Abstract
Objective. The aim of this study was to identify serum markers that are modulated by an investigational anti-IFN-α mAb, sifalimumab, in adult DM or PM patients.
Methods. In a phase 1b clinical trial, sera were collected from a total of 48 DM or PM adult patients receiving either placebo for 3 months or sifalimumab for 6 months. Samples were tested for 128 selected proteins using a multiplex luminex immunoassay. Muscle biopsies from selected patients were stained for T cell infiltration using an anti-CD3 antibody.
Results. A robust overexpression of multiple serum proteins in DM or PM patients was observed, particularly in patients with an elevated baseline type I IFN gene signature in the blood or muscle. Neutralization of the type I IFN gene signature by sifalimumab resulted in coordinated suppression of T cell-related proteins such as soluble IL-2RA, TNF receptor 2 (TNFR2) and IL-18. Muscle biopsies from two patients with the highest serum protein suppression were selected and found to have a pronounced reduction of muscle T cell infiltration. Down-regulation of IL-2RA correlated with favourable manual muscle test 8 (MMT-8) alterations in sifalimumab-dosed patients.
Conclusion. A reduced level of multiple T cell-associated proteins after sifalimumab but not placebo administration suggests a suppressive effect of blocking type I IFN signalling on T cell activation and chemoattraction that may lead to a reduction of T cell infiltration in the muscle of myositis patients. Further, soluble IL-2RA changes from baseline may serve as a responsive and/or predictive marker for type I IFN-targeted therapy in adult DM or PM patients.
Keywords: dermatomyositis, polymyositis, type I interferon, soluble interleukin-2 receptor, T cell infiltration
Introduction
Idiopathic inflammatory myopathies (IIMs) are rare autoimmune disorders sharing the clinical symptom of muscle weakness and inflammatory cell infiltrates in muscle tissue. DM, PM and IBM are three of the most common inflammatory myopathies [1]. Type I IFNs have been shown to be part of the immunopathogenesis of IIMs [2–7]. Recently a phase 1b trial of an investigational anti-IFN-α monoclonal antibody, sifalimumab, was completed in adult DM or PM patients (NCT00533091). Gene expression experiments from this study demonstrated suppression of a type I IFN gene signature in both blood and muscle tissue by sifalimumab, along with a positive correlative trend between IFN target neutralization and clinical improvement in myositis patients [8].
Beyond activated type I IFN signalling, IIMs are also characterized by chronic infiltration of activated T cells and, to a lesser extent, B cells in muscle tissue of patients. A perivascular distribution of CD4+ T cells and B cells is typical for DM, while PM shows direct attack by activated CD8+ T cells against muscle fibres [9]. Proinflammatory cytokines released from inflammatory cells facilitate the migration of those cells from circulation through cellular adhesion molecules on the endothelial cells in an autoamplificatory mechanism [1]. Type I IFN plays a central role in the dysregulated cytokine and chemokine network involved in myositis pathophysiology. Multiple type I IFN-induced proteins are markedly overexpressed in serum and muscle fibres of DM patients [4, 6, 10], and the recruitment of Th1 cells through IFN-γ-induced protein 10 (IP10) and CXCR3 interaction has been shown to play an important role in DM skin lesions [11].
In this study we evaluated >100 proteins in the serum of 48 adult DM or PM patients and assessed their correlation with type I IFN signature score and clinical assessments at baseline. Then the downstream effects of sifalimumab on serum protein levels were investigated in association with type I IFN signature neutralization rates. Muscle biopsies from selected patients with high suppression of serum markers were stained for T cell infiltration using an anti-CD3 antibody. Finally, correlative trends between protein level changes and favourable manual muscle test 8 (MMT-8) alterations were examined following administration of sifalimumab.
Materials and methods
Study design, subjects, laboratory and clinical measurements
MI-CP151 was a phase 1b randomized, double-blind, placebo-controlled, multicentre study to evaluate the safety and tolerability of sifalimumab in adult patients with DM or PM (NCT00533091). The detailed trial design, randomization scheme and inclusion and exclusion criteria have been published previously [8]. Briefly, 39 patients received sifalimumab for 6 months with every other week dosing, while 12 patients received placebo for 3 months then switched to sifalimumab for 3 months with seven doses beginning at day 98. All patients were pre-screened for type I IFN gene signature score, which was calculated using 13 type I IFN-inducible genes and reported as a median-fold change relative to a pool of normal control samples. Patients were identified as having an elevated type I IFN gene signature (IFN-hi) if their baseline signature score in blood specimens was ≥2 or their gene signature in muscle specimens was ≥5. IFN target modulation after administration of sifalimumab for IFN-hi patients was defined as the median suppression of the 13 type I IFN-inducible transcripts [3].
MMT-8 was assessed on a 0–10 or expanded 0–5 scale to evaluate muscle strength in eight designated muscles, including proximal, distal and axial muscles. The MMT-8 change in response to placebo or sifalimumab was an exploratory endpoint, and reliability testing was not performed on the evaluators. Serum levels of ANAs, anti-ribonucleoprotein (RNP), anti-SSA, anti-Jo-1 and anti-Mi-2 antibodies were assayed using standard methods by Quintiles Laboratories (Durham, NC, USA). The study was approved by the institutional review board at each site and informed patient consent was obtained.
Serum proteomics profiling and muscle biopsy immunostaining
Sera from 25 healthy controls (HCs), 27 DM patients and 21 PM patients prior to administration of either placebo or sifalimumab were collected for the characterization of the serological proteome in myositis patients. To compare the effects of placebo and sifalimumab on peripheral protein markers, we procured patient sera on day 98 after drug administration, before placebo-administered patients were switched to sifalimumab dosing. Selected frozen muscle biopsy samples were used for T cell immunostaining with anti-human CD3 antibody.
Serum proteomics profiling
All serum samples were kept frozen at −80°C before shipment to Rules Based Medicine (RBM, Austin, TX, USA) for a multiplexed immunoassay based on Luminex xMAP technology. Measured proteins were chosen based on the literature as well as an RBM Human MAP panel of markers including chemokines, cytokines, hormones, growth factors and antigens. Samples were processed and analysed at RBM according to standard operating procedure. Each of the measured 128 proteins has an established lowest limit of quantitation (LLOQ) and a normal range based on data from ∼200 HC samples provided by RBM.
