ABSTRACT
Mutations in either of two presenilin genes can cause familial Alzheimer's disease. Presenilins have both proteolysis-dependent functions, as components of the γ-secretase complex, and proteolysis-independent functions in signalling. In this study, we investigate a conserved function of human presenilins in the development of the simple model organism Dictyostelium discoideum. We show that the block in Dictyostelium development caused by the ablation of both Dictyostelium presenilins is rescued by the expression of human presenilin 1, restoring the terminal differentiation of multiple cell types. This developmental role is independent of proteolytic activity, because the mutation of both catalytic aspartates does not affect presenilin ability to rescue development, and the ablation of nicastrin, a γ-secretase component that is crucial for proteolytic activity, does not block development. The role of presenilins during Dictyostelium development is therefore independent of their proteolytic activity. However, presenilin loss in Dictyostelium results in elevated cyclic AMP (cAMP) levels and enhanced stimulation-induced calcium release, suggesting that presenilins regulate these intracellular signalling pathways. Our data suggest that presenilin proteins perform an ancient non-proteolytic role in regulating intracellular signalling and development, and that Dictyostelium is a useful model for analysing human presenilin function.
KEY WORDS: Dictyostelium, γ-secretase, Presenilin
INTRODUCTION
To date, more than 170 different mutations in presenilin 1 (PSEN1) and 13 mutations in presenilin 2 (PSEN2) genes are known to give rise to a familial form of Alzheimer's disease (FAD) (De Strooper and Annaert, 2010). The protein products of the mammalian PSEN genes are components of an aspartate protease complex, γ-secretase (De Strooper, 2003), which is responsible for the regulated, intramembranous cleavage of a number of type 1 transmembrane proteins (Wolfe, 2006), including the Notch receptors (Huppert et al., 2000) and the amyloid precursor protein (APP). It is from APP that β-amyloid (Aβ), the likely causative agent of both the familial and sporadic forms of Alzheimer's disease, is derived (De Strooper et al., 1998; Herreman et al., 2000). Prior to incorporation into the γ-secretase complex, the presenilin 1 protein undergoes autoproteolytic cleavage into two parts (Chávez-Gutiérrez et al., 2008). Presenilin proteins have also been shown to have a non-proteolytic function as a scaffold for the regulation of glycogen synthase kinase 3β (GSK3β)-dependent β-catenin phosphorylation (Kang et al., 1999; Kang et al., 2002). Finally, presenilin proteins have also been implicated in altered calcium signalling (Tu et al., 2006) through an unknown mechanism. Understanding the discrete roles of presenilin proteins thus remains an important goal in understanding basic cell function and the progression of Alzheimer's disease (De Strooper and Annaert, 2010).
Although research into presenilin protein function has often focussed on presenilin-knockout mice (Schaeffer et al., 2011), it has been difficult to determine the precise function of presenilins because the loss of both presenilins leads to embryonic lethality. A range of alternative non-mammalian models have been employed to examine the function of presenilin proteins, including Physcomitrella patens (Khandelwal et al., 2007), Caenorhabditis elegans (Calahorro and Ruiz-Rubio, 2011), Danio rerio (van Tijn et al., 2011) and Drosophila melanogaster (Coen et al., 2012). However, the social amoeba Dictyostelium discoideum is the simplest model organism that possesses two presenilin proteins as well as the other three components of the γ-secretase complex (Boeckeler and Williams, 2007; McMains et al., 2010). Dictyostelium has been extensively used in a range of motility (Janetopoulos and Firtel, 2008), developmental (Loomis and Shaulsky, 2011) and biomedical studies (Chang et al., 2012; Francione and Fisher, 2011; Ludtmann et al., 2011; Myre et al., 2011; Terbach et al., 2011), and has a number of experimental advantages over existing models (Williams et al., 2006). In this study, we employ the advantages of Dictyostelium to explore the role of the human presenilin 1 protein (PSEN1) in development. We generated an isogenic Dictyostelium strain lacking both presenilin A and presenilin B genes (psenA−/psenB−), and found that development was blocked at the aggregation stage prior to morphogenesis. We show that either human PSEN1 or Dictyostelium PsenB can rescue the developmental phenotype of this mutant. Presenilin proteins that are mutated at the catalytic aspartic acid residues retain the ability to rescue the developmental block, demonstrating that the proteolytic activity of presenilin is not required for phenotypic rescue. A conserved functional role for the human protein in Dictyostelium provides new insights into the ancient function(s) of presenilin proteins.
RESULTS
Dictyostelium presenilins share substantial similarity with human presenilins
The social amoeba Dictyostelium discoideum represents one of the earliest branches from the common ancestor of all eukaryotes, and thus provides a useful means for understanding the ancestral eukaryotic genome (Eichinger et al., 2005). It is also a simple widely used model organism for understanding developmental signalling and is increasingly being used to investigate the role of disease-related proteins (Williams et al., 2006). The Dictyostelium genome sequence has identified orthologues of a range of proteins associated with human diseases and conditions (Eichinger et al., 2005), including two presenilin proteins (PsenA and PsenB). Phylogenetic analysis reveals that they are more closely related (sister) to the monophyletic animal presenilin clade than to the distinct monophyletic plant presenilin clade (Fig. 1A). Furthermore, PsenA and PsenB (Fig. 1A) are similar in size and structure to the two human homologues and, like their human counterparts, they contain the conserved catalytic aspartic acid residues for proteolytic activity (Wolfe, 2006) and the GxDG motif for γ-secretase integration (Fig. 1B). A comparison of the predicted transmembrane domains reveals that these regions are 43–50% identical in the Dictyostelium and human proteins. Additionally, more than half (67/112) of the residues in either PSEN1 or PSEN2 that are mutated in FAD are conserved in either Dictyostelium PsenA or PsenB (supplementary material Fig. S1) [see also fig. S2 in McMains et al. (McMains et al., 2010)]. The sequence similarity and predicted common structure between the human and Dictyostelium presenilin proteins suggests a possible conservation of their function in these distantly related species.
Fig. 1.
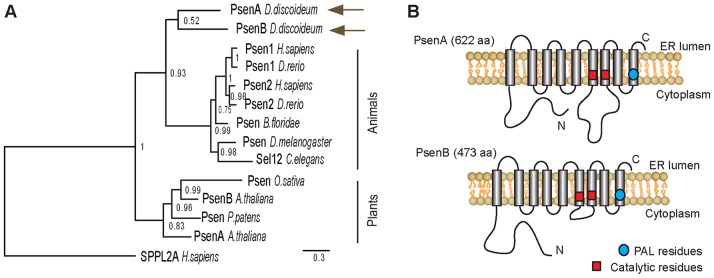
The Dictyostelium PsenA and PsenB proteins show structural similarity to human presenilins. (A) Bayesian-derived phylogeny of presenilin amino-acid-sequence data from animal and plant species and D. discoideum. Human SPPLA2A was used as the outgroup. Numbers adjacent to nodes represent Bayesian posterior probability values. Scale bar: the number of substitutions per site. (B) Schematic of both Dictyostelium presenilin proteins showing the putative orientation in the membrane, cytosolic and transmembrane regions, the conserved catalytic residues and the proline-alanine-leucine (PAL) sequence.
