Abstract
Background and Purpose
Matrix metalloproteinase-9 (MMP-9) is elevated in acute stroke patients who later develop hemorrhagic transformation (HT). It is controversial whether early fluid-attenuated inversion recovery (FLAIR) hyperintensity on brain MRI predicts hemorrhagic transformation (HT). We assessed whether FLAIR hyperintensity was associated with MMP-9 and HT.
Methods
We analyzed a prospectively collected cohort of acute stroke subjects with acute brain MRI images and MMP-9 values within the first 12 hours after stroke onset. FLAIR hyperintensity was measured using a signal intensity ratio between the stroke lesion and corresponding normal contralateral hemisphere. MMP-9 was measured using ELISA. The relationships between FLAIR ratio (FR), MMP-9 and HT were evaluated.
Results
180 subjects were available for analysis. Patients were imaged with brain MRI at 5.6 ± 4.3 hours from last seen well time. MMP-9 blood samples were drawn within 7.7 ± 4.0 hours from last seen well time. The time to MRI (r=0.17, p=0.027) and MMP-9 level (r=0.29, p<0.001) were each associated with FR. The association between MMP-9 and FR remained significant after multivariable adjustment (p<0.001). FR was also associated with HT and symptomatic hemorrhage (p=0.012).
Conclusions
FLAIR ratio correlates with both MMP-9 level and risk of hemorrhage. FLAIR changes in the acute phase of stroke may predict hemorrhagic transformation, possibly as a reflection of altered blood-brain barrier integrity.
Keywords: stroke, brain MRI, matrix metalloproteinase, hemorrhagic transformation
Introduction
T2 fluid-attenuated inversion recovery (FLAIR) hyperintensity has been associated with symptomatic intracerebral hemorrhage (sICH) in several studies,1-3 however this finding has not been substantiated in some reports.4 The underlying basis for the increased T2 signal that comprises FLAIR hypertensity is poorly understood, but may be secondary to vasogenic edema in the setting of blood brain barrier (BBB) breakdown.5 The extent of BBB breakdown may determine the development of hemorrhagic transformation (HT).
Matrix metalloproteinase 9 (MMP-9) is a member of a family of zinc-dependent enzymes that compromises BBB integrity by degrading components of the tight junctions and extracellular matrix, and is thereby implicated in the development of cerebral edema and HT.6, 7 Increased MMP-9 levels are associated with risk of HT in acute ischemic stroke in both experimental models and human subjects, both with and without thrombolysis.8-10
Despite the potential connection to BBB dysfunction, a relationship between MMP-9 and FLAIR hyperintensity has not previously been reported. We hypothesized that FLAIR hyperintensity may serve as an imaging biomarker that reflects altered BBB integrity. The aim of this study was to assess the relationship between FLAIR hyperintensity, MMP and HT after ischemic stroke. We also sought to develop a rapid bedside method for evaluating quantitative FLAIR ratio as a marker for assessing the risk for HT.
Methods
Patients
Subjects for this study participated in a prospective two-center biomarker study of acute ischemic stroke as part of the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) Network. The SPOTRIAS biomarker study enrolled consecutive patients ≥18 years between January 2007 and April 2010, who presented within 9 hours of symptom onset, and with symptoms consistent with ischemic stroke. For the current analysis, patients without a DWI lesion or a lesion volume < 5mL, patients who did not have a T2 fluid-attenuated inversion recovery (FLAIR) sequence available for analysis or with poor technical quality, and those patients who did not have MMP levels were also excluded. Patients with stroke volume <5 mL were excluded due to the technical limitation of accurately measuring a FLAIR value in stroke lesions that did not span more than one axial slice, leading to the risk of volume averaging artifacts. Both subjects with and without intravenous thrombolysis were included since HT is a potential complication in all ischemic stroke.10 No subjects were treated with endovascular thrombolysis. All subjects or their healthcare proxy provided informed consent, and this study was approved by the local institutional review board.
