30.7% 20.0% 12.4% 7.4% 5.6% |
<0.01 |
| Rheumatoid arthritis | 22.3% | 12.7% | <0.01 |
| Asthma/Chronic obstructive pulmonary disease | 10.2% | 13.7% | <0.01 |
| Cough | 9.5% | 11.9% | 0.01 |
| Congestive heart failure | 6.5% | 17.1% | <0.01 |
| Obesity | 1.8% | 3.2% | <0.01 |
| Tobacco use | 3.2% | 5.7% | <0.01 |
| Alcohol abuse | 0.6% | 1.6% | <0.01 |
| Dementia | 1.2% | 1.6% | 0.23 |
| Gait imbalance or a history of falls | 1.9% | 3.3% | <0.01 |
| Coronary artery disease | 10.5% | 19.0% | <0.01 |
| Cerebrovascular disease | 5.5% | 10.5% | <0.01 |
| Peripheral vascular disease | 2.3% | 3.4% | 0.02 |
| Intestinal malabsorption | 0.4% | 1.1% | |
| Chronic renal insufficiency | 9.9% | 33.4% | <0.01 |
| Endocrine disorders affecting bone metabolism | 3.7% | 6.2% | <0.01 |
| Disorders resulting in immunodeficiency | 0.7% | 1.6% | 0.12 |
| Inflammatory bowel disease | 7.1% | 7.2% | <0.01 |
| Osteoporosis | 6.6% | 7.0% | 0.84 |
| History of seizures or syncope | 3.9% | 7.3% | 0.66 |
| Chronic liver disease | 4.5% | 13.2% | <0.01 |
| Nursing home admission in the past 12 months | 3.5% | 9.1% | <0.01 |
| Diabetes | 12.9% | 19.2% | <0.01 |
| Hospitalization in the past 30 days | 14.0% | 56.5% | <0.01 |
| History of GERD or peptic ulcer disease | 7.2% | 13.3% | <0.01 |
| Visits with a physician in the past year | 7.1 | 7.5 | 0.10 |
| Number of medications prescribed in the past year | 7.1 | 7.8 | <0.01 |
| Use of anticoagulation | 3.6% | 6.8% | <0.01 |
| Use of anti–platelet medication | 2.0% | 2.1% | 0.89 |
| Use of NSAIDs in the prior 90 days | 16.5% | 9.5% | <0.01 |
| Use of NSAIDs concurrently | 4.7% | 3.8% | 0.21 |
| Prior use of H2RA | 2.6% | 3.3% | 0.15 |
| Use of immunosuppressive medications | 13.1% | 5.2% | <0.01 |
| Use of thyroid replacement hormone | 10.5% | 8.9% | <0.01 |
| Use of cardiovascular medications | 31.0% | 37.4% | <0.01 |
| Use of bisphosphonates or vitamin D supplements | 11.4% | 7.5% | <0.01 |
Table 2.