Muscle biopsy immunostaining
Selected muscle cryosections (5 μm) were mounted on charged slides, fixed in cold acetone for 10 min and allowed to desiccate overnight. Before staining, endogenous peroxidases were quenched using a peroxidase blocking reagent (Dako North America, Carpinteria, CA, USA), followed by two rinses in 1× Tris-buffered saline (TBS; pH 7.2). A protein blocking solution consisting of 1.5% normal goat serum, 0.5% casein and 1% bovine serum albumin in PBS was added to each slide for 30 min. This was followed by incubation with the rabbit anti-human CD3 antibody (Dako North America) at 0.5 μg/ml for 60 min. The slides were washed with 1× TBS and incubated with a peroxidase-labelled polymer conjugated to goat anti-rabbit immunoglobulin antibody (Envision+; Dako North America) for 30 min, then washed with 1× TBS. Detection was performed using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Vector ImmPACT, Vector Laboratories, Burlingame, CA, USA) as the chromogen. Slides were washed in dH2O, counterstained with Mayer’s haematoxylin, dehydrated and mounted with DPX histology mounting medium (Fluka, Sigma-Aldrich).
Data analysis
Demographic and clinical features of different groups were compared using Student’s t-test for continuous variables or Fisher’s exact test for discrete variables. Negative analytes were defined as proteins with serum levels below the LLOQ in >70% of all samples, and their distributions in different subject groups were compared by the Fisher’s exact test of the number of patients with protein levels higher or lower than its LLOQ. These negative analytes were excluded from further analysis to provide robust assessment of the proteome. Serum levels of positive analytes were log2-transformed for statistical comparison. The global proteomics pattern of all the patients and HCs was examined by principal components analysis (PCA). An analysis of variance (ANOVA) model and Student’s t-test were used to compare serum protein levels among HCs and different groups of myositis patients. Multiple comparison problems were controlled by Benjamin–Hochberg (BH) adjustment [12]. Post-treatment changes were assessed by paired t-test followed by BH adjustment. The percentage change for each analyte of interest was scaled and then subjected to unsupervised hierarchical clustering. For markers of interest, correlation with the type I IFN gene signature score and clinical and laboratory disease activity measurements were calculated by Spearman’s correlation tests and P-values were assessed via the asymptotic t approximation followed by BH adjustment.
Results
Dysregulated proteins in myositis patients
Serum specimens were available at baseline and day 98 after treatment for 27 DM and 21 PM patients. Baseline gene expression screening in blood and muscle specimens indicated that 37 patients had an elevated type I IFN gene signature in blood or muscle tissue (IFN-hi) while the other 11 patients did not (IFN-lo). The clinical and demographic features of the two groups are shown in Table 1. No significant difference was observed in demographic features and immunosuppressive medication profiles between two groups at baseline.
Table 1.
Clinical and demographic features of myositis patients
| Category | Feature | IFN-hi (n = 37) | IFN-lo (n = 11) | P-value |
|---|---|---|---|---|
| Disease type | DM, % | 56.8 | 54.5 | >0.1 |
| Demographic | Age, years | 51 | 50.8 | >0.1 |
| Female, % | 73 | 54.5 | >0.1 | |
| Caucasian, % | 73 | 81.8 | >0.1 | |
| African American, % | 13.5 | 0 | >0.1 | |
| Clinical feature | CK, IU/l | 990 | 1270.1 | >0.1 |
| Aldolase serum, U/l | 17.3 | 16.3 | >0.1 | |
| Anti-nuclear antibody | 1123.8 | 32.7 | 0.0004 | |
| Anti-RNP antibody | 9.6 | 2.5 | >0.1 | |
| Anti-SSA antibody | 36.9 | 15.9 | >0.1 | |
| Anti-Jo-1 antibody | 29.1 | 11.7 | >0.1 | |
| MMT-8 | 116.4 | 127.1 | 0.06 | |
| MDAAT global disease activity | 4.2 | 4.0 | >0.1 | |
| MDAAT muscular disease activity | 4.3 | 4.2 | >0.1 | |
| MDAAT global other disease activity | 0.21 | 0.03 | >0.1 | |
| CLINHAQ disability index score | 1.4 | 1.3 | >0.1 | |
| CLINHAQ physical function score | 1.4 | 1.3 | >0.1 | |
| Medication, % (mean dose, mg) | AZA | 16.2 (112.5) | 36.4 (115) | >0.1 |
| Immunoglobulin | 27 (21.8) | 18.2 (51) | >0.1 | |
| Prednisone | 75.7 (17) | 72.7 (11.9) | >0.1 | |
| MTX | 40.5 (14.8) | 45.4 (17.1) | >0.1 |
CK: creatine kinase; MDAAT: Myositis Disease Activity Assessment Tool; CLINHAQ: Clinical Health Assessment Questionnaire.
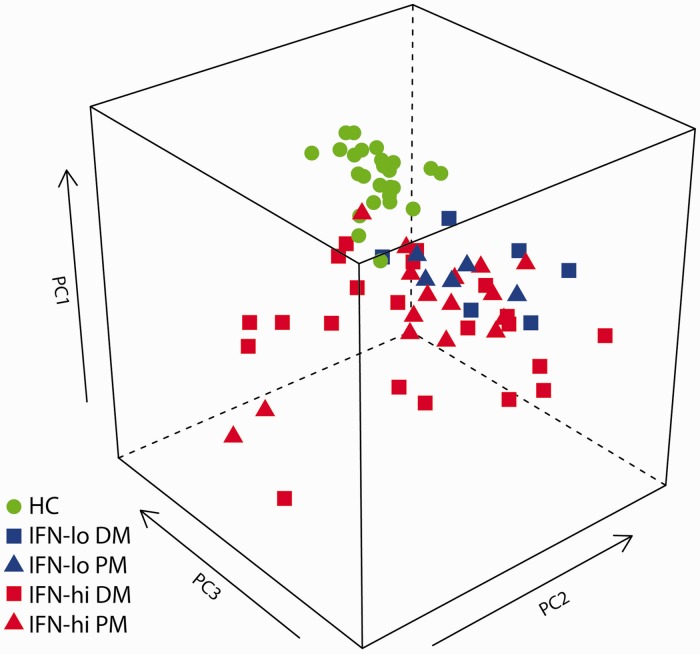
Of the 128 proteins evaluated, 28 negative analytes had serum levels below the LLOQ in >70% of all samples. No significant difference was found for the distributions of the 28 negative analytes among HCs and IFN-lo and IFN-hi patients. PCA of 100 positive analytes shows that HCs are clearly separated from myositis patients (Fig. 1), similar to what is observed in PCA space when using IFN-inducible genes measured in the blood of SLE subjects or normal HCs [13]. Interestingly, 11 IFN-lo patients seemed to group together next to the HCs, while IFN-hi patients deviated more from the HCs. In contrast, DM and PM patients were mixed with each other without a clear partitioning pattern.
