ABSTRACT
The key regulators of endothelial differentiation that is induced by shear stress are mostly unclear. Human atonal homolog 6 (Hath6 or ATOH8) is an endothelial-selective and shear-stress-responsive transcription factor. In this study, we sought to elucidate the role of Hath6 in the endothelial specification of embryonic stem cells. In a stepwise human embryonic stem cell to endothelial cell (hESC-EC) induction system, Hath6 mRNA was upregulated synchronously with endothelial determination. Subsequently, gain-of-function and loss-of-function studies of Hath6 were performed using the hESC-EC induction model and endothelial cell lines. The overexpression of Hath6, which mimics shear stress treatment, resulted in an increased CD45−CD31+KDR+ population, a higher tubular-structure-formation capacity and increased endothelial-specific gene expression. By contrast, the knockdown of Hath6 mRNA markedly decreased endothelial differentiation. Hath6 also facilitated the maturation of endothelial cells in terms of endothelial gene expression, tubular-structure formation and cell migration. We further demonstrated that the gene encoding eNOS is a direct target of Hath6 through a reporter system assay and western blot analysis, and that the inhibition of eNOS diminishes hESC-EC differentiation. These results suggest that eNOS plays a key role in linking Hath6 to the endothelial phenotype. Further in situ hybridization studies in zebrafish and mouse embryos indicated that homologs of Hath6 are involved in vasculogenesis and angiogenesis. This study provides the first confirmation of the positive impact of Hath6 on human embryonic endothelial differentiation and function. Moreover, we present a potential signaling pathway through which shear stress stimulates endothelial differentiation.
KEY WORDS: Hath6, Endothelial differentiation, Human embryonic stem cells
INTRODUCTION
Endothelial progenitor cells (EPCs) contribute to vascular repair and postnatal neovascularization. The understanding of vascular developmental events will shed light on the induction of embryonic and adult stem cells toward the endothelial lineage and will therefore translate these insights on vascular development to clinical practice. The key regulators of endothelial development include cytokines, extracellular matrices, cell–cell interactions, hypoxia and biomechanical forces, such as fluid shear stress (Hsiai and Wu, 2008; Jain, 2003). However, the mechanisms underlying endothelial specialization and endothelium-specific regulation are currently unclear. To this end, blood-vessel development during embryogenesis in an animal model and the in vitro differentiation of embryonic stem cells to endothelial cells (ESC-EC) were investigated to gain insights into the molecular control of endothelial differentiation.
The cardiovascular system is the first system to develop during embryogenesis. Fluid shear stress generated by blood flow has been shown in studies of the development of both zebrafish and mouse embryos to play an important role in the determination and function of the vascular system (Hove et al., 2003; Nonaka et al., 2002). Additionally, an increasing body of evidence suggests that shear stress can promote ESC commitment to the endothelial cell lineage. Studies conducted by Yamamoto et al. demonstrated that shear stress selectively promotes the differentiation of Flk-1-positive ESCs into the endothelial lineage (Yamamoto et al., 2005). Furthermore, Zeng et al. showed that shear stress plays a pivotal role in the differentiation of ESCs toward the endothelial lineage and demonstrated that the Flk-1–PI3K–Akt–HDAC3–p53–p21 pathway mediates this process (Zeng et al., 2006). Hence, understanding the effects of fluid shear stress on ESCs will aid attempts to promote the in vitro commitment of ESCs to form EPCs and will improve the potential therapeutic applications of these cells.
Hath6 (ATOH8), an endothelial-selective basic helix-loop-helix (bHLH) transcription factor, was first identified as a flow-responsive gene through a transcriptional-profile analysis of human umbilical vein endothelial cells (HUVECs) exposed to sustained laminar shear stress (LSS) (Wasserman et al., 2002). Hath6 is a member of the atonal-related protein family, and its murine analog, Math6 (or ATOH8), has been reported to be an important regulator of the development of neurons, as well as the pancreas and kidney, during early embryonic development (Inoue et al., 2001; Lynn et al., 2008; Ross et al., 2006; Yao et al., 2010). Based on these observations, we hypothesized that Hath6 acts as a shear-stress-responsive transcription factor to mediate the transcriptional events necessary for endothelial differentiation and phenotypic modulation. In this study, the Hath6 gene was modified in ESCs and endothelial cells to test our hypothesis.
RESULTS
The expression of Hath6 mRNA is primarily stimulated by shear stress
Cultured HUVECs were exposed to a variety of biochemical and biomechanical stimuli to determine the dominant regulatory factors of Hath6 mRNA expression in vitro. Under all of these conditions, a marked increase in the level of Hath6 transcript was observed after 4 h or 24 h of exposure to LSS (greater than tenfold at 4 h, Fig. 1A), whereas 24 h of exposure to tumor necrosis factor alpha (TNF-α) resulted in a mild (less than twofold) Hath6 induction. This upregulation was augmented by co-incubation with interferon gamma (IFN-γ). Four hours of treatment with vascular endothelial growth factor (VEGF) resulted in a twofold upregulation of Hath6 mRNA. Accordingly, of the biomechanical and biochemical stimuli tested, the foremost stimulator of Hath6 is shear stress, followed by the combination of IFN-γ with TNF-α and then by VEGF.
Fig. 1.

Expression of Hath6 in HUVECs and hESCs exposed to biomechanical and biochemical stimuli. (A) HUVECs were exposed to LSS (10 dynes/cm2), TGF-β1 (5 ng/ml), IFN-γ (150 U/ml), IL-1β (10 U/ml), IFN-γ/IL-1β (150 U/ml, 10 U/ml), TNF-α (200 U/ml), IFN-γ/TNF-α (150 U/ml, 200 U/ml), VEGF (50 ng/ml), basic-FGF (50 ng/ml), HGF (40 ng/ml) or H2O2 (10−5 M) for 4 or 24 h. The fold inductions of Hath6 were normalized to the untreated control. (B) Hath6 was upregulated in hESCs after 12 or 24 h exposure to spontaneous differentiation medium. The RT-PCR results show that the expression of Hath6 was more responsive to LSS than to treatment with the differentiation medium. *P = 0.028476 for static-12 h versus LSS-12 h, **P = 0.007755 for static-24 h versus LSS-24 h (n = 6).
ESCs have been reported to be induced to differentiate along an endothelial lineage when exposed to shear stress (Yamamoto et al., 2005; Zeng et al., 2006). To determine whether Hath6 is involved in this process, human ESCs (hESCs) were exposed to sustained LSS in differentiation medium. Compared with static incubation, LSS treatment and soluble-factor stimuli significantly induced Hath6 expression (Fig. 1B). Based on these findings, we hypothesized that a direct Hath6 upregulation through gene modification might mimic the endothelial induction that is observed following exposure to shear-stress or a combination of cytokines.
Endogenous expression of Hath6 is increased during ESC-EC differentiation and is highly correlated with the expression of endothelial markers
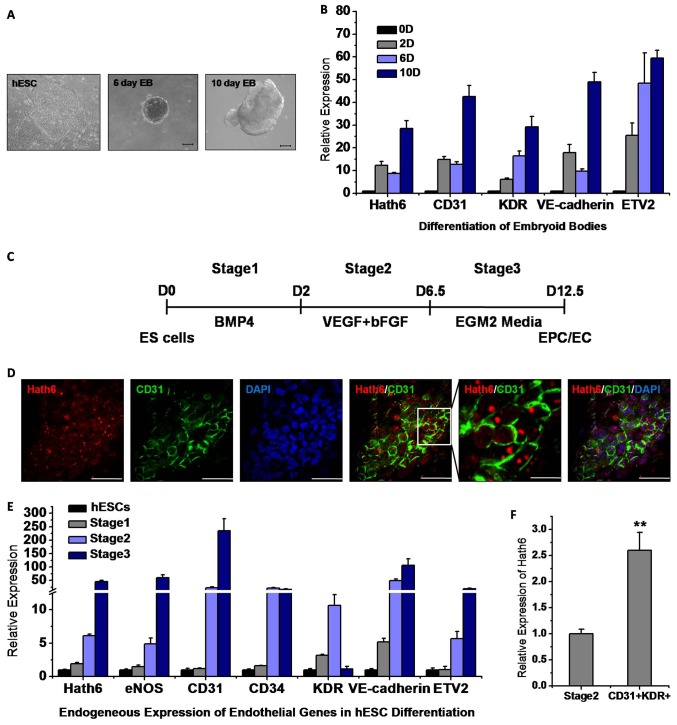
To determine whether Hath6 is involved in endothelial differentiation, the endogenous expression of Hath6 was monitored during the formation of embryoid bodies (EBs) (Fig. 2A) and during a specific three-stage hESC endothelial-differentiation protocol (Fig. 2C; supplementary material Fig. S1). During EB formation, as shown in Fig. 2B, Hath6, CD31 (PECAM-1), KDR (VEGF receptor-2 or Flk-1), VE-cadherin (vascular endothelial cadherin, CD144 or cadherin-5) and ETS translocation variant 2 (ETV2, a homolog of zebrafish Etsrp) were gradually and synchronously upregulated. In a separate experiment, hESCs were directly differentiated into endothelial-lineage cells using a modified three-stage protocol involving treatment with cytokines (Fig. 2C; supplementary material Fig. S1) (Yu et al., 2010; Zhang et al., 2008). The first stage [induction with bone morphogenetic protein 4 (BMP4)] resulted in mesendoderm initiation; this was followed by hemato-vascular-precursor specification in stage two (10–30% of the cells were KDR+CD133+) and endothelial enrichment in stage three (the percentage of CD31+ cells increased to 20–40%; supplementary material Fig. S1). Compared with undifferentiated hESCs, a sixfold upregulation of Hath6 was detected in stage two, and this upregulation was markedly increased by 45-fold in late-stage EPCs (stage three; Fig. 2E). The upregulation of other endothelial-related genes, such as eNOS (endothelial nitric oxide synthase), CD31, CD34, KDR and VE-cadherin, was also detected in both stage two and stage three cells at the onset of Hath6 upregulation. The high correlation of Hath6 expression with endothelial-cell-restricted ETV2 (Sumanas et al., 2008; Sumanas and Lin, 2006) (supplementary material Fig. S2) and the colocalization of Hath6 with the endothelial surface marker CD31 (Fig. 2D) together suggest that Hath6 is enriched in endothelial cells during development.