Unadjusted and fully adjusted associations between patient characteristics and PPI initiation
| Unadjusted OR | 95% CI | Adjusted OR | 95% CI | |
|---|---|---|---|---|
| Age in yrs (continuous) | 0.99 (per yr increase) | 0.99–1.00 | 1.00 | 1.00–1.01 |
| Gender (female) | 0.73** | 0.65–0.83 | 1.00 | 0.86–1.15 |
| Geographic region Midwest† Northeast Southeast West |
-- 1.21 1.08 1.26** |
-- 0.99–1.47 0.88–1.32 1.07–1.49 |
-- 1.43** 0.99 1.31** |
-- 1.11–1.83 0.79–1.24 1.08–1.57 |
| Corticosteroid dose category (mg) 0–<5† 5–<10 10–<20 20–<30 30–<40 40–<50 50–<60 60–<70 70–<79 ≥80 |
-- 0.62** 0.82 1.21 1.39* 1.54** 4.20** 3.17** 4.72** 3.68** |
-- 0.48–0.79 0.66–1.00 0.96–1.53 1.05–1.83 1.20–1.99 2.91–6.05 2.50–4.02 2.27–9.82 2.67–5.08 |
-- 0.77 0.92 0.97 1.08 1.34 3.53** 2.05** 2.85* 2.13** |
-- 0.57–1.03 0.71–1.19 0.73–1.30 0.77–1.51 0.98–1.82 2.31–5.40 1.52–2.76 1.26–6.47 1.45–3.12 |
| Number of co–morbid conditions‡ | 1.44** | 1.38–1.51 | 1.21** | 1.13–1.28 |
| Rheumatoid arthritis | 0.51** | 0.42–0.61 | 0.82 | 0.66–1.01 |
| Asthma/Chronic obstructive pulmonary disease | 1.40** | 1.16–1.68 | 0.91 | 0.74–1.13 |
| Cough | 1.28* | 1.06–1.56 | 0.89 | 0.71–1.11 |
| Congestive heart failure | 2.99** | 2.51–3.57 | 1.25 | 0.99–1.57 |
| Obesity | 1.81** | 1.26–2.61 | 1.14 | 0.76–1.71 |
| Tobacco use | 1.81** | 1.37–2.39 | 1.15 | 0.84–1.56 |
| Alcohol abuse | 2.77** | 1.63–4.71 | 1.08 | 0.59–1.57 |
| Dementia | 1.32 | 0.80–2.19 | 0.80 | 0.46–1.39 |
| Gait imbalance or a history of falls | 1.81** | 1.26–2.59 | 0.96 | 0.63–1.45 |
| Coronary artery disease | 1.99** | 1.69–2.34 | 1.21 | 0.97–1.50 |
| Cerebrovascular disease | 2.01** | 1.63–2.49 | 1.11 | 0.86–1.43 |
| Peripheral vascular disease | 1.51* | 1.06–2.15 | 0.86 | 0.58–1.28 |
| Intestinal malabsorption | 2.71** | 1.44–5.08 | 1.05 | 0.52–2.13 |
| Chronic renal insufficiency | 4.55** | 3.94–5.23 | 2.06** | 1.73–2.46 |
| Endocrine disorders affecting bone metabolism | 1.73** | 1.32–2.26 | 0.89 | 0.66–1.20 |
| Disorders resulting in immunodeficiency | 2.31** | 1.37–3.89 | 1.27 | 0.71–2.26 |
| Inflammatory bowel disease | 1.03 | 0.80–1.31 | 0.88 | 0.67–1.16 |
| Osteoporosis | 1.06 | 0.83–1.36 | 1.25 | 0.94–1.66 |
| History of seizures or syncope | 1.94** | 1.52–2.49 | 0.95 | 0.72–1.26 |
| Chronic liver disease | 3.21** | 2.64–3.91 | 1.82** | 1.45–2.28 |
| Nursing home admission in the past 12 months | 2.75** | 2.18–3.45 | 1.24 | 0.93–1.64 |
| Diabetes | 1.60** | 1.36–1.88 | 1.10 | 0.90–1.33 |
| Hospitalization in the past 30 days | 7.94** | 6.96–9.06 | 4.71** | 4.02–5.52 |
| History of GERD or peptic ulcer disease | 1.97** | 1.63–2.38 | 1.54** | 1.24–1.91 |
| Visits with a physician in the past year | 1.01 | 1.00–1.01 | 1.00 | 0.99–1.01 |
| Number of medications prescribed in the past year | 1.02** | 1.01–1.04 | 1.00 | 0.98–1.01 |
| Use of anticoagulation | 1.91** | 1.48–2.48 | 1.24 | 0.92–1.67 |
| Use of anti–platelet medication | 1.03 | 0.66–1.60 | 0.57* | 0.35–0.93 |
| Use of NSAIDs in prior 90 days | 0.53** | 0.43–0.66 | 0.82 | 0.64–1.03 |
| Concurrent use of NSAIDs | 0.81 | 0.59–1.12 | 1.89** | 1.32–2.70 |
| Prior use of H2RA | 1.30 | 0.91–1.85 | 1.00 | 0.67–1.47 |
| Use of immunosuppressive medications | 0.37** | 0.28–0.48 | 0.61** | 0.46–0.83 |
| Use of thyroid replacement hormone | 0.84 | 0.68–1.04 | 0.86 | 0.67–1.09 |
| Use of cardiovascular medications | 1.33** | 1.17–1.52 | 0.90 | 0.76–1.07 |
| Use of bisphosphonates or vitamin D supplements | 0.63** | 0.50–0.80 | 0.85 | 0.65–1.11 |
p-value <0.05
p-value <0.01
reference group
Analyzed in separate model adjusted for age, gender, region, hospitalization, nursing home residency, frequency of physician visits, number of drugs prescribed in the past year, corticosteroid dose, and the use of the following medications: anticoagulants, anti-platelet agents, NSAIDs (prior and concurrent use separately), drugs to prevent bone loss, cardiovascular drugs, H2 receptor antagonists, thyroid hormone, and immunosuppressants other than corticosteroids.