Fig. 1.
Principal component analysis of HCs and myositis patients by serum protein levels
PCA was performed using serum levels of 100 proteins across 25 HCs and 37 DM or PM patients with high (IFN-hi) and 11 patients with low (IFN-lo) type I IFN gene signature scores at baseline.
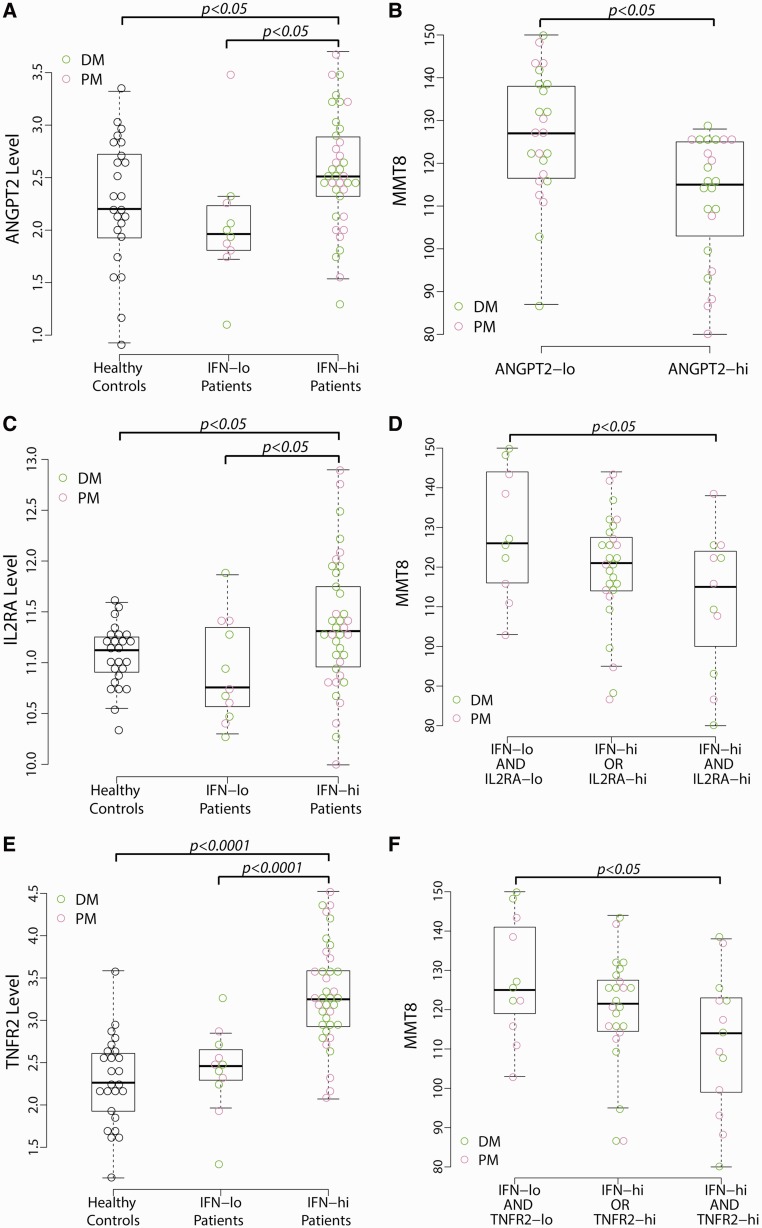
Next we identified dysregulated proteins in DM or PM patients in association with type I IFN status (see supplementary Table S1, available at Rheumatology Online). Protein levels were compared between HCs and all myositis patients. Forty-three proteins were significantly higher in DM or PM patients than HCs (BH P < 0.05). Then we used an ANOVA model to identify proteins with different levels among HCs and IFN-hi and IFN-lo myositis patients. Forty-two proteins demonstrated more than 1.5-fold higher median concentrations in IFN-hi patients than IFN-lo patients or HCs (BH P < 0.05). In total, by combing two lists of proteins identified above, 47 unique proteins demonstrated dysregulated serum levels in IFN-hi and/or all myositis patients, among which 15 are inducible by type I IFN [14]. Pairwise comparisons demonstrated that 23 proteins had significantly higher concentrations in IFN-hi (P < 0.05) but not IFN-lo patients compared with HCs, suggesting a unique proteomic feature of IFN-hi myositis patients. Twenty cytokines were up-regulated in our DM or PM patient cohort, many of which have been reported to be overexpressed in muscle tissue sections of IIMs, such as IL-18, monocyte chemoattractant protein 1 (MCP-1), and B-cell activating factor (BAFF) [15, 16]. Several soluble cytokine receptors and adhesion molecules [IL-2RA, TNF receptor 1 (TNFR1), TNFR2, and vascular cell adhesion molecule 1 (VCAM-1)] demonstrated elevated levels in DM or PM patients, which was consistent with previous reports [17–19]. Our results also indicated overexpression of ANGPT2 and soluble B2M, which has been reported in SLE and SSc, but not IIMs [20–22]. Elevated serum ANGPT2 levels were only seen in the subset of IFN-hi patients (Fig. 2A).
Fig. 2.