Presenilins function redundantly in development
Presenilin proteins have previously been shown to play a key role in Dictyostelium development (McMains et al., 2010). In wild-type cells, starvation triggers the aggregation of ∼100,000 individual amoebae that progress through a series of multicellular morphological stages over a 24-h period, ultimately giving rise to a fruiting body comprising spores held within a sorus suspended above the substratum by a stalk composed of dead vacuolated cells (Fig. 2) (Williams et al., 2006). To test whether PsenA and PsenB act redundantly, we ablated each Dictyostelium gene individually using homologous integration of a knockout cassette (Faix et al., 2004) (supplementary material Fig. S2). The ablation of either Dictyostelium gene individually did not alter this basic developmental morphology under our conditions (supplementary material Fig. S3A), in contrast to earlier reports showing a developmental block following psenB ablation (McMains et al., 2010), which might be accounted for by a difference in parental strain or by differing experimental conditions. However, the removal of both genes (generating a line hereafter referred to as psenA−/psenB− cells) caused a strong block in development (Fig. 2). The psenA−/psenB− cells aggregated to complete early development, forming a rounded structure attached to the substratum, but morphogenesis halted at the beginning of tip formation. This supports the role previously suggested for Dictyostelium presenilins in development (McMains et al., 2010) and demonstrates that the presenilin homologues have redundant functions, consistent with what is observed in mammals (Feng et al., 2004; Kim et al., 2011).
Fig. 2.
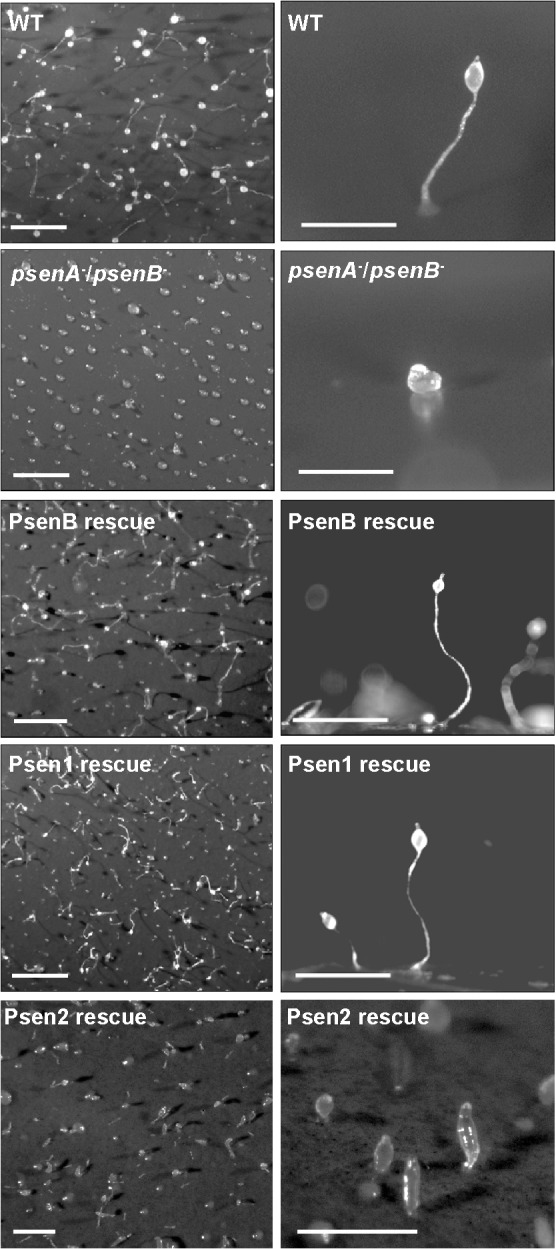
Dictyostelium presenilin proteins redundantly control multicellular development that is complemented by expression of human presenilin 1. Following starvation, wild-type (WT) cells undergo development over a 24-h period, leading to the formation of fruiting bodies; left, aerial view at low magnification; right, side view at higher magnification. Ablation of both presenilin genes (psenA−/psenB−) in one cell line gives rise to a block in fruiting-body formation, leading to a small round structure lacking a stalk. Overexpression of GFP-tagged Dictyostelium PsenB or human PSEN1 in these cells restores development, whereas PSEN2 expression partially rescues development. Scale bars: 1 mm.
Human PSEN1 rescues the defective development of psenA−/psenB− cells
To investigate whether Dictyostelium and human presenilin proteins have conserved functions, we expressed the PsenB and PSEN1 proteins tagged with an N-terminal GFP (Veltman et al., 2009) in the psenA−/psenB− genetic background. Expression of either PsenB or PSEN1 reversed the morphological block in development in this mutant (Fig. 2), resulting in the formation of wild-type fruiting bodies composed of both a stalk and a fully formed sorus (Fig. 2). We also expressed the human PSEN2 protein tagged with an N-terminal GFP, and found that development was partially rescued, so that it progressed beyond the block at the tipped mound observed in psenA−/psenB− cells, to the early culminant (Fig. 2). Because PSEN2 expression did not fully complement development in the psenA−/psenB− genetic background, our subsequent analyses were restricted to human PSEN1. This developmental complementation by PSEN1 in this model organism suggests that the function of PSEN1 is conserved from humans to Dictyostelium.
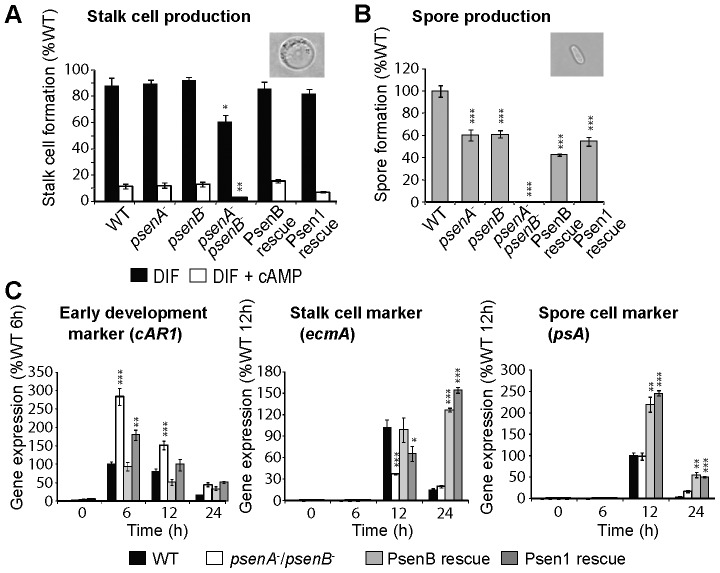
Our previous study also identified altered development in Dictyostelium lacking presenilin activity (McMains et al., 2010), so we assessed whether the Dictyostelium and human presenilin proteins could complement these defects in stalk- and spore-cell production and developmental-marker expression (Fig. 3). Stalk-cell formation was induced in low-density cultures in the presence of differentiation induction factor-1 (DIF-1) (Fig. 3A) (Williams et al., 1999). The ablation of either psenA or psenB had no effect on DIF-1-induced stalk-cell formation, whereas the psenA−/psenB− mutant, although still able to form stalk cells, showed a significant 31% reduction in stalk-cell production (P<0.05), mirroring the developmental phenotype of each mutant. We also analysed the inhibition of this developmental pathway by cAMP, which leads to a glycogen-synthase-kinase-A (GSKA)-dependent block in stalk-cell production (GSKA is the Dictyostelium homologue of mammalian GSK3β) (Williams et al., 1999). The ablation of either Dictyostelium presenilin gene had no effect on cAMP-mediated inhibition of stalk-cell differentiation compared with wild-type cells, whereas the presenilin double-null mutant showed a significant 84% increase in cAMP-dependent stalk-cell inhibition compared with wild-type cells (P<0.004), which is consistent with the absence of a stalk in the fruiting body after 24 h. Furthermore, complementing the psenA−/psenB− mutant with PsenB and PSEN1 restored stalk-cell production to levels close to that achieved by wild-type cells either in the presence or absence of cAMP.