Fig. 2.
Endogenous expression of Hath6 during hESC differentiation. (A) Morphology of differentiated hESCs on days 0, 6 and 10 of embryoid body (EB) formation. Scale bar: 200 µm. (B) The real-time PCR results show the upregulation of Hath6, as well as endothelial-related genes, including CD31, KDR, VE-cadherin and ETV2, during the spontaneous differentiation of hESCs into EBs. Each data point represents the average of six experiments. Gene expression was first normalized to the expression of the housekeeping gene GAPDH. The fold induction of each tested gene was then normalized to the expression in undifferentiated hESCs. The data represent the means±s.d., n = 6. (C) Schematic of a three-step hESC-EC induction system. (D) Immunofluorescence staining observed by confocal microscopy indicates the co-expression of Hath6 (red) and the endothelial cell marker CD31 (green) in cells at stage two of hESC differentiation. Scale bars: 50 µm. A magnified view of the co-expression of Hath6 and CD31 is shown in the second panel from the right. Scale bar: 20 µm. The images are representative of three independent experiments. (E) Expression of Hath6 during endothelial differentiation. The RT-PCR results illustrate the increasing expression of Hath6, eNOS, CD31, VE-cadherin and ETV2 during phase differentiation, especially at stage three (n = 6). The expression of each tested gene was normalized to the expression in undifferentiated hESCs. The highest expression levels of CD34 and KDR in our differentiation system were observed at stage two. The data represent the means±s.d. Compared with undifferentiated hESCs, none of the genes shows a significant difference in expression in the stage one cells. Compared with the results for stage one, all of the genes in the stage two cells show a significant increase in expression (P<0.05). Compared with the results for stage two, all of the genes except KDR show a significant increase in expression at stage three (P<0.05). (F) Hath6 is enriched in CD31+KDR+ endothelial progenitor cells isolated from stage two hESC derivations (2.6-fold, **P<0.01).
To further define the direct correlation of Hath6 expression with the emergence of the endothelial lineage, FACS-sorted CD45−CD31+KDR+ endothelial cells from induction stage two were examined using real-time quantitative PCR (RT-PCR). Fig. 2F shows the enriched expression of Hath6 in CD45−CD31+KDR+ cells compared with the total population of stage two cells (2.6-fold, **P<0.01). A search of the GEO Profiles database (Li et al., 2009) also revealed that, compared with undifferentiated hESCs, Hath6 was upregulated by 39-fold in hESC-derived endothelial cells that were isolated based on the expression of the endothelial marker CD31+VE-cadherin+ (GEO accession GSE20013). Collectively, these data suggest that Hath6 is involved in the in vitro differentiation of endothelial cells from hESCs.
Hath6 enhances endothelial lineage commitment
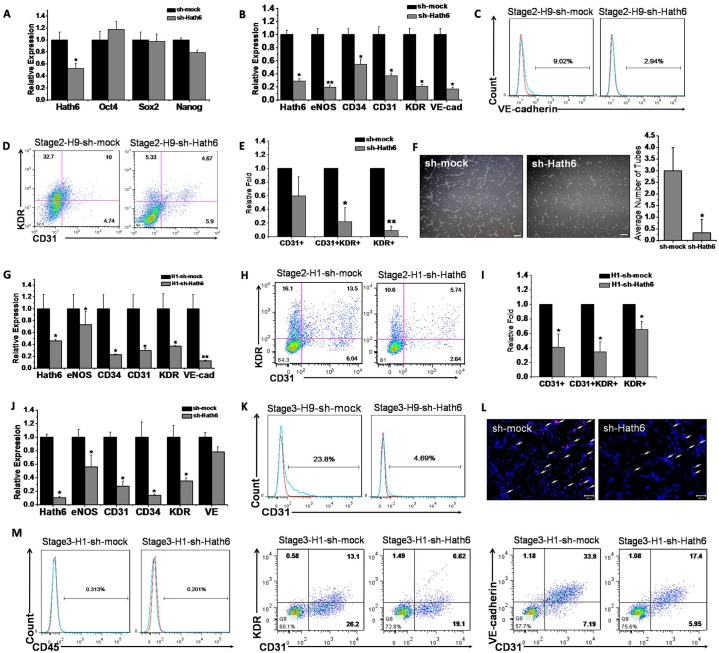
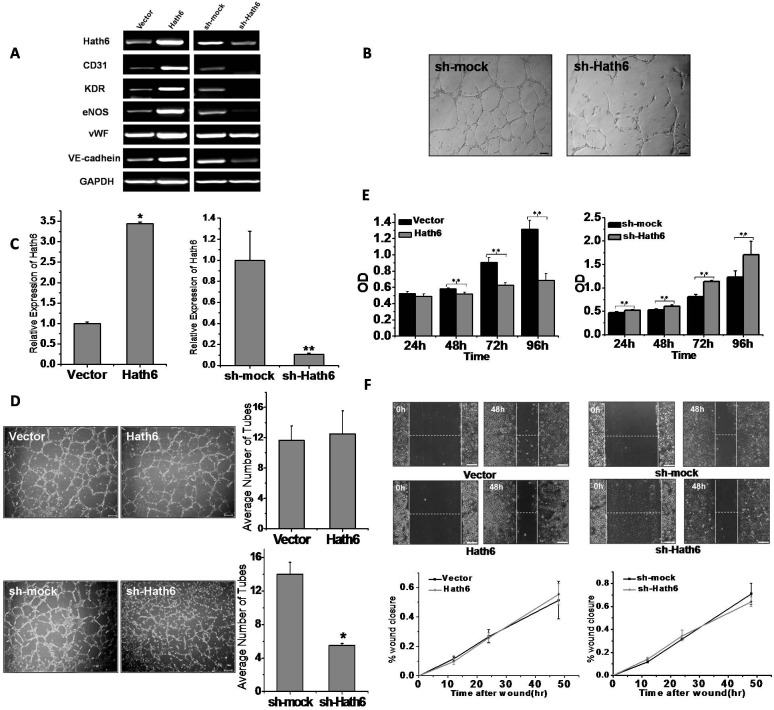
To gain insights into the role of Hath6 in endothelial differentiation, endothelial induction was performed using a stable Hath6-overexpressing H9 hESC line, based on a modified stepwise differentiation protocol (Zhang et al., 2008). The expression of exogenous Hath6 was confirmed by detection of the myc-tag and the overexpression of Hath6 in both undifferentiated hESCs and stage-two cells was confirmed using an anti-Hath6 antibody (Fig. 3A,D). The overexpression of Hath6 did not have a significant effect on hESC pluripotency because the cells retained their regular characteristics even after 20 passages (data not shown) and the expression of most pluripotency markers remained stable over the experimental period (Fig. 3B). However, after 6.5 days of induction (Fig. 2C, by the end of stage two), at the onset of endothelial differentiation (when the expression of endothelial-related genes was detected), RT-PCR analysis indicated that the cells overexpressing Hath6 exhibited a markedly increased expression of endothelial genes compared with the vector-only control cells (Fig. 3C). The FACS data showed that Hath6 overexpression resulted in markedly higher levels of CD31+KDR+ EPCs compared with the vector-only transfection control (1.65-fold, P = 0.034; Fig. 3E,F). An additional FACS assay demonstrated that the expression of VE-cadherin, an endothelial marker, was also upregulated and that this upregulation was correlated with the overexpression of Hath6 at stage two of the differentiation protocol (Fig. 3H).
Fig. 3.