Dysregulated ANGPT2, IL-2RA and TNFR2 levels in myositis patients
(A) Serum angiopoietin-2 (ANGPT2) levels are higher in myositis patients with an elevated type I IFN signature score (IFN-hi) than in those without an elevated IFN signature (IFN-lo) and HCs. The y-axis represents the log2-transformed serum level of ANGPT2 (ng/ml). (B) Myositis patients with ANGPT2 levels higher than the median value (>5.4 ng/ml) show significantly lower MMT-8 scores than those patients with ANGPT2 levels less than the median value (≤5.4 ng/ml). (C) IFN-hi patients show significantly higher soluble IL-2 receptor (IL-2RA) levels than IFN-lo patients and HCs. The y-axis represents the log2-transformed serum levels of IL-2RA (pg/ml). (D) IFN-hi patients with IL-2RA levels higher than the upper limit of the normal range (2970 pg/ml) had significantly lower MMT-8 levels than IFN-lo patients with IL-2RA levels within the normal range. (E) Higher TNFR2 levels in IFN-hi patients than in IFN-lo patients and HCs. The y-axis represents the log2-transformed serum levels of TNFR2 (ng/ml). (F) IFN-hi patients with TNFR2 levels higher than the upper limit of the normal range (11 ng/ml) had significantly lower MMT-8 levels than IFN-lo patients with TNFR2 levels within the normal range.
Correlation of serum protein levels with clinical assessments in myositis patients
We assessed the association between the 47 dysregulated proteins in myositis patients with both MMT-8 scores and ANA levels using Spearman’s correlation analysis (Table 2). The association with anti-RNP, anti-SSA, anti-Jo-1 and anti-Mi-2 antibodies was not assessed since too few patients were positive for those autoantibodies. Interestingly, we found a significant correlation between serum ANGPT2 level and MMT-8 score, which has not been reported before in myositis patients. Higher ANGPT2 levels was associated with worse disease activity as determined by lower MMT-8 scores (ρ = −0.49; BH P < 0.05). When separating patients into two groups using the median value of serum ANGPT2 levels, a significant difference was observed for MMT-8 scores between these groups (Fig. 2B).
Table 2.
Correlation of serum protein levels with IFN activity and disease severity
| Blood IFN score | Muscle IFN score | ANA | MMT-8 | |
|---|---|---|---|---|
| CCL21 (6Ckine) | 0.50** | 0.18 | 0.51** | −0.01 |
| ANGPT2 | 0.39* | 0.34 | 0.28 | −0.49* |
| TNFSF13B (BAFF) | 0.48** | 0.36 | 0.42* | −0.08 |
| CXCL13 (BLC) | 0.50** | 0.53** | 0.40* | −0.05 |
| B2M | 0.64*** | 0.55** | 0.39* | 0.06 |
| IL18 | 0.48** | 0.29 | 0.39* | 0.19 |
| IL2RA | 0.48** | 0.33 | 0.54** | −0.12 |
| CCL8 (MCP2) | 0.64*** | 0.55** | 0.46* | −0.17 |
| TNFRSF1B (TNFR2) | 0.63*** | 0.42* | 0.47* | −0.12 |
| Blood IFN score | 0.47* | −0.16 | ||
| Muscle IFN score | 0.18 | −0.17 |
Correlations of serum protein levels with blood and muscle IFN gene signature scores, ANA levels and MMT-8 scores were assessed by Spearman’s correlation coefficients. Bold represents a type I IFN-inducible gene. *BH P < 0.05; **BH P < 0.01; ***BH P < 0.001.
Eight proteins demonstrated significant correlation with ANA levels, including four IFN-inducible proteins—IL-2RA, MCP-2, BAFF and B2M. All eight proteins and ANGPT2 were positively correlated with blood IFN signature score, four of which also correlated with muscle IFN score (Table 2). Type I IFN status, disease activity and serum levels of these proteins seem to be interrelated in myositis patients. It is possible that the combination of IFN signature score and serum protein level may be associated with clinical disease measures better than either marker alone. Thus we investigated whether those patients with elevated type I IFN status and higher serum levels of identified proteins had different MMT-8 scores from other patients. Three groups of patients were defined for each protein based on its normal range. One group of patients had both elevated blood IFN signatures and protein levels higher than the upper limit of the normal range for each individual protein. The second group showed either high IFN signatures or high protein levels, but not both. The third group had neither high IFN signatures nor high protein levels. Fig. 2D shows that IFN-hi patients with higher IL-2RA levels had significantly lower MMT-8 scores and thus worse clinical symptoms than patients with neither elevated IFN signatures nor higher levels of the protein marker. The MMT-8 scores for those patients with either higher IL-2RA or IFN scores were in between the previous two groups of patients. The same was true for TNFR2 (Fig. 2F). Both proteins were significantly higher in IFN-hi patients than IFN-lo patients and HCs (Fig. 2C and E). These results suggest the association of MMT-8 score with a combination of type I IFN signature and IL-2RA or TNFR2 levels in myositis patients.
Effects of an anti-IFN-α mAb on serum protein levels of DM or PM patients
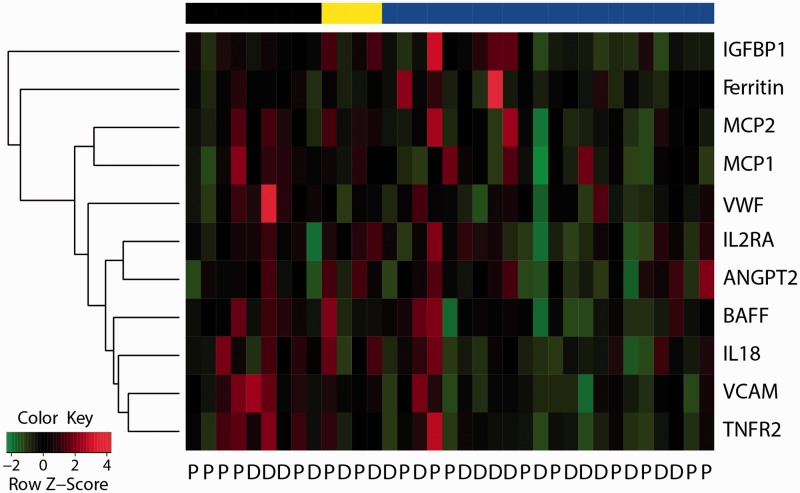
Post-treatment changes for all 47 dysregulated serum proteins in myositis patients were assessed by paired t-test. Eleven proteins demonstrated reduced serum levels by sifalimumab, while no protein showed a significant change in placebo-administered patients. Five of these proteins are inducible by type I IFN, including IL-2RA, MCP-1, MCP-2, BAFF and ferritin. A previous report demonstrated a trend of correlation among MMT-8 improvement, blood and muscle type I IFN target neutralization in patients with >20% target neutralization at day 98 [8]. As shown in Fig. 3, high protein suppression was observed mostly in sifalimumab-administered patients with >20% IFN target neutralization in blood or muscle, but less so in those with a low IFN signature score or <20% IFN target neutralization in blood. Nevertheless, a few IFN-hi patients with >20% target neutralization displayed an increase in those five IFN-inducible proteins. The discordance between mRNA and protein level regulation may reflect the complex post-transcriptional regulation, protein secretion, receptor shedding and negative feedback processes involved in serum proteome regulation.