Fig. 3.
Expression of PsenB or PSEN1 can rescue the block in multiple differentiation pathways resulting from the ablation of presenilin activity in the psenA−/psenB− mutant. (A) Stalk-cell production is unaffected by ablation of either presenilin individually but is reduced when both presenilins are removed and is restored by expression of PsenB or PSEN1. (B) Spore production is reduced by ablation of either presenilin gene individually, is blocked in psenA−/psenB− mutants and is restored by expression of PsenB or PSEN1. Insets show light microscopy images of the cell type analysed in each panel. (C) The expression of developmentally regulated genes (0, 6, 12 and 24 h post-starvation), using quantitative transcriptional analysis for the early developmental marker cAR1 and the cell-type-specific marker genes (pre-stalk, ecmA; pre-spore, psA) in wild-type and in psenA−/psenB− cells, and following complementation with PsenB or PSEN1. Increased cAR1 expression and reduced ecmA expression caused by the loss of both presenilin genes is restored by the expression of PsenB or PSEN1. All data are presented as the mean ± standard deviation. *P<0.05, **P<0.01, ***P<0.001.
Because Dictyostelium presenilin proteins have previously been shown to regulate spore production in monolayer assays (McMains et al., 2010), we also assessed whether expressing the Dictyostelium and human proteins complemented this aspect of multicellular development (Fig. 3B). The ablation of either psenA or psenB caused a 40% reduction in spore production during multicellular development. However, the psenA−/psenB− mutant showed a complete loss of spore formation. The expression of either PsenB or PSEN1 restored spore production to the level of single knockouts, confirming the functionality of the human presenilin protein in Dictyostelium development. Together with the effects on stalk-cell differentiation and the observed developmental arrest at the initiation of tip formation, our results indicate that presenilin proteins are required not for cell-type choice but for differentiation along both the stalk and spore pathways.
Finally, as an independent verification of developmental complementation, we used quantitative transcriptional analysis (qPCR) to monitor the expression of developmental and cell-type markers in the psenA−/psenB− mutant and following complementation with the Dictyostelium and human proteins (Fig. 3C). The cAMP receptor 1 (cAR1) gene is expressed in wild-type cells in early development (Saxe, III et al., 1991), whereas a threefold increase in expression in early (6 h) development and elevated expression in mid–late (12–24 h) development was observed in the psenA−/psenB− mutant compared with wild-type cells. In addition, the pre-stalk-cell marker ecmA (Harwood, 2008) showed a highly significant reduction in expression in the psenA−/psenB− mutant in early and mid-development, whereas the pre-spore-cell marker psA (Harwood, 2008) showed only a small elevation in the double mutant compared with wild-type cells after 24 h of development. Complementation with PsenB returned marker expression to wild-type levels. Complementation with PSEN1 only partially rescued marker expression, with levels not reaching those of either wild-type cells or psenA−/psenB− cells complemented with PsenB, suggesting a reduced efficacy of the human protein in Dictyostelium. Also, expression of either human or Dictyostelium proteins caused an elevation of late (24 h) stalk-cell-marker expression and mid-late (12–24 h) spore-cell-marker expression, suggesting a common controlling role for both presenilin proteins in terminal differentiation. These data suggest that the differentiation of pre-stalk and pre-spore cells in the mound preceding tip formation is not dramatically impaired by the loss of presenilin (but is enhanced by the ectopic expression of either the Dictyostelium or human presenilin protein). By contrast, the further differentiation to mature stalk and spore cells is blocked, as is morphogenesis beyond the beginning of tip formation. These data also confirm the functionality of the human presenilin 1 protein in stalk- and spore-cell differentiation in Dictyostelium.
The presenilins are predominantly localised at the endoplasmic reticulum in Dictyostelium
We next examined the subcellular localisation of the expressed Dictyostelium and human proteins in the psenA−/psenB− mutant. In mammals, presenilins are localised on the endoplasmic reticulum (ER) (Walter et al., 1996) and nuclear membrane (Li et al., 1997). In Dictyostelium, presenilin subcellular localisation was determined by fixing cells expressing GFP-tagged versions of PsenB and PSEN1 to reveal that both proteins were located on a fine interlaced network of membrane structures resembling the ER and nuclear envelope (Fig. 4). Colocalisation with an ER-specific marker, calnexin (Müller-Taubenberger et al., 2001), confirmed that both the human and Dictyostelium presenilin proteins localise to the ER and nuclear envelope in D. discoideum. These data once again confirm the similar behaviour of human and Dictyostelium presenilin proteins.
Fig. 4.
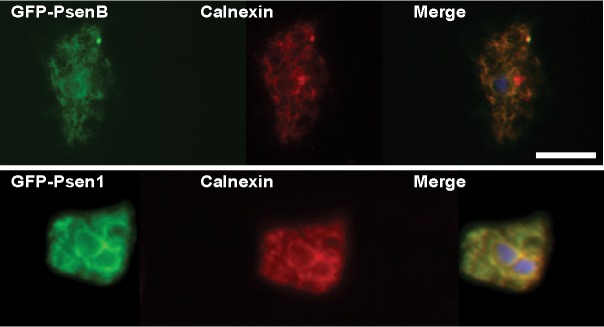
The Dictyostelium and human presenilins localise to the ER in Dictyostelium cells. The GFP tag on PsenB and PSEN1 proteins indicates that both proteins colocalise with the ER marker calnexin (labelled with a specific antibody). Blue, 4′,6-diamidino-2-phenylindole (DAPI) staining. Scale bar: 10 µm.
The proteolytic activity of presenilins is dispensable for development
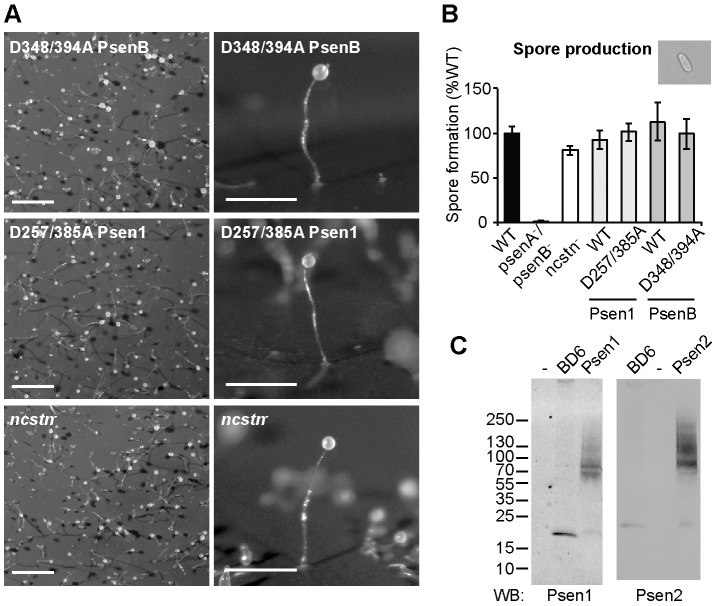
We next investigated the molecular mechanism of presenilin function in Dictyostelium. The human presenilin proteins have been ascribed either a proteolytic function within the γ-secretase complex, dependent upon two crucial catalytic aspartic-acid residues in the protein (Yamasaki et al., 2006), or a structural role related to the docking of intracellular signalling components (Fluhrer et al., 2004; Tesco and Tanzi, 2000; Twomey and McCarthy, 2006). The Dictyostelium presenilin proteins have been shown to proteolytically process recombinant human APP in an identical manner to APP processing in mammalian cells (McMains et al., 2010). This is probably due to γ-secretase activity because the proteolytic ability is lost upon ablation of the γ-secretase components nicastrin (Ncstn) and anterior pharynx defective 1 (Aph1) (McMains et al., 2010). To establish a role for γ-secretase proteolytic activity in Dictyostelium development, we mutated the catalytic aspartic-acid residues to alanines in both Dictyostelium and human proteins [D348A/D394A in PsenB or D257A/D385A in PSEN1 (Tesco and Tanzi, 2000); see supplementary material Fig. S1] and examined whether these mutant presenilin proteins could complement the defective fruiting-body formation of psenA−/psenB− cells. Expression of either the Dictyostelium or human double aspartic-acid-mutated protein rescued Dictyostelium morphological development, enabling wild-type fruiting bodies to form (Fig. 5A). Furthermore, ablation of the ncstn gene, which is necessary for γ-secretase proteolytic ability (Edbauer et al., 2003), had no effect on fruiting-body development (Fig. 5A). As an independent verification of the normal developmental morphology that we observed in these experiments, we measured spore production in the complemented psenA−/psenB− cells or the ncstn− mutant and confirmed that spore production was similar to the wild-type cells (Fig. 5B). These results suggest that presenilin proteins do not require proteolytic activity, presumably as part of the γ-secretase complex, to function in Dictyostelium development.