Overexpression of Hath6 in hESCs stimulates endothelial commitment. (A) Western blot detection of the myc-tag indicated the expression of exogenous Hath6, and probing with the anti-Hath6 antibody confirmed the overexpression of Hath6. (B) The overexpression of Hath6 in hESCs does not affect the expression of pluripotency markers, including Oct4, Sox2 and Nanog (n = 9). The fold induction of each gene was normalized to expression in the vector-transfected controls. *P<0.05. (C–H) Results of the stepwise induction of H9 hESCs at the end of stage two. (C) RT-PCR showing the expression levels of Hath6 and endothelial-related genes, including eNOS, CD34, CD31, KDR and VE-cadherin (VE). These genes were all significantly upregulated compared with the vector-transfected control (n = 6). (D) Western blot indicates the overexpression of Hath6 at stage two. (E) FACS analysis indicates an increased percentage of KDR+ cells, CD31+KDR+ cells and VE-cadherin+ cells (H) as a result of the overexpression of Hath6. The red and blue lines indicate the IgG isotype control and specific antibody staining, respectively. (F) Statistical analysis shows a significant difference in Hath6-overexpressing cells compared with the vector-transfected control cells (*P = 0.049, 0.034 and 0.027, respectively, n = 3). (G) The angiogenesis assay and the counting of closed tube-like structures per visual field (n = 3) revealed that Hath6-overexpressing hESCs at stage two form better tube-like structures on Matrigel compared with the vector-transfected control group. *P<0.05. Scale bar: 200 µm. (I–K) Results of H9 hESC stage three induction. At the end of differentiation stage three, (I) RT-PCR (n = 6) and (J) FACS analyses showed the upregulation of endothelial genes in the Hath6-overexpressing H9 cells compared with the control cells. The results of the RT-PCR analysis show a significant difference compared with the control. The red and blue lines indicate the IgG isotype control and specific antibody staining, respectively. (K) The uptake of DiI–Ac-LDL by Hath6-overexpressing hESCs at stage three was higher than that observed in the control. The arrows indicate the cells that have taken up DiI−Ac-LDL. Scale bars: 100 µm.
Tubule-like structure formation on a Matrigel matrix is the most frequently used criterion for endothelial-cell quantification. The overexpression of Hath6 promoted the formation of tubular structures beginning on day 6.5 of ESC-EC induction (3.17-fold, Fig. 3G), suggesting that Hath6 contributes to endothelial lineage commitment.
At differentiation phase three, RT-PCR analysis (Fig. 3I), FACS experiments (Fig. 3J) and a DiI–Ac-LDL-incorporation assay (which measures the uptake of DiI-labeled acetylated low-density lipoprotein) (Fig. 3K) provided additional evidence that Hath6 contributes to the endothelial differentiation process. Similar results were found in a mouse ESC-EC differentiation model (supplementary material Fig. S4), as described previously (Li et al., 2007).
Endothelial differentiation is seriously impaired by Hath6 knockdown
Although Hath6 overexpression exerted a positive effect on ESC-EC differentiation, it is not clear whether this gene is indispensable for endothelial cell differentiation. Two Hath6-knockdown hESC lines (H9 and H1) were used to further determine the function of this gene. Sh-mock served as a transfection control, which does not alter stem cell properties markedly (supplementary material Fig. S3). The expression of the ‘stemness’ genes (Oct4, Sox2 and Nanog) and the ability to undergo teratoma formation were not disturbed before the induction of endothelial cell differentiation (Fig. 4A; supplementary material Fig. S5). As expected, the decreased expression of Hath6 impaired endothelial differentiation. RT-PCR detected decreased expression of eNOS, CD34, CD31, KDR and VE-cadherin at induction stage two in both the H9 and H1 lines (Fig. 4B,G). FACS analysis revealed that the knockdown of Hath6 mRNA resulted in a sharply decreased VE-cadherin+KDR+ population (10.92-fold, P = 0.0017) and a decreased CD31+KDR+ population (4.54-fold, P = 0.022; Fig. 4C–E,H,I) compared with the short hairpin (sh)-mock control. The tubular-formation assay and quantification of the number of closed cord structures per visual field showed blocked tubule-like structures when Hath6 mRNA was downregulated (9.09-fold, Fig. 4F).
Fig. 4.
The downregulation of Hath6 in both H9 and H1 hESCs inhibits the development of endothelial characteristics during differentiation. (A) The downregulation of Hath6 in H9 hESCs does not affect the expression of pluripotency markers, including Oct4, Sox2 and Nanog (n = 3). (B–F) Results from the stepwise induction of H9 hESCs from the end of stage two. (B) The expression of Hath6 and endothelial-related genes, including eNOS, CD34, CD31, KDR and VE-cadherin, in shHath6-H9-hESC-EPCs was significantly downregulated compared with the mock control (n = 6). (C–E) FACS analyses of CD31 and KDR expression at H9-hESC differentiation stage two. The numbers indicate a decreased percentage of VE-cadherin+ cells (C), KDR+ cells and CD31+KDR+ cells (D) due to the downregulation of Hath6. (E) Statistical analysis shows a significant difference in these cells compared with the control (n = 6). (F) The angiogenesis assay and the counting of closed tube-like structures per visual field (n = 3) revealed that siHath6-H9-EPCs cannot form tube-like structures on Matrigel, in contrast to the control group. Scale bar: 200 µm. (G–I) Results of H1 hESC stage two induction. (G) RT-PCR (n = 6) and (H) FACS analyses indicate that the downregulation of endothelial-specific genes correlated with the knockdown of the Hath6 gene. (I) The statistical analysis showed a significant difference in these cells compared with the mock control (n = 3). (J–L) Results of H9 hESC stage three induction. (J) RT-PCR (n = 6) and (K) FACS analyses show the downregulation of endothelial genes in sh-Hath6-H9-hESCs compared with the mock control. The RT-PCR analysis shows that the endothelial-specific genes, with the exception of VE-cadherin, are significantly decreased compared to the control. (L) The uptake of DiI–Ac-LDL by sh-Hath6-hESCs at stage three was lower than that observed in sh-mock-hESCs. The arrows indicate cells that have taken up DiI–Ac-LDL. Scale bar: 100 µm. (M) Results of H1 hESC stage three induction. After culture in EGM2 medium for 6 days, FACS analysis indicates that most of the hESC derivations are CD45-negative. The downregulation of Hath6 decreases the percentage of CD45−CD31+KDR+ cells, CD45−CD31+VE-cadherin+ cells and total CD31+ cells, as determined by FACS analysis. *P<0.05, **P<0.01. In panels C,K,M the red and blue lines indicate the IgG isotype control and specific antibody staining, respectively.
At differentiation stage three, in endothelial-specific medium (EGM2), RT-PCR results were consistent with those seen for stage two samples (Fig. 4J), showing that endothelial gene expression is suppressed following Hath6 knockdown. The metabolism of Ac-LDL is one of the special characteristics of endothelial cells. The DiI–Ac-LDL-incorporation assay indicated that fewer cells are able to perform uptake of LDL after the knockdown of Hath6 (Fig. 4L).
To eliminate interference from hematopoietic cells, the anti-CD45 antibody was used to exclude hematopoietic lineages at this stage of development. Both H9 and H1 derivations were cultured in EGM2 medium for 6 days. Subsequently, a FACS analysis revealed decreased CD45−CD31+KDR+ (H1-sh-Hath6), CD45−CD31+VE-cadherin+ (H1-sh-Hath6) and total CD31+ (H9-sh-Hath6) endothelial populations (23.8% for sh-mock versus 4.69% for sh-Hath6) in the sh-Hath6 lines (Fig. 4K,M). Thus, Hath6 seems to play an important role in the endothelial differentiation of hESCs.
Hath6 modulates endothelial proliferation, migration and tubule-like structure formation
To further investigate the role of Hath6 in endothelial phenotypic modulation, we performed Hath6 gain- and loss-of-function studies in endothelial cells. We established ECV-304 (Fig. 5C) and HUVEC cell lines that either stably overexpressed Hath6 mRNA or stably expressed sh-Hath6, leading to a reduction in Hath6 mRNA levels. Similar to the findings in hESCs (Fig. 3I; Fig. 4J), the expression of CD31, KDR, VE-cadherin, von Willebrand factor (vWF) and eNOS was positively correlated with the expression of Hath6 mRNA (Fig. 5A). In the tubule-like structure formation assay, both HUVECs (Fig. 5B) and ECV-304 cells with reduced Hath6 expression exhibited a significantly decreased ability to form tube-like structures but higher Hath6 expression did not reverse this effect (Fig. 5D). Hath6 also played a role in the regulation of endothelial proliferation: an increased expression of Hath6 attenuated ECV-304 cell proliferation and the knockdown of Hath6 increased the growth rate (Fig. 5E).
Fig. 5.