After multivariable adjustment (Table 2), chronic kidney disease (OR 2.06, 95% CI 1.73–2.46), chronic liver disease (OR 1.82, 95% CI 1.45–2.28), and a history of GERD or gastric ulcer disease (OR 1.54, 95% CI 1.24–1.91) remained significantly associated with PPI initiation.. Recent hospitalization (OR 4.71, 95% CI 4.02–5.52) was strongly associated with increased PPI use.
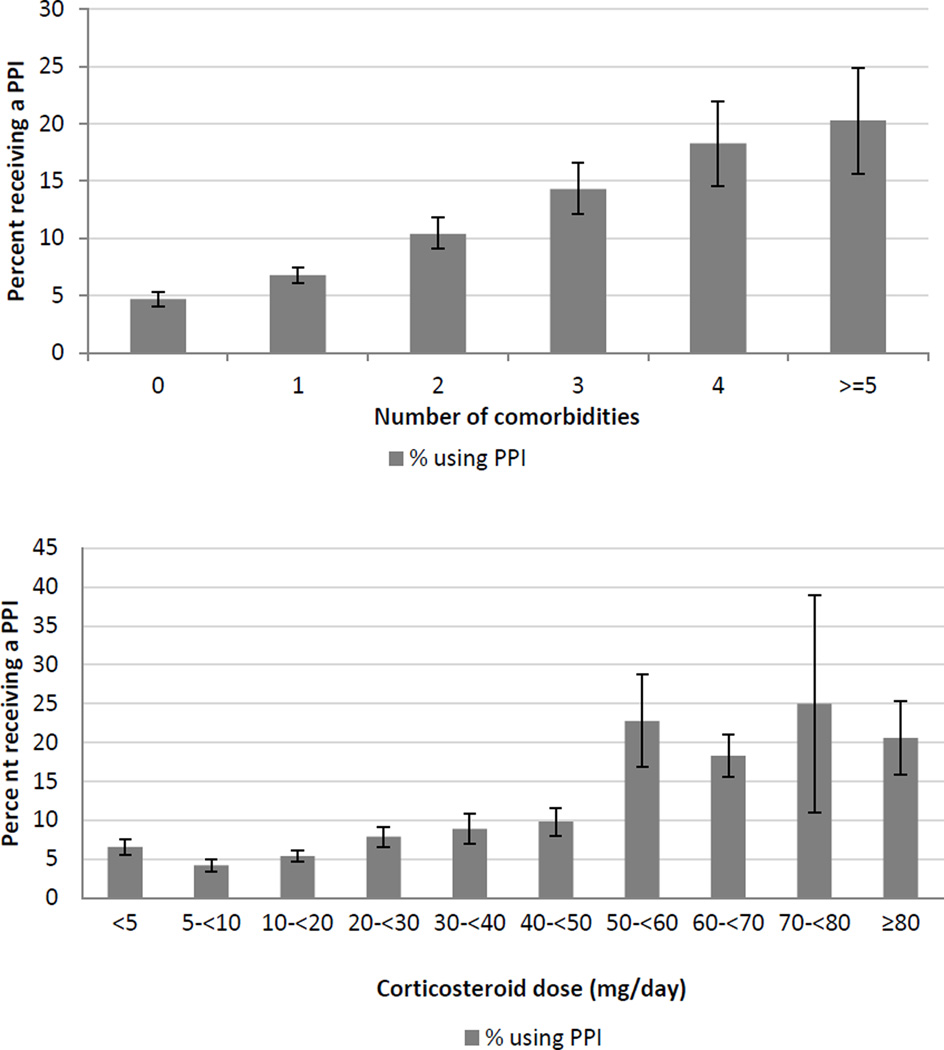
When we examined the number of co-morbid diagnoses as a risk factor, a clear relationship between the increasing burden of co-morbid illness and PPI use was observed (see Figure 1 and Table 2) with an adjusted linear OR of 1.21 (95% CI 1.13–1.28) for each additional co-morbid diagnosis.