Fig. 5.
Dictyostelium fruiting-body development does not require the proteolytic activity of presenilins or a complete γ-secretase complex. (A) Expression of Dictyostelium PsenBD348A/D394A or human PSEN1D257A/D385A, lacking the key catalytic aspartic-acid residues that are necessary for proteolytic activity, rescued Dictyostelium development in psenA−/psenB− cells. The ablation of nicastrin (ncstn−) had no effect on Dictyostelium fruiting-body (developmental) morphology. Images show low-magnification aerial view (left) and high-magnification side view (right). Scale bars: 1mm. (B) Spore production in psenA−/psenB− cells is restored by expression of either Dictyostelium or human presenilins mutated at the two catalytic aspartic-acid residues and is unaffected by the ablation of nicastrin (ncstn−). WT, wild type. Results show means±s.e.m. Inset shows a light microscopy image of the cell type analysed. (C) Both human presenilins, when expressed in Dictyostelium psenA−/psenB− cells, undergo endoproteolysis to yield a single band of 20–25 kDa, corresponding to the C-terminal fragment, as demonstrated by western analysis. Molecular-mass markers in kilodaltons are indicated on the left; -, untransformed psenA−/psenB− control; BD6, mouse-blastocyst-like cells showing processing of endogenous mouse presenilins. The antibodies used for western blotting (WB) are indicated below the blots.
The proteolytic activity of mammalian presenilins depends on their PEN2-dependent autoproteolytic cleavage at the intracellular loop, giving rise to a 34-kDa N-terminal and a 22-kDa C-terminal fragment (CTF) (Ahn et al., 2010; Haass and De Strooper, 1999). We therefore probed protein extracts from psenA−/psenB− cells transformed with GFP-tagged wild-type PSEN1 and PSEN2 by western blotting, to ascertain whether cleavage occurred. This analysis showed that a band of 20–25 kDa corresponding to the CTF was detected for both proteins (Fig. 5C), indicating that endoproteolysis of both the human presenilins occurs in Dictyostelium. This result suggests that these human proteins can form an active protease complex together with the remaining Dictyostelium γ-secretase components.
Presenilins function in cAMP signalling and calcium homeostasis in Dictyostelium
We then investigated two signalling pathways that might be controlled by presenilin activity during Dictyostelium development. A role for presenilins in cAMP signalling was likely, based upon two observations: first, psenA−/psenB− cells do not show streaming during early development (where a visible trail of aggregating cells is seen leading to a mound; supplementary material Movie 1 and supplementary material Fig. S3B) – a phenotype associated with altered cAMP signalling (Garcia and Parent, 2008; Veltman and van Haastert, 2008); and second, the mutant cells also show increased sensitivity to cAMP-induced stalk-cell inhibition in late development (Fig. 3A). Therefore, we assessed intracellular cAMP levels during early development in wild-type cells, the psenA−/psenB− mutant and double-mutant cells complemented with PsenB or PSEN1 (Fig. 6A). No significant difference was found between the different strains during growth (t = 0 h) or early development (t = 4 h). However, at t = 8 h, the psenA−/psenB− mutant showed significantly higher cAMP levels than wild-type cells (P<0.001). This was rescued following complementation with PsenB and reduced following complementation with PSEN1, and both proteins rescued the lack of streaming observed in early development (supplementary material Fig. S3B). These data demonstrate a role for the Dictyostelium presenilin proteins in regulating cAMP signalling, an effect that has also been seen in mammals (Müller et al., 2011; Wang et al., 2011). Once again, these results demonstrate that the Dictyostelium and human proteins have overlapping functions and perform a conserved role in Dictyostelium development.
Fig. 6.
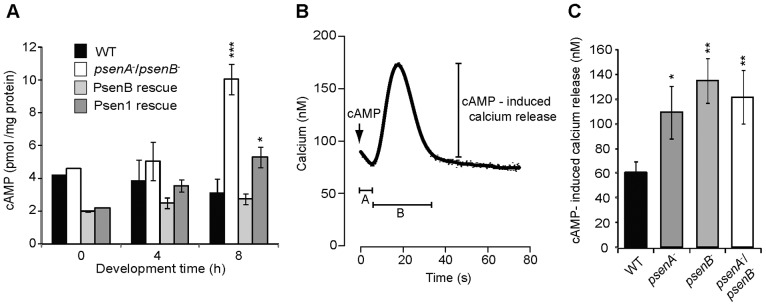
Loss of presenilin proteins in Dictyostelium alters cAMP and calcium levels. (A) cAMP levels in cells during growth (t = 0 h) and early development (t = 4 and 8 h) in wild-type (WT) cells and psenA−/psenB− cells with and without complementation (rescue) with PsenB or PSEN1. Data are derived from five independent experiments and show a statistically significant increase in cAMP in psenA−/psenB− and the PSEN1-rescue cells at 8 h only. (B) Schematic representation of the calcium-response recording prior to and upon 50 nM cAMP stimulation; A, the time until calcium response after cAMP stimulation; B, the length of the calcium response. The magnitude of the cAMP-induced calcium release is also shown. Data for A and B are presented in supplementary material Fig. S4. (C) The magnitude of the calcium response in wild-type and presenilin-mutant cells. The magnitude of the calcium response is significantly increased in psenA−, psenB− and psenA−/psenB− cells when compared to wild-type cells. Values shown are means±s.e.m, n = 5. *P<0.05, **P<0.01, ***P<0.001.