The overexpression or knockdown of Hath6 in endothelial cells affects the endothelial phenotypes. (A) The overexpression or knockdown of Hath6 in HUVECs results in the upregulation or downregulation, respectively, of some endothelial-related genes, including CD31, KDR, eNOS, vWF and VE-cadherin (GAPDH was used as the loading control). (B) The angiogenesis assay indicates that siHath6-HUVECs cannot form tube-like structures on Matrigel compared to the control group. Scale bars: 100 µm. (C) RT-PCR analysis showed the overexpression and knockdown of Hath6 in genetically modified ECV-304 cells (*P = 0.018, **P = 0.0076, n = 9). The expression of each tested gene was normalized to its expression in the vector-transfected control cells and mock-transfected control cells. (D) The angiogenesis assay revealed the influence of Hath6 overexpression and Hath6 knockdown on the formation of tube-like structures on Matrigel. Scale bars: 200 µm. The siHath6-endothelial cells exhibit serious impairment of their tube-formation ability (*P = 0.014, n = 9), whereas Hath6 overexpression has no significant effect. An image from a representative experiment is shown on the left. The quantification of the average number of tubes per field of view at 48 h is shown on the right. (E) The proliferation of Hath6-endothelial cells and shHath6-endothelial cells was detected by CCK8 (at 96 h, **P = 0.0049 in the left graph and **P = 6.3E−07 in the right graph, n = 8). Hath6 overexpression impairs endothelial cell proliferation, and its knockdown promotes proliferation. (F) The effect of Hath6 overexpression or knockdown on endothelial cell migration. The dashed lines indicate the width of a ‘wound’. The upper panels show one representative experiment. The percent wound closure is shown as the mean±s.e.m. in the lower panel (n = 4, scale bars: 200 µm). No significant difference was detected between the experimental groups 48 h after wound formation.
The wound-healing assay is commonly used to evaluate endothelial cell migration (Coomber and Gotlieb, 1990). At 48 h after the wounds were made, cells that were overexpressing Hath6 mRNA showed an increase in the rate of healing, with more narrow scrapes compared with the control group and the Hath6-knockdown group (Fig. 5F, P = 0.75). The cell proliferation rate was also positively correlated with the speed of wound healing and, given the extremely low proliferation of ECV-304-Hath6 cells, Hath6 might promote more cell migration than these results suggest. Collectively, our results demonstrate that endothelial cells with higher Hath6 mRNA levels behave similar to mature cells, which are characterized by relatively low cell proliferation, fast migration, high capability of forming tube-like structures and greater endothelial-specific gene expression.
Hath6 is a direct transcriptional regulator of eNOS
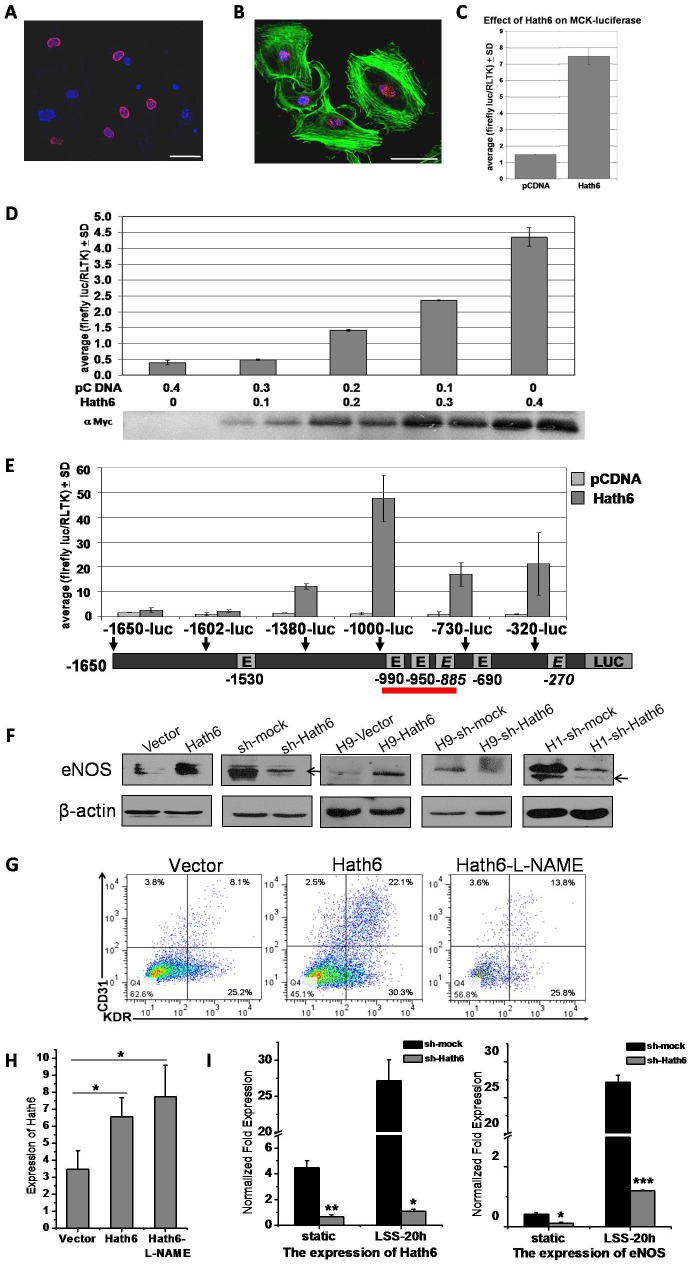
A previous study reported that Hath6 is a bHLH transcription factor that belongs to the atonal-related protein family (Wasserman et al., 2002). Therefore, we verified this function and examined which targets mediate the Hath6-dependent regulation of endothelial differentiation and maturation. To investigate the role of this gene as a transcription factor, the nuclear localization of Hath6 was first confirmed using an immunofluorescence assay in Hath6–myc-transfected COS7 cells (Fig. 6A). The localization of endogenous Hath6 was then assessed in HUVECs using antisera against Hath6 (Fig. 6B).
Fig. 6.
The expression of eNOS is directly regulated by the new endothelial cell transcription regulator Hath6. (A) Nuclear expression of Hath6 in transiently transfected COS7 cells. A prominent nuclear (DAPI, blue) expression of Hath6–myc (red) was observed, with little cytoplasmic staining. Scale bar: 100 µm. (B) Endogenous Hath6 localizes to the HUVEC nucleus as ‘nuclear speckles’ (red). HUVEC nuclei and the cytoskeleton were counterstained with DAPI (blue) and phalloidin (green), respectively. Scale bar: 10 µm. (C) Hath6 is capable of activating the transcription of the E-box motif. MCK promoter driving firefly luciferase was transiently transfected into 293T cells along with vector expressing Hath6 or an empty vector. A Renilla luciferase construct served as a control for transfection efficiency. (D) eNOS–luciferase activity increased with higher amounts of transfected Hath6 cDNA in BAECs. The expression of transfected Hath6 was detected by western blotting. (E) Deletion analysis of the eNOS promoter. Luciferase reporter constructs with different deletions within the 1650-bp promoter are shown. For each construct, the length of the fragment upstream of the transcription start site is shown above the schematic and the potential E-box sites are shown. All of the transfection experiments were repeated at least three times in BAECs and the luciferase activity was normalized to the internal Renilla control. According to the luciferase assay, the construct from −1000 to −730 (indicated by the red line) exhibited the maximal luciferase activity. (F) Hath6 modulates the expression of eNOS in ECV-304 cells (the first two lines) and endothelial cells derived from hESCs, as detected by western blot analysis. The expression of eNOS shows upregulation with the overexpression of Hath6, and the decreased expression of eNOS corresponds to a knockdown of Hath6. Arrows indicate bands corresponding to the eNOS protein. (G,H) FACS analysis of CD31 and KDR expression at the end of hESC differentiation stage two, after treatment with 50 µM L-NAME during stages one and two. The numbers indicate a decreased percentage of CD31+, KDR+ and CD31+ KDR+ cells due to the inhibition of eNOS activity. The RT-PCR results show that L-NAME does not affect the expression of Hath6 (n = 3). (I) The RT-PCR results indicate that the constant knockdown of Hath6 in hESCs results in the marked suppression of eNOS upregulation after LSS treatment (n = 3). *P<0.05, **P<0.01, ***P<0.001.
The function of Hath6 as a bHLH transcription factor was confirmed using a reporter system containing the muscle creatine kinase (MCK) promoter fused to the firefly luciferase gene. It has been shown that the MCK promoter contains a consensus sequence called an E-box that can be recognized and bound specifically by bHLH transcription factors (Nguyen et al., 2003). Fig. 6C indicates that the relative luciferase activity of the E-box reporter was significantly increased by co-transfection with the Hath6 overexpression vector.
The targets that were regulated by Hath6 were then investigated. The identification of Hath6 targets was limited to genes that are specific to endothelial cells, that might be upregulated by shear stress and that contain an E-box in their promoters. Based on the profile of shear stress-responsive genes in endothelial cells (Braddock et al., 1998; Chiu et al., 2005; Topper et al., 1996), we hypothesized that the eNOS gene is directly regulated by Hath6.