Figure 1.
Percentage of new corticosteroid users initiating PPI therapy according to the number of co-morbid conditions present in the year prior to treatment (top) and corticosteroid dose (bottom)
When we examined the use of concomitant medications as risk factors in multivariable models, we observed that both the use of immunosuppressants other than corticosteroids (OR 0.62, 95% CI 0.46–0.84) and the use of anti-platelet medications (OR 0.57, 95% CI 0.35–0.93) were associated with less frequent PPI use. Patients who filled a prescription for an NSAID in the first 30 days after initiating corticosteroid therapy were more likely to also receive a PPI (adjusted OR 1.89, 95% CI 1.32–2.70); however, despite being more likely than non-NSAID users to initiate PPI therapy, the proportion of NSAID users filling a PPI prescription was small (6.3%, 95% CI 4.4–8.2%). Among those patients prescribed NSAIDs who were also over the age of 70 and thus at further increased risk of GI toxicity, PPI use was even lower (1.2%, CI 0–3.6%).
We observed a relationship between corticosteroid dose and PPI therapy; however, this relationship was not strictly linear (see Figure 1). The dose categories less than 40 mg were not associated with increased PPI use compared to the lowest dose category. At doses of 40 mg/day, the odds of PPI initiation began to rise (OR 1.34, 95% CI 0.98–1.82 for doses of 40–<50 mg). As shown in Figure 1, above 50 mg/day the frequency of PPI initiation more than doubled (22.8% vs. 9.8% for doses of 50–<60 mg/day compared to 40–<50 mg/day) and the odds of receiving a PPI remained consistently elevated across all subsequent dose categories (Table 2).
We did observe evidence of regional variation in the use of PPIs: the Midwest (referent) and Southeast regions (OR 0.99, 95% CI 0.79–1.24) demonstrated similar use of PPIs in this population, while patients enrolled in plans serving the West (OR 1.31, 95% CI 1.08–1.57) and Northeast (OR 1.43, 95% CI 1.11–1.83) regions were more likely to use PPIs.
When we restricted the cohort to patients over the age of 18, the results were not changed (data not shown.)
Discussion
The role of proton pump inhibitors in the routine prophylaxis of patients taking corticosteroid therapy is unclear. Some studies have demonstrated that corticosteroids are associated with the development of gastrointestinal ulcers,20, 21, 23 while others have failed to find an association after adjusting for confounders.19, 22 We have not found any published studies that have examined whether PPI therapy has a protective effect among corticosteroid users in the absence of concomitant NSAID use. As a result, no clear consensus exists regarding the routine use of PPIs in this population. Similarly, the possibility of additive or synergistic toxicities related to co-therapy with PPI’s and corticosteroids has not been verified. However, in light of growing evidence that PPI therapy may be associated with significant adverse events including fractures and community acquired pneumonia, defining the factors that influence the decision to prescribe PPIs may help identify populations in which the risks of current practice outweigh the uncertain benefits of acid suppression.