Because both human and Dictyostelium presenilin proteins are localised to the ER and presenilin ablation has been shown to alter calcium signalling in mammals (Tu et al., 2006), we also analysed whether calcium levels were affected in the presenilin mutants. In these experiments, cells expressing apoaequorin were loaded with coelenterazine and calcium levels were measured by luminescence in resting cells and following cAMP stimulation (Fig. 6). This analysis showed that the resting calcium levels, the cAMP-dependent time until calcium response and the duration of the calcium response were not significantly different in the single- and double-presenilin-null cells when compared to wild-type cells (supplementary material Fig. S4). However, the magnitude of the cAMP-induced calcium response was 2–2.5-fold greater in psenA−, psenB− and psenA−/psenB− cells (P<0.05, P<0.01 and P<0.01, respectively) when compared to the wild-type cells (Fig. 6C). These results suggest a role for presenilin proteins in Dictyostelium calcium homeostasis.
Dictyostelium presenilins can cleave mammalian substrates
The conserved function for the human presenilin 1 protein in Dictyostelium development prompted us to examine whether the reverse is true; that is, whether Dictyostelium presenilins can function in mammalian cells. We tested the ability of presenilin proteins to function in the human γ-secretase complex to cleave Notch-1 within the transmembrane domain, as measured by the activation of a construct containing a Notch-and-CBF1-dependent luciferase reporter gene (Hooper et al., 2006). We transfected mouse-blastocyst-derived cells lacking all copies of both presenilin genes [BD8 cells (Donoviel et al., 1999)] with constructs expressing either PsenA, PsenB (both with and without FLAG tags) or human PSEN1, an S2-cleaved but membrane-tethered form of human Notch-1 (ΔEN1) and the CBF1-luc reporter construct. Both Dictyostelium presenilin proteins showed significant activity in their ability to participate in γ-secretase cleavage of the S3 cleavage site in ΔEN1, and to induce reporter activation (Fig. 7), indicating that the Dictyostelium presenilin proteins are functionally active in mammalian cells, presumably as part of the γ-secretase complex. These results are in agreement with our previous demonstration of the cleavage of an N-terminally truncated human APP in Dictyostelium (McMains et al., 2010).
Fig. 7.
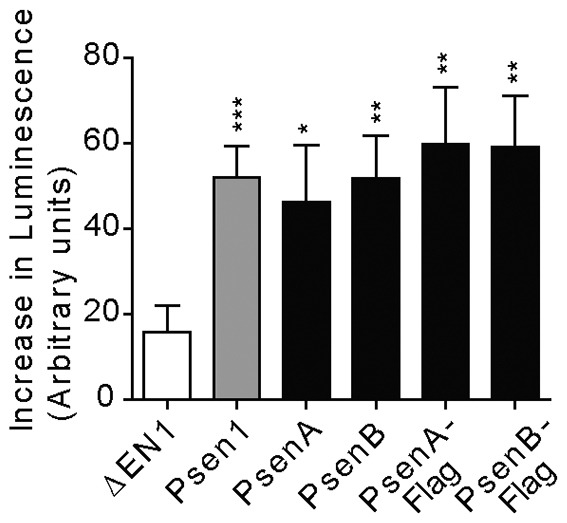
The Dictyostelium presenilins are functional in mammalian cells. The PsenA and PsenB proteins were expressed in mouse-blastocyst-derived BD8 cells that have both psen1 and psen2 ablated. γ-secretase activity was assessed by the fold increase in luminescence resulting from cleavage of the substrate ΔEN1 (a membrane-tethered Notch1 lacking the extracellular domain) by transfected human presenilin 1 (WT) or Dictyostelium presenilin proteins (PsenA and PsenB) with or without a C-terminal FLAG tag. Results show means±s.e.m. Data represent two to three independent experiments carried out in duplicate (n = 4–6). *P<0.05, **P<0.01, ***P<0.001.
DISCUSSION
There is a pressing need for new models in presenilin research owing to the lethality of double-presenilin-null mutants in mammals (Shen et al., 1997), which makes research in the absence of background presenilin activity highly problematic. Alternative non-mammalian model systems (Calahorro and Ruiz-Rubio, 2011; Coen et al., 2012; Khandelwal et al., 2007; van Tijn et al., 2011) have been used to provide some insight into presenilin function, but studies in these models are complicated by difficulties in gene ablation or in the overexpression of tagged proteins in stable isogenic cell lines to enable cell-signalling and biochemistry studies. This study in Dictyostelium, in combination with our previous work (McMains et al., 2010), thus demonstrates the experimental benefits provided by this novel model for the analysis of presenilin function.
We have addressed the conservation of function of the Dictyostelium and human presenilins in development by analysing their ability to rescue the block mid-way through development (at the mound stage) observed in a psenA−/psenB− mutant (Fig. 2). The ability of PSEN1 to efficiently restore morphological development strongly supports the conclusion that the human and Dictyostelium presenilins share a conserved and evolutionarily ancient function. In addition, the ability of PsenB to rescue the development of the double-null mutant suggests that there is functional redundancy between the Dictyostelium presenilins, even though their starkly different expression profiles (McMains et al., 2010), with psenB expressed highly in growth and psenA expressed highly during later development, would suggest specific functions for each presenilin. The reverse is also true: APP expressed ectopically in Dictyostelium is cleaved efficiently by the endogenous γ-secretase complex and in a similar way as in mammalian cell lines (McMains et al., 2010), and we show that Dictyostelium presenilins can cleave mammalian Notch 1 when they are expressed in Psen1−/Psen2− mouse-blastocyst-like cells (Fig. 7). Thus, it appears that human presenilin can be incorporated into the Dictyostelium γ-secretase complex and vice versa, and that both of these complexes are proteolytically active. Additionally, the shared cellular localisation [on the ER and nuclear envelope (Walter et al., 1996)] in both species and a shared function in regulating intracellular (cAMP) signalling (Müller et al., 2011; Wang et al., 2011) and calcium homeostasis (Berridge, 2011) further support a common function for presenilins in mammals and Dictyostelium (Figs 4,6). These observations together suggest that presenilins might have a conserved function throughout multiple kingdoms of life and across millennia of evolution.
Despite a conserved function of the Dictyostelium and human presenilin proteins in a range of cellular processes, the physiological function of the proteins in the development of the two species appears to be different. Mutating the catalytic aspartic-acid residues of either PsenB or PSEN1 did not remove the ability of these proteins to rescue the morphological defect of the psenA−/psenB− mutant during development (Fig. 5A). Also, ablation of nicastrin, which completely blocks γ-secretase proteolytic activity in mammalian models (Edbauer et al., 2003), did not block Dictyostelium development, unlike the strong effect observed when both psenA and psenB are disrupted. Thus, these data showing that presenilin proteolytic activity is dispensable for development suggest an archaic structural role for presenilin proteins in this amoeba, which is independent of proteolytic function. In support of this, a study in the moss Physcomitrella patens showed that both human presenilin 1 and the PSEN1D385A variant were able to rescue the growth defect observed in the moss presenilin-null mutant (Khandelwal et al., 2007). However, it appears that certain growth and other developmental aspects in Dictyostelium require the other components of the γ-secretase complex (McMains et al., 2010). Our data thus provide evidence for a highly conserved structural role for presenilin proteins in development.
The conserved cellular role for human presenilin 1 that we have shown in Dictyostelium will enable a more thorough analysis of the cellular function of the wild-type protein in isogenic cell lines. Multiple gram weights of identical cells can be employed for biochemical analysis in this model, and a range of genetic approaches [including suppression screens and pharmocogenetics (Williams, 2005)] can be employed to better understand wild-type presenilin function in cells. Dictyostelium can also be used to analyse the >170 presenilin mutations that give rise to FAD (Parks and Curtis, 2007) in relation to basic cellular functions (such as calcium regulation) and in relation to γ-secretase activity [we can monitor the cleavage of human APP in Dictyostelium, giving rise to a potential change in the Aβ40∶Aβ42 ratio associated with Alzheimer's disease progression (McMains et al., 2010)]. This new presenilin model could provide an important versatile system for future research on FAD and drug-development studies in translational research.