Fig. 3.
Effects of sifalimumab administration on serum protein levels in myositis patients
Eleven proteins had significantly reduced levels at day 98 after administration of sifalimumab in comparison with pre-treatment by paired t-test (BH P < 0.05). Percentage of change at day 98 relative to pre-treatment was scaled to have a mean of 0 and a s.d. of 1 for each protein across sifalimumab-administered patients. The black bar on the top denotes IFN-lo patients. The yellow bar shows four IFN-hi patients with ≤20% IFN target neutralization rate in blood. The blue bar denotes 20 IFN-hi patients with >20% IFN target neutralization rate in blood and two IFN-hi patients with >20% IFN neutralization rate in muscle (missing blood data). The normalized value of protein change at day 98 was used to order 11 proteins by the complete hierarchical clustering method.
IL-2RA, IL-18, TNFR2, BAFF, MCP-1 and MCP-2 are involved in T cell regulation [23–27] and their baseline levels have been shown to correlate with type I IFN status and clinical activities of DM or PM patients (Table 2 and Fig. 2). All these proteins demonstrated significantly reduced levels after sifalimumab, but not placebo, administration (Fig. 3). Functional enrichment analysis [Database for Annotation, Visualization, and Integrated Discovery (DAVID)] [28] indicated that positive regulation of T cell proliferation and T cell activation are the only overrepresented gene ontology biological process terms among 11 sifalimumab-regulated proteins when using the 47 up-regulated proteins at baseline as the background gene list (P < 0.05). The coordinated down-regulation of T cell–associated proteins by sifalimumab suggests the link between blocking type I IFN signalling and suppression of T cell function.
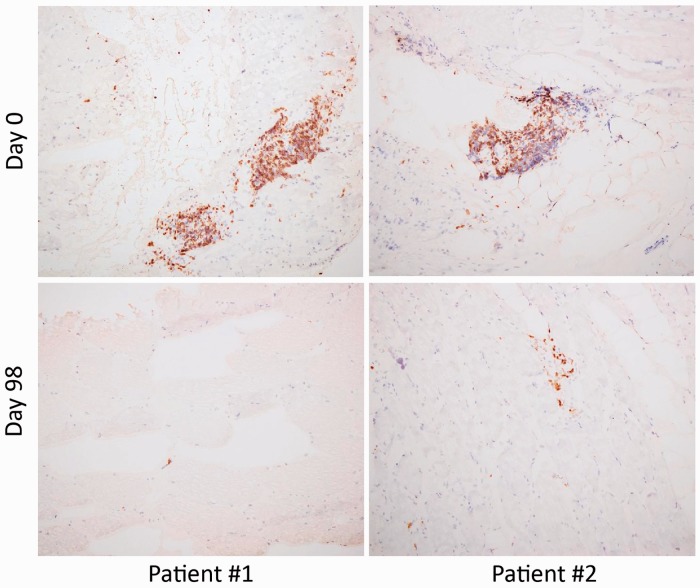
To investigate the effects of sifalimumab on muscle T cell infiltration, we used anti-CD3 antibody to stain muscle biopsies from two patients with the highest median suppression of T cell-associated proteins. Fig. 4 demonstrates pronounced reduction of CD3+ cells in muscle at day 98 after sifalimumab administration for those two patients. Serum levels of MCP-1 and MCP-2 were suppressed by 74.9% and 81.2%, respectively, for patient 1, and the suppression rates of two chemokines for patient 2 were 45.1% and 43.3%, respectively. Serum level reductions for IL-2RA, IL-18, TNFR2 and BAFF ranged from 30 to 55% for these two patients.
Fig. 4.
Suppression of muscle-infiltrating T cells by sifalimumab in myositis patients
Immunohistochemistry results with anti-CD3 antibody are shown for paired muscle biopsies at baseline and day 98 after administration of sifalimumab from two patients with the highest reduction of T cell-associated proteins. Pronounced decreases in CD3 staining are seen at day 98 in both patients.
Correlation between IL-2RA suppression and favourable MMT-8 alteration after sifalimumab administration
To evaluate the clinical relevance of serum proteins suppressed by sifalimumab, we assessed the association between protein level change and MMT-8 alteration in sifalimumab-administered patients. A significant correlation was observed between IL-2RA down-regulation at day 98 and MMT-8 up-regulation at day 196 post-administration (ρ = −0.39, P = 0.02). A > 15% improvement in MMT-8 score is considered a viable clinical endpoint for efficacy in DM and PM. Thus we classified patients into two groups: ≥15% (n = 13) and <15% improvement (n = 23) in MMT-8 at day 196 compared to pre-dose. IL-2RA change at day 98 after sifalimumab administration was significantly different between these two groups (Fig. 5A). On the other hand, MMT-8 improvement was higher in sifalimumab-administered patients with greater IL-2RA reduction than those with lesser IL-2RA reduction at the threshold from 0 to 40% in 5% increments. A significant difference in MMT-8 change was observed at the thresholds of 5%, 30% (Fig. 5B) and 35% reduction of IL-2RA at day 98. Our previous report demonstrated a correlative trend between type I IFN target neutralization and MMT-8 improvement at day 98 [8]. Here we assessed the association between the percentage of change in blood type I IFN score at day 98 and MMT-8 score at day 196 for all sifalimumab-administered patients. A trend of correlation was also evident among these two measurements (ρ = −0.31, P = 0.08).
Fig. 5.