We observed that PPI use in the first 30 days was associated with several co-morbid conditions as well as the overall burden of co-morbid illness. We also observed that PPI use was strongly associated with the highest daily doses (> 50 mg/day) of corticosteroids. Of the three specific comorbidities identified in this analysis as being associated with PPI use, two (chronic liver disease and chronic renal insufficiency) have also been identified as independent risk factors for community acquired pneumonia.26 Similarly, chronic liver disease and chronic renal disease are widely recognized risk factors for osteoporosis, and the number of co-morbid chronic illnesses has been identified as a risk factor for falls.25 While the precise dose-toxicity relationship of corticosteroids has not been well defined in higher dose ranges, it is generally accepted that increased daily doses also increase the risk of adverse events including fracture and infection.31, 32 The observed associations between PPI use and comorbidities and between PPI use and high corticosteroid dose therefore suggest that in a population of patients already at increased risk for fractures and infection because of their chronic corticosteroid exposure, PPIs are preferentially prescribed to patients with additional independent risk factors for fracture and community acquired pneumonia. This is of particular interest given that these highly morbid outcomes have also been associated with PPI exposure in other populations. Additionally, although the overall use of PPI’s in this population is low, it exceeds 20% among those in the highest corticosteroid dose categories and with the greatest number of co-morbid illnesses.
It is theoretically possible that the identified factors that increase the risk of pneumonia and fracture are also risk factors for gastrointestinal complications associated with corticosteroid use, and that PPI use among patients with these risk factors is therefore appropriate. However, this possibility is not clearly supported by existing literature. Luo and colleagues examined a cohort of corticosteroid users and only identified age, smoking history, and concomitant NSAID use as significantly associated with GI toxicity.33 Hernandez-Diaz and Rodriguez found that the risk of gastrointestinal ulcer formation was higher among patients receiving corticosteroid doses >30 mg/day with a reported OR of 3.3 compared to non-users; however, this finding was based on a very small subset of patients from a larger case-control study.20 Nonetheless, it is possible that the use of PPIs in a select population of high-dose corticosteroid users may reduce the risk of GI ulcer formation and bleeding. Prevention of GI toxicity may not be an optimal strategy, however, if it is also associated with an increased risk of other, potentially more common and equally morbid outcomes. Defining the risks and benefits of PPI therapy among corticosteroid users is therefore a critical next step in defining the best use of these drugs.
The combination of NSAIDs and corticosteroids has been consistently identified as a risk factor for adverse gastrointestinal events,20, 28, 33 and gastroprotection is recommended for patients receiving both NSAIDs and corticosteroids.18 Despite the significantly increased OR for PPI use among NSAID users, however, the use of PPIs in NSAID-corticosteroid recipients was very low (6.3%) even among those patients with the additional risk factor of advanced age (1.2%). It is possible that some NSAID users were considered low risk because of a short intended duration of use; however, the median duration of NSAID exposure among this group was 8 months. Additionally, existing evidence suggests that ulcer formation after aspirin or NSAID exposure occurs rapidly and is not dependent upon duration of therapy.34, 35 Furthermore, our findings are consistent with past studies that demonstrate that the use of gastroprotection among chronic NSAID users is often very low.36–38 We also found that patients prescribed anti-platelet medications in addition to corticosteroids were less likely to receive a PPI. These results suggest that the use of PPIs could be more aggressively targeted to patients at risk for gastrointestinal toxicity.
Our study findings are also consistent with past work that highlights the role of in-patient hospitalization in the initiation of PPI therapy. In our study, being hospitalized in the 30 days prior to initiating corticosteroid therapy was the strongest independent risk factor for concomitant PPI initiation. The inappropriate use of PPIs for stress ulcer prophylaxis in the in-patient setting as well as reflexive continuation of PPI therapy at discharge have been well-documented in the literature.39–41 These practices have clear economic implications and may also introduce an increased risk of complications among patients recently discharged from the hospital.