Bovine aortic endothelial cells (BAECs) were transiently transfected with an eNOS-promoter-driven luciferase reporter construct, Renilla luciferase (transfection efficiency control) and different amounts of empty vector (pCDNA) or myc-tagged Hath6. The total transfected Hath6 and/or pCDNA cDNA remained constant. The eNOS-luciferase activity increased with increasing amounts of transfected Hath6. A western blot of the transfected wells (two transfections per condition) confirmed that the amount of Hath6 protein expression increased with increasing amounts of transfected Hath6 cDNA. The promoter-reporter gene assay indicated that the expression of eNOS-luciferase was dependent on the amount of Hath6 cDNA (Fig. 6D). A series of truncated portions of the eNOS promoter were then constructed to determine its key interaction sites with Hath6. The luciferase reporter assay indicates that the promoter region −1000 to −730 seems to mediate the Hath6 induction of eNOS (Fig. 6E).
Furthermore, the expression and activity of eNOS that is regulated by Hath6 was explored in the endothelial cell line and in EPCs derived from hESCs. Consistent with the alteration in mRNA levels detected by RT-PCR (Fig. 2E; Fig. 3C,I; Fig. 4B,G,J; Fig. 5A), a western blot analysis showed that the expression of eNOS was positively correlated with that of Hath6 both in ECV-304 cells (the first two lines) and in EPCs differentiated from hESCs (Fig. 6F).
Finally, to further validate the participation of eNOS in Hath6 signaling, Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), a small-molecule eNOS inhibitor (Chen et al., 2012; Go et al., 2012), was applied to the step-wise endothelial differentiation system. FACS analysis (Fig. 6G) showed that the addition of L-NAME returned the efficiency of endothelial differentiation to the basal level even when the Hath6 gene was overexpressed (Fig. 6H). The stable knockdown of Hath6 mRNA resulted in a marked suppression of eNOS upregulation, as was generally detected in hESCs after LSS treatment (Fig. 6I). Thus, Hath6 modulates endothelial differentiation and function through eNOS signals.
A homolog of Hath6 is involved in embryonic neovascularization
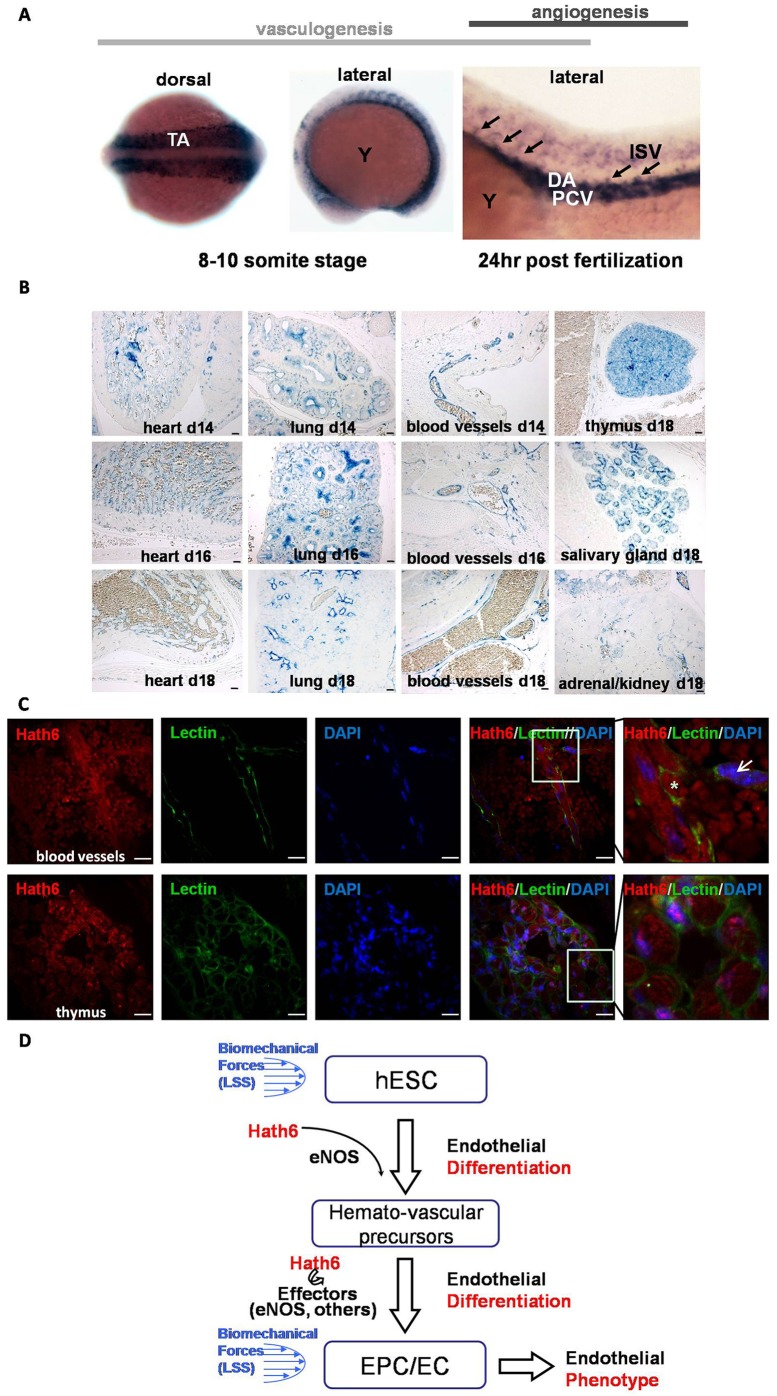
Although the endothelial-specific expression of Hath6 has been shown in human umbilical vessel and tissue staining (Wasserman et al., 2002), it is not clear whether Hath6 is involved in vasculogenesis and/or angiogenesis. To determine the in vivo role of Hath6, we studied the expression profile of a homolog of the Hath6 gene during zebrafish and mouse embryogenesis. The in situ hybridization of ath6 was performed in the developing zebrafish embryos as described previously (Hauptmann and Gerster, 1994). During the early embryonic 8–10 somite stage, ath6 expression was observed in both somitic tissues and trunk angioblasts, which are two parallel stripes that migrate and coalesce in the midline to form the dorsal aorta (DA) and the posterior cardinal vein (PCV) during the process of vasculogenesis (Fig. 7A, left and middle panels). Later, during angiogenesis (24 h post-fertilization), ath6 expression was observed in both the DA and the PCV (Fig. 7A, right panel). Furthermore, ath6 also seemed to be expressed in the angiogenic sprouts of the intersegmental vessels (ISVs) that originate from the DA at this time point. These studies suggest that Ath6 might play important roles in both vasculogenesis and angiogenesis in zebrafish.
Fig. 7.
Expression of Hath6 (Atoh8) in zebrafish and mouse embryos. (A) Whole-mount RNA in situ hybridization was performed on staged zebrafish embryos with an ath6 (zebrafish homolog of human Hath6) anti-sense riboprobe. A probe for ath6 was employed for in situ hybridization on embryos at the 8–10 somite stage (the left image shows the dorsal view and the middle image shows the lateral view) and 24 hours post-fertilization (the right image, lateral view). A positive signal is shown as dark blue. Representative intersegmental arteries are denoted by black arrows. DA, dorsal aorta; ISV, intersegmental vessel; PCV, posterior cardinal vein; TA, trunk angioblasts; Y, yolk). (B) Expression of Math6, the murine homolog of Hath6, during embryonic development in vivo. In situ hybridization was performed on murine embryos at d14–18. An anti-sense digoxigenin-labeled riboprobe for Math6 was employed for in situ hybridization on sections of murine embryos. Tyramide-mediated signal amplification was used to detect probe hybridization. Sense control probes did not yield a signal (data not shown). Math6 was detected in the perivascular and blood-vessel wall; however, staining in non-endothelial tissues is also observed. Scale bars: 100 µm. (C) Confocal microscopy of sections from an E18 mouse embryo indicates the colocalization of Hath6 (red) with the endothelial marker fluorescein griffonia (bandeiraea) simplicifolia lectin II (GSLII, green). The upper panels show blood vessels with enucleated red blood cells (red autofluorescence without blue nuclear staining; indicated with an asterisk). The nuclear staining of Hath6 and the membrane staining with GSLII on the same cell is indicated with an arrow. The lower panels show images of the thymus. The white-boxed area is magnified in the right-most panel. Scale bars: 20 µm. (D) A working model of Hath6 in endothelial differentiation and function. Treatment with biomechanical forces induces Hath6 mRNA expression in hESCs or endothelial cells, which results in increased differentiation of hemato-vascular precursors, endothelial progenitors and endothelial cells, and endothelial phenotype. eNOS is one of the mediators of Hath6 function.