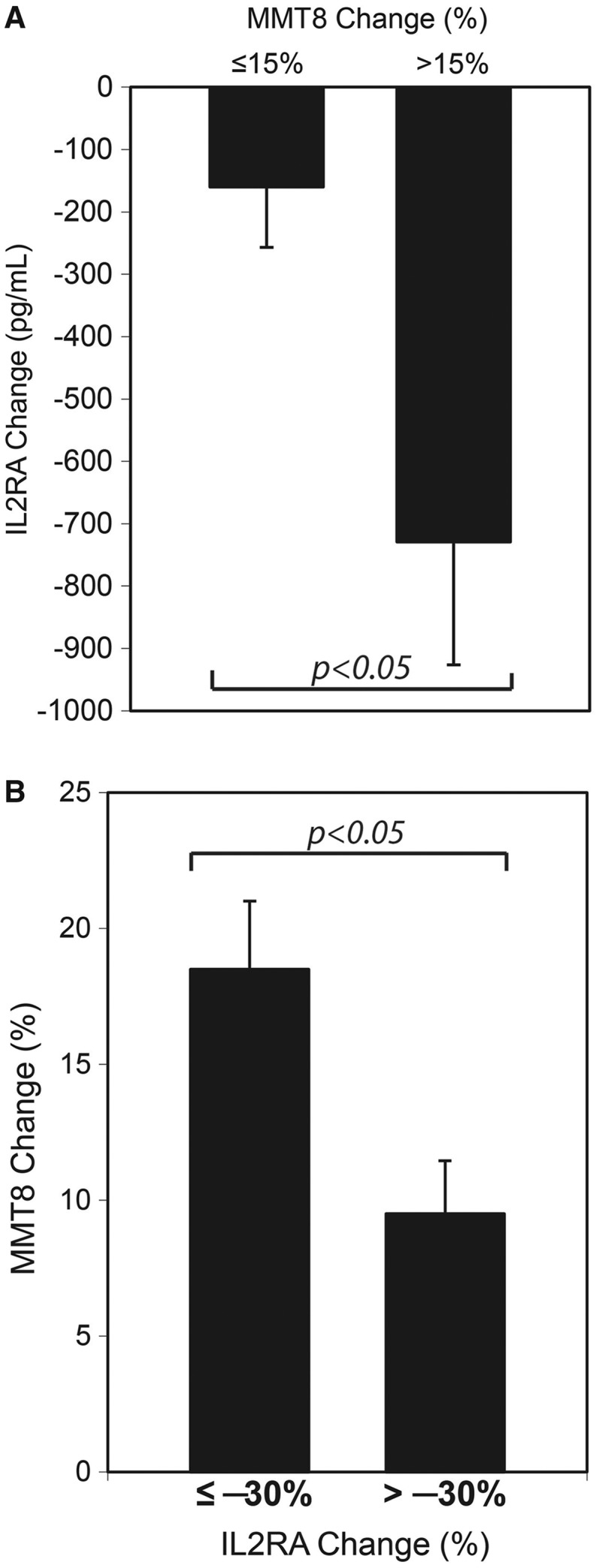
Association between soluble IL-2RA reduction and favourable MMT-8 alterations after sifalimumab administration in myositis patients
(A) Paired t-test shows that soluble IL-2 receptor (IL2RA) level at day 98 was significantly different from that at day 0 for patients with ≥15% MMT-8 improvement (n = 13; P < 0.05), but not those with <15% MMT-8 improvement (n = 23) at day 196 after administration of sifalimumab. The absolute change of IL-2RA level (pg/ml) at day 98 was also significantly different between two groups of patients with different MMT-8 improvement rates (P < 0.05). (B) MMT-8 level at day 196 was significantly different from that pre-treatment for patients with >30% reduction of IL-2RA level at day 98 (P < 0.001), but not those with <30% reduction of IL-2RA level at day 98 relative to pre-dose. Direct comparison of MMT-8 change between two groups of patients with different IL-2RA changes also showed a significant difference (P < 0.05).
Discussion
In this study we performed a survey of the serological proteome in DM and PM patients. Our results suggest a distinct proteome profile of IFN-hi patients compared with IFN-lo patients and HCs, while the global proteomics pattern seems to be more similar between DM and PM patients. Multiple proteins are elevated in IFN-hi patients only, but not IFN-lo patients compared with HCs. Overexpressed proteins in myositis patients are involved in various biological processes, including regulation of T cell proliferation and activation. The soluble form of IL-2RA is predominantly released by proteolytic cleavage from the activated T cell surface, mainly the Th1 subset of CD4+ lymphocytes [24]. TNFR2 plays an important co-stimulatory role for T cells, and the shedding of TNFR2 has been shown to be increased in patients with immunological disorders [29]. IL-18 is a Th1 cytokine that induces proliferation and differentiation of naive T cells. Previous reports have demonstrated overexpression of three T cell-associated proteins in the serum of IIM patients [15–18, 30–32]. BAFF, MCP-1 and MCP-2 are also involved in T cell regulation and chemotaxis, among other functions. Elevated BAFF expression in muscle tissue has been shown to be associated with an increased number of CD4+ T cells in DM [15].
Immunomodulatory functions of type I IFN include the control of Th1/Th2 function and the regulation of CD8+ cytotoxic T cell activity and memory. IFN-α has been shown to induce IL-12 receptor and IL-2RA gene expression in human T cells, thus promoting a Th1-type immune response [33, 34]. Increased serum soluble IL-2RA levels have also been reported following IFN-α therapy in patients with chronic hepatitis C [35]. Our results demonstrated that sifalimumab, but not placebo, administration reduced IL-2RA, TNFR2, IL-18, BAFF, MCP-1 and MCP-2 levels in adult DM or PM patients, which suggests a suppressive effect of type I IFN neutralization on T cell activation and/or chemoattraction that may reduce T cell infiltration in the muscle of myositis patients. CD3 staining results confirmed the suppression of muscle-infiltrating T cells in two selected patients with the highest down-regulation of T cell-associated proteins after administration of sifalimumab. Furthermore, IL-2RA levels correlate with disease activity at baseline and the post-treatment down-regulation correlates with favourable MMT-8 alteration in sifalimumab-administered patients. It is possible that IL-2RA and type I IFN gene signature changes from baseline may serve as a responsive and/or predictive marker of changes in disease activity for IFN-targeted therapy in DM or PM patients. The predictive value of IL-2RA and/or type I IFN gene signatures needs to be confirmed in a larger future study with sufficient statistical power as well as reliability testing in the evaluation of MMT-8 or other clinical measures of response. Serum samples should also be tested prior to day 98 to enable identification of earlier association between biomarker levels and clinical measures of response.