This study has several potential limitations. First, we defined PPI initiation as occurring in the first 30 days of corticosteroid initiation. It is therefore possible that PPIs were started in some patients to treat dyspepsia related to corticosteroid therapy rather than as prophylaxis for GI toxicity. This is unlikely to explain all or even a majority of PPI use given the observed associations with co-morbid conditions and region which are more likely to reflect physician’s perception of risk or regional variations in practice patterns than variations in symptomatic dyspepsia. Second, we used insurance claims to define co-morbid diseases, which may be subject to misclassification. Systematic misclassification seems unlikely, however, and any resulting bias is likely to be towards the null. Third, we did not differentiate between traditional non-selective NSAIDs and COX-2 selective NSAIDs. We did this for two reasons. Dividing the NSAID users into smaller subsets would have resulted in very small numbers for analysis, particularly among the COX-2 selective group. Also, it is not clear from existing literature that COX-2 selective NSAIDs are by themselves adequate gastroprotection in a population of patients also using chronic corticosteroids. Finally, because we used prescription claims to define drug exposure, our analysis does not include users of over-the-counter PPIs or NSAIDs. However, a secondary analysis of PPI use by index year did not show any reduction in PPI use after omeprazole was approved for over-the-counter use at the end of 2003, suggesting the majority of PPI use remained by prescription. Also, regarding NSAID users, our primary focus was to define the association between PPI use and risk factors likely to be known to the prescribing physician.
Conclusions
The initiation of PPI therapy among new corticosteroid users is uncommon overall, but significantly more common in important sub-populations. Patients with selected co-morbidities, a higher burden of total co-morbid illness, and the highest doses of corticosteroids are the most likely to be prescribed a PPI within 30 days of starting steroid therapy. Many of these factors are also risk factors for fracture and community acquired pneumonia; however, it is currently unknown whether co-therapy with a PPI and corticosteroids results in an increased risk of these adverse events. Additionally, the use of PPIs is, perhaps unsurprisingly, more strongly influenced by recent hospitalization than by known risk factors for gastrointestinal toxicity associated with corticosteroids, most notably the concomitant use of prescription NSAIDs. A clearer understanding of the potential benefits of PPI therapy in patients at risk for GI toxicity as well as the potential risks for significant toxicities associated with the combination of PPIs with corticosteroids is needed.
Supplementary Material
Take home messages.
Proton pump inhibitors are not widely prescribed to corticosteroid users with additional risk factors for gastrointestinal toxicity and in whom gastroprotection is therefore indicated
Proton pump inhibitors are preferentially prescribed to corticosteroid users with known risk factors for fractures and community acquired pneumonia, outcomes that have been associated with both corticosteroid and proton pump inhibitor therapy
Whether current prescribing practices increase the risk of additive or synergistic toxicity merits further investigation especially among patients at low risk for gastrointestinal complications of corticosteroid therapy.
Acknowledgments
Funding: This study was funded by T32GM075766 (JM), R01AG025152 (SH) and a fellowship training grant from HealthCore, Inc. (JM)
Footnotes
Conflict of interest:
JM has no conflicts to declare. During the time this work was being undertaken, PW and GD were full-time employees of HealthCore, Inc. SK has received research funding from Astra Zeneca to study a drug unrelated to the current study. SH has received funding from Astra-Zeneca and Takeda unrelated to this topic and has consulted for Astra-Zeneca, Teva, and Wyeth.