Math6, the murine Hath6 homolog, was also detected with an antisense riboprobe in the perivascular and blood-vessel walls and in non-endothelial tissues in embryonic day (E)14–18 murine embryos (Fig. 7B). Higher-magnification immunofluorescence staining of E18-mouse-tissue sections demonstrates the colocalization of Hath6 with the endothelial marker fluorescein-labelled griffonia (bandeiraea) simplicifolia lectin II (GSLII) in vessel-rich tissues (Fig. 7C). The early expression of Hath6 in vascular systems suggests that this gene might play an important role in vascularization and angiogenesis.
DISCUSSION
In the present study, we used a hESC-EC differentiation model to determine the role of Hath6 in endothelial differentiation. In general, animal models rather than human subjects are used in development and gene-function studies owing to ethical issues. However, the species variation of ATOH8 might indicate that it has different functions in different cellular contexts, especially for Hath6, as previously suggested (Chen et al., 2011). The findings from hESC and EC studies emphasized the significance of Hath6 in the control of endothelial-specific gene expression, cell-fate determination and possibly the genetic program that permits mechanotransduction.
Our characterization of the Hath6 gene was primarily based on sequence homology. The Hath6 protein is the first shear-stress-responsive bHLH protein identified in cultured endothelial cells that is selectively expressed in the mature vascular endothelium in vivo (Wasserman et al., 2002). HLH proteins are a family of more than 350 evolutionarily conserved proteins found in organisms ranging in complexity from humans to yeast (Ledent and Vervoort, 2001; Massari and Murre, 2000). These proteins are characterized by a HLH motif that mediates their homo- or hetero-dimerization and DNA-binding properties. All of these proteins act as transcription factors and play essential roles in fundamental cellular processes, such as proliferation, differentiation and cell-fate determination. Individual HLH transcription factors, as well as the Hath6 homologs Ath6 and Math6, have been implicated in the development of the nervous, muscular, pancreatic, hematopoietic and cardiovascular systems (Inoue et al., 2001; Lynn et al., 2008; Ross et al., 2006; Wang et al., 2009; Yao et al., 2010). In this study, the involvement of Hath6 in vascular development was shown by detection of the Hath6 homolog signal in endothelial-enriched tissues from zebrafish and mouse embryos. A previous study also demonstrated that morpholino-reduced ath6 expression in zebrafish results in circulation defects (Yao et al., 2010). Moreover, the inactivation of the Math6 gene during the embryonic stage is lethal for mice, which suggests the importance of Hath6 in embryogenesis (Lynn et al., 2008). We also demonstrated that Hath6 is capable of modulating the transcription of several genes that play important roles in the endothelial phenotype, including eNOS, CD31, KDR and VE-cadherin. Therefore, it is reasonable to infer that Hath6 might play an important role in embryonic endothelial differentiation.
A cytokine cocktail and flow shear stress seem to efficiently induce the endothelial differentiation of ESCs (Wang et al., 2007; Yamamoto et al., 2005; Zeng et al., 2006). In this report, we first confirmed that both of these stimuli can boost Hath6 mRNA expression (Figs 1, 2). Subsequently, the upregulation of Hath6 enhanced endothelial determination (Fig. 3). To define the signal that is acting upstream of Hath6, endothelial cells were treated with different stimuli and the Hath6 response was evaluated. Although the combination of IFN-γ and TNF-β as well as oxidative stress can promote Hath6 expression, shear stress seemed to be the key stimulator of Hath6 expression. A possible pattern of Hath6 regulation during embryogenesis is the following: at the initiation of endothelial commitment in the absence of blood flow, soluble factors trigger Hath6 expression; subsequently, shear stress becomes the leading factor in Hath6 stimulation. Our data also provide the first demonstration that Hath6 might be upregulated within 12 h in hESCs treated with LSS. Accordingly, the Hath6 gene was directly modulated in the subsequent experiments to investigate its role in the efficient endothelial induction of hESCs. Using Hath6-overexpressing and -knockdown hESCs and endothelial cells, the expression of endothelial functional markers, such as eNOS, CD31, KDR and VE-cadherin, was demonstrated to be positively correlated with the level of Hath6. The significant increase in the expression of these endothelial markers with an increase in the level of Hath6 expression suggests that Hath6 might play a role at an early stage in the process of differentiation and the accumulation of Hath6 transcripts in the cell during differentiation might contribute to later endothelial determination and maturation. Collectively, these data suggest that Hath6 might act as an important link between flow dynamics and the endothelial phenotype.
In endothelial cells, the intracellular signaling cascades that are activated in response to mechanical factors include the nitric oxide (NO), Akt and reactive oxygen species (ROS) pathways (Lehoux et al., 2006). NO is produced by the endothelial isoform of NO synthase (NOS), also known as endothelial NOS (eNOS), which has high endothelial specificity (Teichert et al., 2000). NO production in response to flow-dependent shear forces applied to the surface of endothelial cells is a fundamental mechanism of the regulation of vascular tone, peripheral resistance and tissue perfusion (Balligand et al., 2009). Huang et al. also reported a positive effect of NO signaling on the endothelial differentiation of ESCs (Huang et al., 2010). Accordingly, one might hypothesize that NO signaling is part of the pathway that links shear stress to endothelial differentiation – shear-stress–factor-X–eNOS–NO–endothelial differentiation. In this study, a Hath6-regulated element termed the E-box was found in the eNOS promoter and the direct transcriptional control of eNOS by Hath6 was confirmed through a luciferase-reporter analysis. Furthermore, we demonstrated a high correlation between the expression of Hath6 and eNOS during hESC-EC differentiation, the upregulation of eNOS by Hath6 overexpression and the downregulation of eNOS with Hath6 knockdown. When the activity of eNOS was blocked by L-NAME, the positive effect of Hath6 on endothelial differentiation was diminished consistently. In addition, when Hath6 expression was blocked by shRNA, the response of eNOS to shear stress was significantly suppressed. Hence, we hypothesize that Hath6 regulates eNOS expression and subsequently modulates endothelial differentiation and function.
Histone deacetylase is also commonly reported to respond to shear stress in endothelial cells (Cheng et al., 2012; Zeng et al., 2006). The Hath6-regulated E-box element was identified in the gene encoding the histone deacetylase Sirt1; therefore, we tested the expression of this gene after Hath6 overexpression or knockdown. Although a positive correlation between the expression levels of the two genes was detected in endothelial cells, this correlation was not detected in the ESCs (supplementary material Fig. S6). Because our study demonstrated that Hath6 is indispensable for endothelial determination, whereas the knockout of eNOS or Sirt1 is not lethal in mouse (Sumanas et al., 2008; Yu et al., 2010), additional studies need to be conducted to fully identify the signaling network of Hath6 that is involved in the regulation of hESC-EC differentiation.
It is now generally accepted that the commitment of ESCs to endothelial cells occurs through several stages, involving differentiation of the mesoderm, hemato-vascular precursor and endothelial progenitor cell (Ferguson et al., 2005; Le Bras et al., 2010). In this study, hESCs were first induced into the mesoderm stage as described previously (Zhang et al., 2008). Although the positive effect of Hath6 on embryonic endothelial-cell differentiation was detected early at this stage, the tendency of endothelial lineages to accumulate was accompanied by endothelial specialization. The greatest change caused by manipulation of the Hath6 gene was found at differentiation-induction stage two, when most of the endothelial-committed cells are hemato-vascular precursors or EPCs. The eNOS signaling pathways might be key regulators in EPC specialization.
In conclusion, our findings uncover the properties and function of the Hath6 gene: Hath6 encodes an endothelial-selective, shear-stress-responsive bHLH transcription factor that plays a pivotal role in the regulation of hESC-EC differentiation and endothelial function, and eNOS is a direct target of Hath6. A working model of the role of Hath6 in endothelial differentiation is summarized in Fig. 7D. The present study also provides insights into the key regulators of hESC endothelial-cell differentiation for future stem-cell-based regenerative therapies.
MATERIALS AND METHODS
Culturing ECV-304 cells, HUVECs and hESCs
Human endothelial cell line ECV-304, HUVECs and the undifferentiated hESCs were cultured as described previously (Lejoly-Boisseau et al., 1999; Zhang et al., 2008).
In vitro differentiation of hESC-ECs
(1) Spontaneous differentiation of hESCs
hESCs were passaged by incubating with 0.5 mg/ml type IV collagenase for 5–15 min at 37°C and were washed with PBS twice. Then the cells were plated in ultra-low-adherence six-well plates in DMEM (high glucose) with 20% fetal bovine serum (FBS) plus 0.1 mM NEAA, 1 mM L-glutamine and 0.1 mM β-mercaptoethanol. The media was changed every 2 days.