MATERIALS AND METHODS
Phylogenetic and structural analysis
MEGA4 was employed to align protein sequence data (Kumar et al., 2008), with further edits and the removal of highly variable regions conducted in Se-ALv2.0 (Rambaut, 1996). The phylogenetic relationships within this dataset were estimated by Bayesian analyses, using human signal-peptide-peptidase-like-2A (SPLLA2; Q8TCT8) as the outgroup, because presenilins and this protein belong to different subgroups of the peptidase A22 family (A22A and A22B, respectively). Bayesian trees were constructed in MrBayes 3.2 (Ronquist and Huelsenbeck, 2003) with gamma distribution, proportion of invariable sites and implementation of the ‘aamodelpr = mixed prior’ that allowed selection for the optimum substitution model. Two independent runs using four chains (three heated, one cold) were run for 1×106 generations, with sampling every 1×103 generations and a burn-in period of 250 trees. Nodal support was determined by approximate posterior probabilities performed in MrBayes. Stabilisation and convergence between runs was assessed using Tracer 1.5 (Rambaut and Drummond, 2009). Protein structural analysis was based upon the human protein structure (Parks and Curtis, 2007).
Presenilin-mutant cell lines and cell-type-differentiation analysis
Presenilin- and nicastrin-null mutants were generated by homologous integration using the Cre-Lox system (Faix et al., 2004). Wild-type psenB and human psen1 (isoform 2) full-length cDNAs were ligated into pDM317 and pDM448 N-terminal-GFP expression constructs under control of the act15 promoter (Veltman et al., 2009). Wild-type human psen2 (isoform 1) with a D. discoideum codon bias was synthesised by MWG-Biotech (Germany) and ligated into the pDM448 N-terminal-GFP expression construct. Stalk-cell development was conducted as described previously (Williams et al., 1999). Spore production was assessed by the lysis of mature fruiting bodies (24 h) in 0.1% NP40 or 0.3% Triton X-100 and counting spores with a haemocytometer.
cAMP assays
Cells were grown in HL-5 medium containing selection antibiotics where appropriate. Cells were developed on non-nutrient (NN) agar (0.68 g/l KH2PO4, 0.89 g/l Na2HPO4•2H2O pH 6.5), 15 g/l agar], and samples were harvested in PB (0.68 g/l KH2PO4, 0.89 g/l Na2HPO4•2H2O pH 6.5) at 0, 4 and 8 h of development. 5×107 cells were resuspended in 200 µl of PB and were lysed with 300 µl 3.5% (v/v) HClO4. The lysates were neutralised by the addition of an equal volume of 50% saturated KHCO3 and 200 µl of cAMP-assay buffer (4 mM EDTA in 150 mM sodium phosphate pH 7.5). The lysates were centrifuged for 5 min at 3000 g to precipitate protein and KClO4. cAMP was assayed in 20 µl and 40 µl of the supernatant fraction by isotope-dilution assay, using purified protein kinase A regulatory subunit (PKA-R) from beef muscle as a cAMP-binding protein and [2,8-3H]cAMP as the competitor. The data represent the mean and s.e.m. of five experiments performed in triplicate. The data from individual measurements were not normally distributed and significant differences between datasets were therefore estimated by Kruskal–Wallis ANOVA on ranks.
Calcium assays
Calcium homeostasis in Dictyostelium cells was measured by using the calcium-sensitive aequorin approach in which strains were transformed with an apoaequorin-expressing plasmid (pPROF120) that allows real-time assay of cytosolic free Ca2+ levels (Allan and Fisher, 2009). Briefly, 108 cells were incubated in 5 ml of MES development buffer [10 mM 2-(N-morpholino)ethanesulfonic acid (MES, pH 6.2), 10 mM KCl, 0.25 mM CaCl2] containing coelenterazine-h (0.5 µg/ml dissolved in 20% w/v Pluronic F-127) for 7 h in shaking suspension, followed by washing with MES development buffer to remove residual coelenterazine-h. The coelenterazine-h allows in vivo reconstitution of the functional photoprotein, which upon calcium binding produces luminescence that can be detected with a photometer. In order to measure calcium influx upon cAMP stimulation, the total possible light emission was determined to normalise the aequorin luminescence signals. These values allowed calculation of an in vitro calcium concentration-effect curve upon 1 µM cAMP stimulation. All measurements were carried out in a New Brunswick ATP Photometer, as described previously (Allan and Fisher, 2009), and analysed using the R statistical package (R Core Team, 2012, http://www.R-project.org/).
Immunofluorescence and western analysis
Monoclonal antibodies against calnexin were kindly provided by Annette Müller-Taubenberger (Müller-Taubenberger et al., 2001). For immunofluorescence labelling, cell lines expressing the D. discoideum or human genes were grown overnight in low-fluorescence medium, fixed, probed with anti-calnexin antibodies and labelled with goat anti-mouse-IgG (NEB 4409s; Alexa Fluor 555; Ipswich, MA). Rabbit monoclonal anti-presenilin-1 D39D1 antibody (#5643) was obtained from Cell Signaling Technology (Danvers, MA) and anti-presenilin-2 (EP1515Y) antibody (ab51249) was obtained from Abcam (Cambridge, MA) and used at 1∶1000 for western analysis.
Quantitative RT-PCR
Total RNA was extracted from Dictyostelium using the High Pure RNA Isolation kit (11828665001; Roche; Welwyn Garden City, UK) and cDNA was amplified using the First Strand cDNA Synthesis Kit (K1612; Fermentas; Loughborough, UK). Real-time amplification with SYBR Green (Sigma, S4438) was performed in a Rotor-Gene 6000 (Qiagen Ltd, Manchester, UK). Triplicate samples were collected at each time point with two RT-PCR technical replicates and the level of transcription was quantified using the 2−ΔΔCT method (Livak and Schmittgen, 2001). PCR primer pairs were designed using Primer3 (Rozen and Skaletsky, 2000).
Notch cleavage assay
An S2-cleaved form of human Notch 1 that requires γ-secretase-dependent S3 cleavage was used in a CBF1-reporter-gene assay as described previously (Hooper et al., 2006). The CBF1 reporter contains four tandem consensus DNA-binding sites in the promoter driving luciferase expression (a gift fromGerry Weinmaster, UCLA Medical School, CA). Presenilin-dependent generation of the Notch intracellular domain is required to activate CBF1-dependent transcription. Full-length Dictyostelium presenilin was expressed from the pFLAG-CMV5a vector (Sigma-Aldrich St Louis, MO).
Supplementary Material
Acknowledgments
G.P.O. (funded by the Dr Hadwen Trust) and R.S.B.W. did not participate in experiments involving animals, or cells or tissues from animals or from human embryos. The Dr Hadwen Trust is the UK leading medical research charity that funds and promotes exclusively human-relevant research that encourages the progress of medicine with the replacement of the use of animals in research.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
The project was conceived and designed by R.S.B.W., with additional experimental design input by M.H.R.L. M.H.R.L. and G.P.O. performed the majority of the experiments. C.S. and Z.-H.C. performed the cAMP experiments. C.A. and M.H.R.L. performed the calcium experiments. R.K. and M.H.R.L. performed the mammalian experiments. S.B. performed the phylogenetic analysis. P.W.B., A.R.K., P.F. and R.K. contributed reagents, materials or analysis tools and provided manuscript comments. R.S.B.W., M.H.R.L. and G.P.O. wrote the paper. G.P.O. did not participate in experiments involving animals, or cells or tissues from animals or from human embryos.