References Cited
- 1.Chiu HF, Huang YW, Chang CC, Yang CY. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf. Nov;19(11):1131–1136. doi: 10.1002/pds.2026. [DOI] [PubMed] [Google Scholar]
- 2.Corley DA, Kubo A, Zhao W, Quesenberry C. Proton Pump Inhibitors and Histamine-2 Receptor Antagonists Are Associated With Hip Fractures Among At-Risk Patients. Gastroenterology. 139(1):93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries F, Cooper A, Cockle S, van Staa TP, Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporosis International. 2009;20(12):1989–1998. doi: 10.1007/s00198-009-0891-4. [DOI] [PubMed] [Google Scholar]
- 4.Gray SL, LaCroix AZ, Larson J, et al. Proton Pump Inhibitor Use, Hip Fracture, and Change in Bone Mineral Density in Postmenopausal Women: Results From the Women's Health Initiative. Arch Intern Med. 2010 May 10;170(9):765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok CS, Yeong JK-Y, Loke YK. Meta-analysis: Risk of fractures with acid-suppressing medication. Bone. doi: 10.1016/j.bone.2010.12.015. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y-X, Lewis JD, Epstein S, Metz DC. Long-term Proton Pump Inhibitor Therapy and Risk of Hip Fracture. JAMA: The Journal of the American Medical Association. 2006 Dec 27;296(24):2947–2953. doi: 10.1001/jama.296.24.2947. 2006. [DOI] [PubMed] [Google Scholar]
- 7.Yu E, Blackwell T, Ensrud K, et al. Acid-Suppressive Medications and Risk of Bone Loss and Fracture in Older Adults. Calcified Tissue International. 2008;83(4):251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eom C-S, Jeon CY, Lim J-W, Cho E-G, Park SM, Lee K-S. Use of acid-suppressive drugs and risk of pneumonia: systematic review and meta-analysis. CMAJ. 2010 Dec 20; doi: 10.1503/cmaj.092129. cmaj.092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Alimentary Pharmacology & Therapeutics. 31(11):1165–1177. doi: 10.1111/j.1365-2036.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 10.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of communityacquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004 Oct 27;292(16):1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 11.Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJP, Gibson JE. Risk of communityacquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case–control study. Pharmacoepidemiology and Drug Safety. 2009;18(4):269–275. doi: 10.1002/pds.1715. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez LA, Ruigomez A, Wallander MA, Johansson S. Acid-suppressive drugs and communityacquired pneumonia. Epidemiology. 2009 Nov;20(6):800–806. doi: 10.1097/EDE.0b013e3181b5f27d. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008 Sep 16;149(6):391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 14.Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Jackson LA. Use of proton pump inhibitors and H2 blockers and risk of pneumonia in older adults: a population-based case-control study. Pharmacoepidemiology and Drug Safety. 19(8):792–802. doi: 10.1002/pds.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008 Aug;28(8):951–959. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- 16.Pouwels S, Lalmohamed A, Souverein P, et al. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporosis International. :1–8. doi: 10.1007/s00198-010-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartnell NR, Flanagan PS, MacKinnon NJ, Bakowsky VS. Use of gastrointestinal preventive therapy among elderly persons receiving antiarthritic agents in Nova Scotia, Canada. The American Journal of Geriatric Pharmacotherapy. 2004;2(3):171–180. doi: 10.1016/j.amjopharm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Chan FK, Abraham NS, Scheiman JM, Laine L. Management of patients on nonsteroidal antiinflammatory drugs: a clinical practice recommendation from the First International Working Party on Gastrointestinal and Cardiovascular Effects of Nonsteroidal Anti-inflammatory Drugs and Anti-platelet Agents. Am J Gastroenterol. 2008 Nov;103(11):2908–2918. doi: 10.1111/j.1572-0241.2008.02200.x. [DOI] [PubMed] [Google Scholar]