(2) Phase differentiation of hESCs
hESCs were induced into endothelial lineage cells using a three-step protocol. Before induction, hESCs were passaged onto Matrigel-coated plates as small colonies and were cultured in mTeSR (Stem Cells) overnight. On the second day, the hESCs were washed with PBS and first differentiated in basal medium [75% IMDM (Gibco) and 25% F12 medium (Gibco) supplemented with 1% insulin-transferrin-selenium (Gibco), 0.05% BSA (Amresco) and 1% nonessential amino acid (Invitrogen)] and 25 ng/ml recombinant human (rh)BMP-4 (R&D Systems) for 48 h. Next, the cells were washed with PBS and differentiated in basal medium with 50 ng/ml rhVEGF (Peprotech) and 50 ng/ml rhbFGF (Millipore) for 4.5 days for stage two differentiation. For stage three differentiation, the cells were washed with PBS, passaged with 0.25% trypsin at a 1∶2 ratio and cultured in EGM2 media (Lonza) for 6 days.
Differentiation of mouse ESC-ECs
Mouse ESC differentiation was performed as described previously (Li et al., 2007). Briefly, 3×104 cells per well were transferred to collagen-IV-coated six-well plates (Becton Dickinson), cultured for 4 days and FACS analyzed for KDR and CD31 expression. Spontaneous differentiation of mouse ESCs was performed with protocols described previously (Vittet et al., 1996), followed by tubular structure formation assay at day 14.
LSS treatment
hESCs seeded on Matrigel-coated 3.5-cm dishes were assembled in a parallel-plate flow chamber (GlycoTech) and treated with flow at 12 dyne/cm2 in spontaneous differentiation medium for 12 or 24 h. A static culture with the same medium was used as the control.
Vector construction and transfections
The Hath6 gene with a Myc–His tag in the pcDNA4-Hath6 plasmid was inserted into the MCS of pcDNA3.1-puro to generate pHath6–myc-puro with restriction sites. The empty vector was used as a transfection control. A lentivirus transfer vector encoding shRNA targeting the 5′-AGTTCCTACTCGTCAATTT-3′ segment of the Hath6 gene was constructed as described previously, and an shRNA targeting the 5′-GTACGCGGAATACTTCGAA-3′ segment of luciferase was used as the mock control (Yu et al., 2010). The plasmids and lentivirus were separately transfected into ECV-304, HUVECs and hESCs using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Stable clones were selected with puromycin (1 µg/ml for ECV-304 and 0.5 µg/ml for hESCs).
Cell proliferation assays
For the proliferation assay, the cells were trypsinized and seeded at a concentration of 1×103 cells/well in 200 µl of medium onto 96-well plates. Every day, 20 µl of CCK-8 solution (Dojindo Laboratories, Japan) was added to each well in eight repeated experiments and the plates were incubated for 1 h in a humidified atmosphere with 5% CO2 at 37°C. The absorbance at 490 nm was then measured using a Bio-Rad microplate reader. The growth curve of each cell type was plotted based on the average absorbance measured each day.
Endothelium wound-migration assay
A confluent monolayer area of ECV-304 cells in a six-well plate was selected and 5×20-mm wounds were made with a rubber scraper. After washing three times with PBS to remove all loose or dead cells, the cells were cultured in standard medium. Cells that migrated to the scrape line were photographed using a microscope (NIKON, Japan) (Wang et al., 2006) at the same sites at 0 and 48 h after the wound was made.
Matrigel-induced capillary-tube formation
To make capillary tubes, 48-well plates were coated with 15 µl/well Matrigel and allowed to stand for 30 min at 37°C to form a gel layer. After gel formation, HUVEC, ECV-304 cells and hESC-derived cells at differentiation stage two were trypsinized and seeded in triplicate at a concentration of 5×103–1×104 cells/well in 200 µl EGM2 media. The plates were incubated in 5% CO2 at 37°C for 48 h and photographed using a microscope (OLYMPUS, Japan).
Reverse transcript polymerase chain reaction (PCR) and RT-PCR
Cells were harvested with Trizol (Invitrogen). RNA (0.8 µg) was reverse transcribed using a PrimeScript RT-PCR kit (TaKaRa Bio) according to the manufacturer's instructions. RT-PCR with SYBR Green Master Mix (Applied Biosystems) was performed with a multicolor thermocycler (Bio-Rad). PCR was performed in triplicate for each sample. All data are shown as the mean±s.d. The primers and reaction conditions are listed in supplementary material Table S1.
Immunofluorescence
The cells were fixed with 4% paraformaldehyde for 10 min at room temperature and then treated with ice-cold acetone for 5 min. The cells or cryosections were then washed three times with 0.125% Triton X-100 in PBS for 10 min and blocked with 10% FBS in PBS for 30 min. The cells or sections were incubated with anti-human CD31 (Biolegend), anti-human Brachyury (R&D Systems) or anti-human-and-mouse Hath6 (Santa Cruz) antibody overnight and then with the corresponding secondary antibody. Sections of the E18 mouse embryo were incubated with fluorescein griffonia (bandeiraea) simplicifolia lectin II (Vector) to distinguish the vessels. Finally, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min. After being washed with PBS, the cells were examined by confocal microscopy (Ultra VIEW VoX).
Fluorescence-activated cell sorting (FACS)
Cells were dissociated into a single cell suspension and washed three times in PBS. Then the cells were stained with either anti-human CD31−APC (eBioscience) or anti-human CD31–Alexa-Fluor-488 (BD Biosciences), anti-human CD133−APC (Miltenyi Biotech), anti-human CD45−FITC (BD Biosciences), anti-human CD144−PE (VE-Cadherin, BD Biosciences) or anti-human CD309−PE (VEGFR-2; BD Biosciences) at 5 µl/3×105 cells for 40 min on ice. Subsequently, the cells were washed twice with PBS and were resuspended in 2% paraformaldehyde. The samples were run on a FACS Calibur machine (BD Biosciences) and the data were analyzed using FlowJo software.
Western blot
Protein extracts were subjected to 12% or 10% SDS-PAGE and transferred onto PVDF membranes. Next, the membranes were probed with antibodies against human eNOS (Santa Cruz), Sirt1 (Santa Cruz), Hath6 (Abcam) or c-myc (Santa Cruz) and then incubated with an HRP-conjugated anti-mouse secondary antibody (Santa Cruz) or HRP-conjugated anti-rabbit secondary antibody (Santa Cruz). After washing, the proteins were visualized using an ECL kit (Santa Cruz).
Teratoma formation
To track teratoma formation in vivo, 1×106 H1-sh-Hath6 or H1-sh-mock hESCs were suspended in 50 µl PBS and mixed with 50 µl of Matrigel for subcutaneous injection into the left or right dorsal flanks of NOD/SCID mice, respectively (n = 4, one spot for each group per mouse). Six weeks after injection, all animals were euthanized according to the guidelines for laboratory animal treatment approved by the Beijing Experimental Animal Management Center. Teratomas were harvested, fixed with 4% paraformaldehyde and then embedded with paraffin, sectioned and stained with hematoxylin and eosin.
Statistical analysis
The data are presented as the mean from at least three separate experiments. Student's t-test was used for statistical evaluation and a P value less than 0.05 was considered statistically significant. Unless specified, the data are expressed as the mean±standard deviation.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
X.P., J.C.W. and S.M.W. conceived the idea for this project; X.X., F.F., S.M.W., J.T., F.C., Z.L. and K.D.W. performed the experiments; X.X. and F.F. wrote and edited the manuscript; B.W. and W.Y. supervised the work.