Funding
This work was supported by the Alzheimer's Society UK (R.K.); by the Intramural Research Programs of the National Institutes of Health; and the National Institute of Diabetes and Digestive and Kidney Diseases (A.R.K.); and by an Alzheimer's Research UK PhD studentship to R.S.B.W. M.H.R.L. received a Central Research Fund and Helen Shackleton travel scholarship. G.P.O. is funded by the Dr Hadwen Trust (DHT). Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.140939/-/DC1
References
- Ahn K., Shelton C. C., Tian Y., Zhang X., Gilchrist M. L., Sisodia S. S., Li Y. M. (2010). Activation and intrinsic gamma-secretase activity of presenilin 1. Proc. Natl. Acad. Sci. USA 107, 21435–21440 10.1073/pnas.1013246107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan C. Y., Fisher P. R. (2009). In vivo measurements of cytosolic calcium in Dictyostelium discoideum. Methods Mol. Biol. 571, 291–308 10.1007/978--1--60761--198--1_20 [DOI] [PubMed] [Google Scholar]
- Berridge M. J. (2011). Calcium signalling and Alzheimer's disease. Neurochem. Res. 36, 1149–1156 10.1007/s11064--010--0371--4 [DOI] [PubMed] [Google Scholar]
- Boeckeler K., Williams R. S. B. (2007). Dictyostelium as a biomedical model. Encyclopedia of Life Sciences. 2007, 1–6 10.1002/9780470015902.a0006038 [DOI] [Google Scholar]
- Calahorro F., Ruiz-Rubio M. (2011). Caenorhabditis elegans as an experimental tool for the study of complex neurological diseases: Parkinson's disease, Alzheimer's disease and autism spectrum disorder. Invert. Neurosci. 11, 73–83 10.1007/s10158--011--0126--1 [DOI] [PubMed] [Google Scholar]
- Chang P., Orabi B., Deranieh R. M., Dham M., Hoeller O., Shimshoni J. A., Yagen B., Bialer M., Greenberg M. L., Walker M. C. et al. (2012). The antiepileptic drug valproic acid and other medium-chain fatty acids acutely reduce phosphoinositide levels independently of inositol in Dictyostelium. Dis. Model. Mech. 5, 115–124 10.1242/dmm.008029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez-Gutiérrez L., Tolia A., Maes E., Li T., Wong P. C., de Strooper B. (2008). Glu(332) in the Nicastrin ectodomain is essential for gamma-secretase complex maturation but not for its activity. J. Biol. Chem. 283, 20096–20105 10.1074/jbc.M803040200 [DOI] [PubMed] [Google Scholar]
- Coen K., Flannagan R. S., Baron S., Carraro-Lacroix L. R., Wang D., Vermeire W., Michiels C., Munck S., Baert V., Sugita S. et al. (2012). Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 198, 23–35 10.1083/jcb.201201076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. (2003). Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron 38, 9–12 10.1016/S0896--6273(03)00205--8 [DOI] [PubMed] [Google Scholar]
- De Strooper B., Annaert W. (2010). Novel research horizons for presenilins and γ-secretases in cell biology and disease. Annu. Rev. Cell Dev. Biol. 26, 235–260 10.1146/annurev--cellbio--100109--104117 [DOI] [PubMed] [Google Scholar]
- De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. (1998). Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 10.1038/34910 [DOI] [PubMed] [Google Scholar]
- Donoviel D. B., Hadjantonakis A. K., Ikeda M., Zheng H., Hyslop P. S., Bernstein A. (1999). Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 13, 2801–2810 10.1101/gad.13.21.2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D., Winkler E., Regula J. T., Pesold B., Steiner H., Haass C. (2003). Reconstitution of gamma-secretase activity. Nat. Cell Biol. 5, 486–488 10.1038/ncb960 [DOI] [PubMed] [Google Scholar]
- Eichinger L., Pachebat J. A., Glöckner G., Rajandream M. A., Sucgang R., Berriman M., Song J., Olsen R., Szafranski K., Xu Q. et al. (2005). The genome of the social amoeba Dictyostelium discoideum. Nature 435, 43–57 10.1038/nature03481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix J., Kreppel L., Shaulsky G., Schleicher M., Kimmel A. R. (2004). A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 32, e143 10.1093/nar/gnh136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R., Wang H., Wang J., Shrom D., Zeng X., Tsien J. Z. (2004). Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer's presenilin-1 and presenilin-2. Proc. Natl. Acad. Sci. USA 101, 8162–8167 10.1073/pnas.0402733101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhrer R., Friedlein A., Haass C., Walter J. (2004). Phosphorylation of presenilin 1 at the caspase recognition site regulates its proteolytic processing and the progression of apoptosis. J. Biol. Chem. 279, 1585–1593 10.1074/jbc.M306653200 [DOI] [PubMed] [Google Scholar]
- Francione L. M., Fisher P. R. (2011). Heteroplasmic mitochondrial disease in Dictyostelium discoideum. Biochem. Pharmacol. 82, 1510–1520 10.1016/j.bcp.2011.07.071 [DOI] [PubMed] [Google Scholar]
- Garcia G. L., Parent C. A. (2008). Signal relay during chemotaxis. J. Microsc. 231, 529–534 10.1111/j.1365--2818.2008.02066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., De Strooper B. (1999). The presenilins in Alzheimer's disease – proteolysis holds the key. Science 286, 916–919 10.1126/science.286.5441.916 [DOI] [PubMed] [Google Scholar]
- Harwood A. J. (2008). Dictyostelium development: a prototypic Wnt pathway? Methods Mol. Biol. 469, 21–32 10.1007/978--1--60327--469--2_2 [DOI] [PubMed] [Google Scholar]
- Herreman A., Serneels L., Annaert W., Collen D., Schoonjans L., De Strooper B. (2000). Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell Biol. 2, 461–462 10.1038/35017105 [DOI] [PubMed] [Google Scholar]
- Hooper C., Tavassoli M., Chapple J. P., Uwanogho D., Goodyear R., Melino G., Lovestone S., Killick R. (2006). TAp73 isoforms antagonize Notch signalling in SH-SY5Y neuroblastomas and in primary neurones. J. Neurochem. 99, 989–999 10.1111/j.1471--4159.2006.04142.x [DOI] [PubMed] [Google Scholar]
- Huppert S. S., Le A., Schroeter E. H., Mumm J. S., Saxena M. T., Milner L. A., Kopan R. (2000). Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature 405, 966–970 10.1038/35016111 [DOI] [PubMed] [Google Scholar]
- Janetopoulos C., Firtel R. A. (2008). Directional sensing during chemotaxis. FEBS Lett. 582, 2075–2085 10.1016/j.febslet.2008.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. E., Soriano S., Frosch M. P., Collins T., Naruse S., Sisodia S. S., Leibowitz G., Levine F., Koo E. H. (1999). Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. J. Neurosci. 19, 4229–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. E., Soriano S., Xia X., Eberhart C. G., De Strooper B., Zheng H., Koo E. H. (2002). Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell 110, 751–762 10.1016/S0092--8674(02)00970--4 [DOI] [PubMed] [Google Scholar]