- 19.Conn HO, Poynard T. Corticosteroids and peptic ulcer: meta-analysis of adverse events during steroid therapy. J Intern Med. 1994 Dec;236(6):619–632. doi: 10.1111/j.1365-2796.1994.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Diaz S, Rodriguez LA. Steroids and risk of upper gastrointestinal complications. Am J Epidemiol. 2001 Jun 1;153(11):1089–1093. doi: 10.1093/aje/153.11.1089. [DOI] [PubMed] [Google Scholar]
- 21.Messer J, Reitman D, Sacks HS, Smith H, Jr, Chalmers TC. Association of adrenocorticosteroid therapy and peptic-ulcer disease. N Engl J Med. 1983 Jul 7;309(1):21–24. doi: 10.1056/NEJM198307073090105. [DOI] [PubMed] [Google Scholar]
- 22.Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991 May 1;114(9):735–740. doi: 10.7326/0003-4819-114-9-735. [DOI] [PubMed] [Google Scholar]
- 23.Weil J, Langman MJ, Wainwright P, et al. Peptic ulcer bleeding: accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut. 2000 Jan;46(1):27–31. doi: 10.1136/gut.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fardet L, Kassar A, Cabane J, Flahault A. Corticosteroid-Induced Adverse Events in Adults. Drug Safety. 2007;30(10):861–881. doi: 10.2165/00002018-200730100-00005. [DOI] [PubMed] [Google Scholar]
- 25.Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ. 2003 Sep 27;327(7417):712–717. doi: 10.1136/bmj.327.7417.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinogradova Y, Hippisley-Cox J, Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. 2009 Oct;59(567):e329–338. doi: 10.3399/bjgp09X472629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunton LC, Lazo JS, Parker KR, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. [Google Scholar]
- 28.Garcia Rodriguez LA, Hernandez-Diaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res. 2001;3(2):98–101. doi: 10.1186/ar146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. Jun;48(6 Suppl):S114–120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001 Nov 1;154(9):854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 31.Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989 Nov-Dec;11(6):954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 32.van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000 Dec;39(12):1383–1389. doi: 10.1093/rheumatology/39.12.1383. [DOI] [PubMed] [Google Scholar]
- 33.Luo JC, Chang FY, Lin HY, et al. The potential risk factors leading to peptic ulcer formation in autoimmune disease patients receiving corticosteroid treatment. Aliment Pharmacol Ther. 2002 Jul;16(7):1241–1248. doi: 10.1046/j.1365-2036.2002.01279.x. [DOI] [PubMed] [Google Scholar]
- 34.Hollenz M, Stolte M, Leodolter A, Labenz J. NSAID-associated dyspepsia and ulcers: a prospective cohort study in primary care. Dig Dis. 2006;24(1–2):189–194. doi: 10.1159/000090321. [DOI] [PubMed] [Google Scholar]
- 35.Nema H, Kato M, Katsurada T, et al. Endoscopic survey of low-dose-aspirin-induced gastroduodenal mucosal injuries in patients with ischemic heart disease. Journal of Gastroenterology and Hepatology. 2008;23:S234–S236. doi: 10.1111/j.1440-1746.2008.05411.x. [DOI] [PubMed] [Google Scholar]
- 36.Sturkenboom MCJM, Burke TA, Dieleman JP, Tangelder MJD, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology. 2003 Nov 1;42(suppl 3):iii23–iii31. doi: 10.1093/rheumatology/keg495. 2003. [DOI] [PubMed] [Google Scholar]
- 37.Suh DC, Hunsche E, Shin HC, Mavros P. Co-prescribing of proton pump inhibitors among chronic users of NSAIDs in the UK. Rheumatology (Oxford) 2008 Apr;47(4):458–463. doi: 10.1093/rheumatology/kem375. [DOI] [PubMed] [Google Scholar]
- 38.Van der Linden MW, Gaugris S, Kuipers EJ, Van den Bemt BJ, van Herk-Sukel MP, Herings RM. Gastroprotection among new chronic users of non-steroidal anti-inflammatory drugs: a study of utilization and adherence in The Netherlands. Curr Med Res Opin. 2009 Jan;25(1):195–204. doi: 10.1185/03007990802632915. [DOI] [PubMed] [Google Scholar]
- 39.Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol. 2006 Oct;101(10):2200–2205. doi: 10.1111/j.1572-0241.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 40.Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000 Nov;95(11):3118–3122. doi: 10.1111/j.1572-0241.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- 41.Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005 May 15;21(10):1203–1209. doi: 10.1111/j.1365-2036.2005.02454.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.