A previous gene expression study has demonstrated multiple biological pathways that were significantly suppressed following administration of sifalimumab in muscle specimens from DM or PM patients [8]. The top 10 most enriched pathways include the IL-2 signalling pathway, integrin signalling pathway and four T cell-associated pathways such as iCOS-iCOSL signalling in T helper cells and CTLA4 signalling in cytotoxic T lymphocytes. In addition to T cell-associated effects, sifalimumab suppressed expression of transcripts involved in B cell development, phagocytosis in macrophages and monocytes in muscle specimen of myositis patients. The neutralization of a leucocyte index, MHC class I and immunoglobulin signature was significantly correlated with target neutralization of the IFN gene signature [8]. These effects of sifalimumab in disease tissue may also be related to cytokine changes we have observed in this study. Most cytokines are pleiotropic and affect many different immune cells and inflammatory pathways. MCP-1 and MCP-2 recruit T cells, dendritic cells, monocytes and various other immune cells to the sites of inflammation. MCP-1 was significantly up-regulated in DM and PM muscle [36], and its main receptor, CCR2, has been detected in macrophages and T cells of DM and PM patients [37]. BAFF acts as a potent B cell activator, and its expression was increased along with an increased number of CD19+ B cells and CD4+ T cells in the muscle tissue of DM patients [15]. Thus suppression of MCP-1, MCP-2 and BAFF by sifalimumab may be partially responsible for neutralization of the leucocyte index, immunoglobulin signature and other pathways following neutralization of the type I IFN pathway in myositis patients.
The Tie2 receptor antagonist, ANGPT2, is a key mediator of endothelial cell activation. High serum ANGPT2 levels have been associated with greater severity and higher activity of SLE and SSc [21, 22]. To our knowledge there has not been any report showing the relevance of ANGPT2 in myositis patients. Von Willebrand factor (VWF) is secreted by endothelial cells, and increased levels in the circulation is presumed to be a marker for endothelial perturbation [38]. VCAM-1 is a cell adhesion molecule that is expressed on blood vessels only after the endothelial cells are stimulated by cytokines. All three proteins can bind to integrins expressed in various types of leucocytes, resulting in adhesion and transmigration of inflammatory cells into multiple organs [39, 40]. Our results demonstrated elevated serum VWF and VCAM-1 levels in myositis patients, which is consistent with previous reports [19, 41]. Moreover, we discovered the association of elevated serum ANGPT2 levels with myositis disease activity for the first time. Sifalimumab administration reduced expression of all three endothelium-related proteins, suggesting endothelial cell suppression by type I IFN neutralization through which lymphocyte infiltration may be affected.
Our results demonstrate a robust overexpression of multiple serum proteins in DM and PM patients, especially those with an elevated type I IFN gene signature at baseline. Sifalimumab but not placebo administration resulted in coordinated suppression of multiple cytokines, adhesion molecules and soluble receptors associated with immune cell activation and movement. In particular, IL-2RA down-regulation correlated with favourable MMT-8 alteration in sifalimumab-administered patients. Together with T cell staining and gene expression profile findings in muscle tissue, our results suggest suppression of activated T cell infiltration into the muscle of myositis patients following inhibition of type I IFN signalling. The link between IL-2RA suppression and favourable MMT-8 alteration suggests the potential of IL-2RA changes from baseline as a responsive and/or predictive marker of changes in disease activity for IFN-targeted therapies in DM and PM patients. Further studies will be needed to test this hypothesis as well as to more extensively investigate the impact of IFN blockade on immune cell infiltration in the muscle of IIM patients.
Rheumatology key messages.
Sifalimumab suppressed expression of multiple T cell-associated proteins in myositis patients.
Soluble IL-2 receptor changes from baseline correlated with favourable MMT-8 alterations in sifalimumab-dosed DM or PM patients.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Anmarie Boutrin for performing immunostaining of the muscle biopsies, Chris Morehouse for providing genomics data and Jichao Sun for providing the tables and listings of the clinical data. They wish to thank Sek Fung, Gabriel Robbie, Stephen Yoo and Micki Hultquist for helping in the review of this manuscript.
Funding: Funding for this study was received from MedImmune.