- Khandelwal A., Chandu D., Roe C. M., Kopan R., Quatrano R. S. (2007). Moonlighting activity of presenilin in plants is independent of gamma-secretase and evolutionarily conserved. Proc. Natl. Acad. Sci. USA 104, 13337–13342 10.1073/pnas.0702038104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Chang A., Dudak A., Federoff H. J., Lim S. T. (2011). Characterization of nectin processing mediated by presenilin-dependent γ-secretase. J. Neurochem. 119, 945–956 10.1111/j.1471--4159.2011.07479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Nei M., Dudley J., Tamura K. (2008). MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9, 299–306 10.1093/bib/bbn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu M., Zhou H., Ma J., Potter H. (1997). Alzheimer presenilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregation. Cell 90, 917–927 10.1016/S0092--8674(00)80356--6 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Shaulsky G. (2011). Developmental changes in transcriptional profiles. Dev. Growth Differ. 53, 567–575 10.1111/j.1440--169X.2010.01241.x [DOI] [PubMed] [Google Scholar]
- Ludtmann M. H., Boeckeler K., Williams R. S. (2011). Molecular pharmacology in a simple model system: implicating MAP kinase and phosphoinositide signalling in bipolar disorder. Semin. Cell Dev. Biol. 22, 105–113 10.1016/j.semcdb.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMains V. C., Myre M., Kreppel L., Kimmel A. R. (2010). Dictyostelium possesses highly diverged presenilin/gamma-secretase that regulates growth and cell-fate specification and can accurately process human APP: a system for functional studies of the presenilin/gamma-secretase complex. Dis. Model. Mech. 3, 581–594 10.1242/dmm.004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Cárdenas C., Mei L., Cheung K. H., Foskett J. K. (2011). Constitutive cAMP response element binding protein (CREB) activation by Alzheimer's disease presenilin-driven inositol trisphosphate receptor (InsP3R) Ca2+ signaling. Proc. Natl. Acad. Sci. USA 108, 13293–13298 10.1073/pnas.1109297108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Taubenberger A., Lupas A. N., Li H., Ecke M., Simmeth E., Gerisch G. (2001). Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. EMBO J. 20, 6772–6782 10.1093/emboj/20.23.6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myre M. A., Lumsden A. L., Thompson M. N., Wasco W., MacDonald M. E., Gusella J. F. (2011). Deficiency of huntingtin has pleiotropic effects in the social amoeba Dictyostelium discoideum. PLoS Genet. 7, e1002052 10.1371/journal.pgen.1002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks A. L., Curtis D. (2007). Presenilin diversifies its portfolio. Trends Genet. 23, 140–150 10.1016/j.tig.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Rambaut A. (1996). Se-ALv2.0a11: sequence alignment editor [Google Scholar]
- Rambaut A., Drummond A. J. (2009). Tracer v1.5 [Google Scholar]
- Ronquist F., Huelsenbeck J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386 [DOI] [PubMed] [Google Scholar]
- Saxe C. L., III, Johnson R., Devreotes P. N., Kimmel A. R. (1991). Multiple genes for cell surface cAMP receptors in Dictyostelium discoideum. Dev. Genet. 12, 6–13 10.1002/dvg.1020120104 [DOI] [PubMed] [Google Scholar]
- Schaeffer E. L., Figueiro M., Gattaz W. F. (2011). Insights into Alzheimer disease pathogenesis from studies in transgenic animal models. Clinics (Sao Paulo) 66, Suppl. 145–54 10.1590/S1807--59322011001300006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Bronson R. T., Chen D. F., Xia W., Selkoe D. J., Tonegawa S. (1997). Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89, 629–639 10.1016/S0092--8674(00)80244--5 [DOI] [PubMed] [Google Scholar]
- Terbach N., Shah R., Kelemen R., Klein P. S., Gordienko D., Brown N. A., Wilkinson C. J., Williams R. S. (2011). Identifying an uptake mechanism for the antiepileptic and bipolar disorder treatment valproic acid using the simple biomedical model Dictyostelium. J. Cell Sci. 124, 2267–2276 10.1242/jcs.084285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G., Tanzi R. E. (2000). GSK3 beta forms a tetrameric complex with endogenous PS1-CTF/NTF and beta-catenin. Effects of the D257/D385A and FAD-linked mutations. Ann. N. Y. Acad. Sci. 920, 227–232 10.1111/j.1749--6632.2000.tb06927.x [DOI] [PubMed] [Google Scholar]
- Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S. F., Hao Y. H., Serneels L., De Strooper B., Yu G., Bezprozvanny I. (2006). Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell 126, 981–993 10.1016/j.cell.2006.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey C., McCarthy J. V. (2006). Presenilin-1 is an unprimed glycogen synthase kinase-3beta substrate. FEBS Lett. 580, 4015–4020 10.1016/j.febslet.2006.06.035 [DOI] [PubMed] [Google Scholar]
- van Tijn P., Kamphuis W., Marlatt M. W., Hol E. M., Lucassen P. J. (2011). Presenilin mouse and zebrafish models for dementia: focus on neurogenesis. Prog. Neurobiol. 93, 149–164 10.1016/j.pneurobio.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Veltman D. M., van Haastert P. J. (2008). The role of cGMP and the rear of the cell in Dictyostelium chemotaxis and cell streaming. J. Cell Sci. 121, 120–127 10.1242/jcs.015602 [DOI] [PubMed] [Google Scholar]
- Veltman D. M., Akar G., Bosgraaf L., Van Haastert P. J. (2009). A new set of small, extrachromosomal expression vectors for Dictyostelium discoideum. Plasmid 61, 110–118 10.1016/j.plasmid.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Walter J., Capell A., Grünberg J., Pesold B., Schindzielorz A., Prior R., Podlisny M. B., Fraser P., Hyslop P. S., Selkoe D. J. et al. (1996). The Alzheimer's disease-associated presenilins are differentially phosphorylated proteins located predominantly within the endoplasmic reticulum. Mol. Med. 2, 673–691 [PMC free article] [PubMed] [Google Scholar]
- Wang D., Yuen E. Y., Zhou Y., Yan Z., Xiang Y. K. (2011). Amyloid beta peptide-(1-42) induces internalization and degradation of beta2 adrenergic receptors in prefrontal cortical neurons. J. Biol. Chem. 286, 31852–31863 10.1074/jbc.M111.244335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S. B. (2005). Pharmacogenetics in model systems: defining a common mechanism of action for mood stabilisers. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1029–1037 10.1016/j.pnpbp.2005.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S. B., Eames M., Ryves W. J., Viggars J., Harwood A. J. (1999). Loss of a prolyl oligopeptidase confers resistance to lithium by elevation of inositol (1,4,5) trisphosphate. EMBO J. 18, 2734–2745 10.1093/emboj/18.10.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S., Boeckeler K., Gräf R., Müller-Taubenberger A., Li Z., Isberg R. R., Wessels D., Soll D. R., Alexander H., Alexander S. (2006). Towards a molecular understanding of human diseases using Dictyostelium discoideum. Trends Mol. Med. 12, 415–424 10.1016/j.molmed.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Wolfe M. S. (2006). The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry 45, 7931–7939 10.1021/bi060799c [DOI] [PubMed] [Google Scholar]
- Yamasaki A., Eimer S., Okochi M., Smialowska A., Kaether C., Baumeister R., Haass C., Steiner H. (2006). The GxGD motif of presenilin contributes to catalytic function and substrate identification of gamma-secretase. J. Neurosci. 26, 3821–3828 10.1523/JNEUROSCI.5354--05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.