Funding
This work was supported by National High Technology Research and Development Program of China [grant number 2011AA020109 to X.P., 2012AA020503 to X.X.]; National Nature Science Foundation of China [grant number 31071303]; and National Institutes of Health [grant number K08HL076191 to S.M.W., U01HL107393 to J.C.W.]. S.M.W. is an employee of Amgen, Inc. S.M.W. declares that he has no competing financial interest in this study. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.136358/-/DC1
References
- Balligand J-L., Feron O., Dessy C. (2009). eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 89, 481–534 10.1152/physrev.00042.2007 [DOI] [PubMed] [Google Scholar]
- Braddock M., Schwachtgen J-L., Houston P., Dickson M. C., Lee M. J., Campbell C. J. (1998). Fluid shear stress modulation of gene expression in endothelial cells. News Physiol Sci. 13, 241–246 [DOI] [PubMed] [Google Scholar]
- Chen J., Dai F., Balakrishnan-Renuka A., Leese F., Schempp W., Schaller F., Hoffmann M. M., Morosan-Puopolo G., Yusuf F., Bisschoff I. J. et al. (2011). Diversification and molecular evolution of ATOH8, a gene encoding a bHLH transcription factor. PLoS ONE 6, e23005 10.1371/journal.pone.0023005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Bakhshi F. R., Shajahan A. N., Sharma T., Mao M., Trane A., Bernatchez P., van Nieuw Amerongen G. P., Bonini M. G., Skidgel R. A. et al. (2012). Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol. Biol. Cell 23, 1388–1398 10.1091/mbc.E11--09--0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B-B., Yan Z-Q., Yao Q-P., Shen B-R., Wang J-Y., Gao L-Z., Li Y-Q., Yuan H-T., Qi Y-X., Jiang Z-L. (2012). Association of SIRT1 expression with shear stress induced endothelial progenitor cell differentiation. J. Cell. Biochem. 113, 3663–3671 10.1002/jcb.24239 [DOI] [PubMed] [Google Scholar]
- Chiu J-J., Lee P-L., Chang S-F., Chen L-J., Lee C-I., Lin K. M., Usami S., Chien S. (2005). Shear stress regulates gene expression in vascular endothelial cells in response to tumor necrosis factor-α: a study of the transcription profile with complementary DNA microarray. J. Biomed. Sci. 12, 481–502 10.1007/s11373--005--4338--4 [DOI] [PubMed] [Google Scholar]
- Coomber B. L., Gotlieb A. I. (1990). In vitro endothelial wound repair. Interaction of cell migration and proliferation. Arterioscler. Thromb. Vasc. Biol. 10, 215–222 10.1161/01.ATV.10.2.215 [DOI] [PubMed] [Google Scholar]
- Ferguson J. E., III, Kelley R. W., Patterson C. (2005). Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 25, 2246–2254 10.1161/01.ATV.0000183609.55154.44 [DOI] [PubMed] [Google Scholar]
- Go Y-M., Lee H-R., Park H. (2012). H(2)S inhibits oscillatory shear stress-induced monocyte binding to endothelial cells via nitric oxide production. Mol. Cells 34, 449–455 10.1007/s10059--012--0200--5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann G., Gerster T. (1994). Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 10, 266 10.1016/0168--9525(90)90008--T [DOI] [PubMed] [Google Scholar]
- Hove J. R., Köster R. W., Forouhar A. S., Acevedo-Bolton G., Fraser S. E., Gharib M. (2003). Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 10.1038/nature01282 [DOI] [PubMed] [Google Scholar]
- Hsiai T. K., Wu J. C. (2008). Hemodynamic forces regulate embryonic stem cell commitment to vascular progenitors. Curr. Cardiol. Rev. 4, 269–274 10.2174/157340308786349471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N. F., Fleissner F., Sun J., Cooke J. P. (2010). Role of nitric oxide signaling in endothelial differentiation of embryonic stem cells. Stem Cells Dev. 19, 1617–1626 10.1089/scd.2009.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue C., Bae S-K., Takatsuka K., Inoue T., Bessho Y., Kageyama R. (2001). Math6, a bHLH gene expressed in the developing nervous system, regulates neuronal versus glial differentiation. Genes Cells 6, 977–986 10.1046/j.1365--2443.2001.00476.x [DOI] [PubMed] [Google Scholar]
- Jain R. K. (2003). Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 10.1038/nm0603--685 [DOI] [PubMed] [Google Scholar]
- Le Bras A., Vijayaraj P., Oettgen P. (2010). Molecular mechanisms of endothelial differentiation. Vasc. Med. 15, 321–331 10.1177/1358863X10371685 [DOI] [PubMed] [Google Scholar]
- Ledent V., Vervoort M. (2001). The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res. 11, 754–770 10.1101/gr.177001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux S., Castier Y., Tedgui A. (2006). Molecular mechanisms of the vascular responses to haemodynamic forces. J. Intern. Med. 259, 381–392 10.1111/j.1365--2796.2006.01624.x [DOI] [PubMed] [Google Scholar]
- Lejoly-Boisseau H., Appriou M., Seigneur M., Pruvost A., Tribouley-Duret J., Tribouley J. (1999). Schistosoma mansoni: in vitro adhesion of parasite eggs to the vascular endothelium. Subsequent inhibition by a monoclonal antibody directed to a carbohydrate epitope. Exp. Parasitol. 91, 20–29 10.1006/expr.1999.4348 [DOI] [PubMed] [Google Scholar]
- Li Z., Wu J. C., Sheikh A. Y., Kraft D., Cao F., Xie X., Patel M., Gambhir S. S., Robbins R. C., Cooke J. P. et al. (2007). Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 116, Suppl, I46-I54 10.1161/CIRCULATIONAHA.106.680561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wilson K. D., Smith B., Kraft D. L., Jia F., Huang M., Xie X., Robbins R. C., Gambhir S. S., Weissman I. L. et al. (2009). Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE 4, e8443 10.1371/journal.pone.0008443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn F. C., Sanchez L., Gomis R., German M. S., Gasa R. (2008). Identification of the bHLH factor Math6 as a novel component of the embryonic pancreas transcriptional network. PLoS ONE 3, e2430 10.1371/journal.pone.0002430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari M. E., Murre C. (2000). Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20, 429–440 10.1128/MCB.20.2.429--440.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q-G. V., Buskin J. N., Himeda C. L., Shield M. A., Hauschka S. D. (2003). Differences in the function of three conserved E-boxes of the muscle creatine kinase gene in cultured myocytes and in transgenic mouse skeletal and cardiac muscle. J. Biol. Chem. 278, 46494–46505 10.1074/jbc.M308194200 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Shiratori H., Saijoh Y., Hamada H. (2002). Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96–99 10.1038/nature00849 [DOI] [PubMed] [Google Scholar]
- Ross M. D., Martinka S., Mukherjee A., Sedor J. R., Vinson C., Bruggeman L. A. (2006). Math6 expression during kidney development and altered expression in a mouse model of glomerulosclerosis. Dev. Dyn. 235, 3102–3109 10.1002/dvdy.20934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S., Lin S. (2006). Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 4, e10 10.1371/journal.pbio.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S., Gomez G., Zhao Y., Park C., Choi K., Lin S. (2008). Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood 111, 4500–4510 10.1182/blood--2007--09--110569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert A. M., Miller T. L., Tai S. C., Wang Y., Bei X., Robb G. B., Phillips M. J., Marsden P. A. (2000). In vivo expression profile of an endothelial nitric oxide synthase promoter-reporter transgene. Am. J. Physiol. 278, H1352–H1361 [DOI] [PubMed] [Google Scholar]
- Topper J. N., Cai J., Falb D., Gimbrone M. A., Jr (1996). Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc. Natl. Acad. Sci. USA 93, 10417–10422 10.1073/pnas.93.19.10417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittet D., Prandini M., Berthier R., Schweitzer A., Martin-Sisteron H., Uzan G., Dejana E. (1996). Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood 88, 3424–3431 [PubMed] [Google Scholar]
- Wang Y., Hu Y., Sun C., Zhang X., He W. (2006). Mechanism of arsenic trioxide inhibiting angiogenesis in multiple myeloma. J. Huazhong Univ. Sci. Technolog. Med. Sci. 26, 43–46 10.1007/BF02828035 [DOI] [PubMed] [Google Scholar]
- Wang Z. Z., Au P., Chen T., Shao Y., Daheron L. M., Bai H., Arzigian M., Fukumura D., Jain R. K., Scadden D. T. (2007). Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat. Biotechnol. 25, 317–318 10.1038/nbt1287 [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen K., Yao Q., Zheng X., Yang Z. (2009). Phylogenetic analysis of zebrafish basic helix-loop-helix transcription factors. J. Mol. Evol. 68, 629–640 10.1007/s00239--009--9232--7 [DOI] [PubMed] [Google Scholar]
- Wasserman S. M., Mehraban F., Komuves L. G., Yang R-B., Tomlinson J. E., Zhang Y., Spriggs F., Topper J. N. (2002). Gene expression profile of human endothelial cells exposed to sustained fluid shear stress. Physiol. Genomics 12, 13–23 [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Sokabe T., Watabe T., Miyazono K., Yamashita J. K., Obi S., Ohura N., Matsushita A., Kamiya A., Ando J. (2005). Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. 288, H1915–H1924 10.1152/ajpheart.00956.2004 [DOI] [PubMed] [Google Scholar]
- Yao J., Zhou J., Liu Q., Lu D., Wang L., Qiao X., Jia W. (2010). Atoh8, a bHLH transcription factor, is required for the development of retina and skeletal muscle in zebrafish. PLoS ONE 5, e10945 10.1371/journal.pone.0010945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Liu Y., Miao Z., Yin M., Lu W., Lv Y., Ding M., Deng H. (2010). Retinoic acid enhances the generation of hematopoietic progenitors from human embryonic stem cell-derived hemato-vascular precursors. Blood 116, 4786–4794 10.1182/blood--2010--01--263335 [DOI] [PubMed] [Google Scholar]
- Zeng L., Xiao Q., Margariti A., Zhang Z., Zampetaki A., Patel S., Capogrossi M. C., Hu Y., Xu Q. (2006). HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J. Cell Biol. 174, 1059–1069 10.1083/jcb.200605113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Li J., Tan Z., Wang C., Liu T., Chen L., Yong J., Jiang W., Sun X., Du L. et al. (2008). Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 111, 1933–1941 10.1182/blood--2007--02--074120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.