Disclosure statement: All authors are full-time employees of MedImmune and have stock in AstraZeneca.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Dalakas MC. Review: an update on inflammatory and autoimmune myopathies. Neuropathol Appl Neurobiol. 2011;37:226–42. doi: 10.1111/j.1365-2990.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SA. Type 1 interferons and myositis. Arthritis Res Ther. 2010;12(Suppl 1):S4. doi: 10.1186/ar2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg SA, Higgs BW, Morehouse C, et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun. 2011;13:207–13. doi: 10.1038/gene.2011.61. [DOI] [PubMed] [Google Scholar]
- 4.Bilgic H, Ytterberg SR, Amin S, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60:3436–46. doi: 10.1002/art.24936. [DOI] [PubMed] [Google Scholar]
- 5.Hengstman GJ, Vogels OJ, ter Laak HJ, et al. Myositis during long-term interferon-alpha treatment. Neurology. 2000;54:2186. doi: 10.1212/wnl.54.11.2186. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SA, Pinkus JL, Pinkus GS, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57:664–78. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- 7.Isenberg DA, Rowe D, Shearer M, et al. Localization of interferons and interleukin 2 in polymyositis and muscular dystrophy. Clin Exp Immunol. 1986;63:450–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Higgs BW, Zhu W, Morehouse C, et al. A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-alpha monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- 10.Salajegheh M, Kong SW, Pinkus JL, et al. Interferon-stimulated gene 15 (ISG15) conjugates proteins in dermatomyositis muscle with perifascicular atrophy. Ann Neurol. 2010;67:53–63. doi: 10.1002/ana.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenzel J, Schmidt R, Proelss J, et al. Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol. 2006;31:576–82. doi: 10.1111/j.1365-2230.2006.02150.x. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Higgs BW, Morehouse C, et al. Development of potential pharmacodynamic and diagnostic markers for anti-IFN-alpha monoclonal antibody trials in systemic lupus erythematosus. Hum Genomics Proteomics. 2009;2009:374312. doi: 10.4061/2009/374312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samarajiwa SA, Forster S, Auchettl K, et al. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res. 2009;37:D852–7. doi: 10.1093/nar/gkn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baek A, Park HJ, Na SJ, et al. The expression of BAFF in the muscles of patients with dermatomyositis. J Neuroimmunol. 2012;249:96–100. doi: 10.1016/j.jneuroim.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 16.De Paepe B, Creus KK, De Bleecker JL. Role of cytokines and chemokines in idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2009;21:610–6. doi: 10.1097/BOR.0b013e3283317b31. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi I, Ono S, Kawamura N, et al. Elevated serum levels of soluble interleukin-2 receptor in juvenile dermatomyositis. Pediatr Int. 2001;43:109–11. doi: 10.1046/j.1442-200x.2001.01367.x. [DOI] [PubMed] [Google Scholar]
- 18.Mielnik P, Chwalinska-Sadowska H, Wiesik-Szewczyk E, et al. Serum concentration of interleukin 15, interleukin 2 receptor and TNF receptor in patients with polymyositis and dermatomyositis: correlation to disease activity. Rheumatol Int. 2012;32:639–43. doi: 10.1007/s00296-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain A, Sharma MC, Sarkar C, et al. Increased expression of cell adhesion molecules in inflammatory myopathies: diagnostic utility and pathogenetic insights. Folia Neuropathol. 2009;47:33–42. [PubMed] [Google Scholar]
- 20.Hermansen ML, Hummelshoj L, Lundsgaard D, et al. Increased serum beta2-microglobulin is associated with clinical and immunological markers of disease activity in systemic lupus erythematosus patients. Lupus. 2012;21:1098–104. doi: 10.1177/0961203312447668. [DOI] [PubMed] [Google Scholar]
- 21.Michalska-Jakubus M, Kowal-Bielecka O, Chodorowska G, et al. Angiopoietins-1 and -2 are differentially expressed in the sera of patients with systemic sclerosis: high angiopoietin-2 levels are associated with greater severity and higher activity of the disease. Rheumatology. 2011;50:746–55. doi: 10.1093/rheumatology/keq392. [DOI] [PubMed] [Google Scholar]
- 22.Kumpers P, David S, Haubitz M, et al. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis. 2009;68:163843. doi: 10.1136/ard.2008.094664. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Hamano R, Subleski JJ, et al. Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3− conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J Immunol. 2010;185:174–82. doi: 10.4049/jimmunol.0903548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caruso C, Candore G, Cigna D, et al. Biological significance of soluble IL-2 receptor. Mediators Inflamm. 1993;2:3–21. doi: 10.1155/S0962935193000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol. 2004;173:4500–9. doi: 10.4049/jimmunol.173.7.4500. [DOI] [PubMed] [Google Scholar]
- 26.Mackay F, Leung H. The role of the BAFF/APRIL system on T cell function. Semin Immunol. 2006;18:284–9. doi: 10.1016/j.smim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Weninger W, Carlsen HS, Goodarzi M, et al. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–48. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Jurewicz AM, Walczak AK, Selmaj KW. Shedding of TNF receptors in multiple sclerosis patients. Neurology. 1999;53:1409–14. doi: 10.1212/wnl.53.7.1409. [DOI] [PubMed] [Google Scholar]
- 30.Tokano Y, Kanai Y, Hashimoto H, et al. Soluble interleukin 2 receptors in patients with polymyositis/dermatomyositis. Ann Rheum Dis. 1992;51:781–2. doi: 10.1136/ard.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucci M, Quatraro C, Dammacco F, et al. Interleukin-18 overexpression as a hallmark of the activity of autoimmune inflammatory myopathies. Clin Exp Immunol. 2006;146:21–31. doi: 10.1111/j.1365-2249.2006.03180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabay C, Gay-Croisier F, Roux-Lombard P, et al. Elevated serum levels of interleukin-1 receptor antagonist in polymyositis/dermatomyositis. A biologic marker of disease activity with a possible role in the lack of acute-phase protein response. Arthritis Rheum. 1994;37:1744–51. doi: 10.1002/art.1780371206. [DOI] [PubMed] [Google Scholar]
- 33.Matikainen S, Sareneva T, Ronni T, et al. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood. 1999;93:1980–91. [PubMed] [Google Scholar]
- 34.Wenner CA, Guler ML, Macatonia SE, et al. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–7. [PubMed] [Google Scholar]
- 35.Sugimura T, Motomura S, Sakai H, et al. Increased serum soluble IL-2 receptor levels following interferon therapy in patients with chronic hepatitis C. Hepatogastroenterology. 1999;46:1827–30. [PubMed] [Google Scholar]
- 36.Liprandi A, Bartoli C, Figarella-Branger D, et al. Local expression of monocyte chemoattractant protein-1 (MCP-1) in idiopathic inflammatory myopathies. Acta Neuropathol. 1999;97:642–8. doi: 10.1007/s004010051041. [DOI] [PubMed] [Google Scholar]
- 37.De Paepe B, De Bleecker JL. Beta-chemokine receptor expression in idiopathic inflammatory myopathies. Muscle Nerve. 2005;31:621–7. doi: 10.1002/mus.20294. [DOI] [PubMed] [Google Scholar]
- 38.Mannucci PM. von Willebrand factor: a marker of endothelial damage? Arterioscler Thromb Vasc Biol. 1998;18:1359–62. doi: 10.1161/01.atv.18.9.1359. [DOI] [PubMed] [Google Scholar]
- 39.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:487–95. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholz A, Lang V, Henschler R, Czabanka M, et al. Angiopoietin-2 promotes myeloid cell infiltration in a beta-integrin-dependent manner. Blood. 2011;118:5050–9. doi: 10.1182/blood-2011-03-343293. [DOI] [PubMed] [Google Scholar]
- 41.Bloom BJ, Tucker LB, Miller LC, et al. von Willebrand factor in juvenile dermatomyositis. J Rheumatol. 1995;22:320–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.