Imaging Analysis
Imaging analyses were performed by trained readers blinded to all clinical and MMP data. The goal was to develop a reliable quantitative estimate of relative FLAIR hyperintensity that could be performed at the bedside. Using standard clinical imaging viewing software, eight regions of interests (ROI) within the stroke lesion were outlined on the T2 FLAIR sequence using the following rule: 2 gray matter ROIs and 2 white matter ROIs each on 2 contiguous slices. The ROIs were mirrored to a similar location on the contralateral hemisphere and the final FR was the average of these eight values (Figure 1). After validating this method against a gold standard (AnalyzeDirect 11.0, Supplementary Material),4, 11 we used it for the final analysis.
Figure 1. Representative example of the method for generating FLAIR ratio.
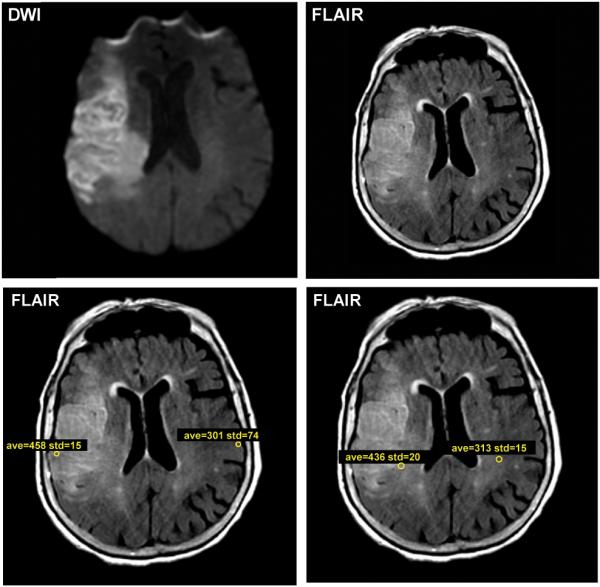
The FLAIR image was aligned with the DWI sequence. Within the stroke lesion on two contiguous slices, regions of interest (ROIs) in the ischemic white matter of the FLAIR sequence were drawn, and ROIs were mirrored onto the contralateral normal white matter (lower lefthand panel). A similar approach was used for gray matter (lower righthand panel). The final FLAIR ratio was calculated as the mean of all lesional FLAIR values to all contralateral FLAIR values, with a final value of 1.53 in this subject.
Two stroke neurologists (R.J. and W.T.K.) independently classified hemorrhagic transformation and symptomatic hemorrhage (sICH) using the European Cooperative Acute Stroke Study (ECASS) III criteria12 based on follow-up CT scan (n=119) or MRI (n=25) on imaging studies obtained 1.6 ± 1.9 days after stroke onset. All cases were adjudicated by consensus. Four subjects had hemorrhage identified on MRI only, which is reported to be more sensitive than CT.13 Therefore, we also performed analyses with and without the subjects who had HT designated on MRI only.
MMP Analysis
Peripheral blood samples were collected in EDTA-containing tubes and plasma was separated from cellular material by centrifugation (1000×g for 15 min) within 60 min of collection. Supernatant was aliquoted into cryo vials and frozen at −70°C (EDTA plasma) until analysis. MMP-9 and MMP-2 analysis was performed using a commercially available ELISA (R&D systems), according to the manufacturer’s instruction. The mean coefficient of variation for these assays is <5%.
Statistical Analysis
Descriptive statistics of baseline variables were performed, and reported as mean ± standard deviation (for normally-distributed continuous data), median with interquartile range (IQR; for non-normal data) and proportions for binary data. Differences in continuous variables were compared using Student’s t test, Wilcoxon rank-sum test or ANOVA, as appropriate. Categorical variables were compared using Fischer’s exact test or Chi-square test. Multivariate logistic regression models were developed with all variables with a univariate p value of <0.20 to determine the independent effects that were associated with FR and HT. Statistical significance was taken at a two-sided p value of <0.05. Statistical analyses were performed with JMP Pro 10 software.
Results
The patient characteristics of the study cohort are shown in Table 1. The original cohort was designed to enroll both stroke and stroke mimics. Of 522 subjects, baseline MMP-9 values were available for 448 subjects. 50 subjects did not have an acute brain MRI performed, 68 subjects did not have an acute lesion visible on imaging, 29 did not have a T2 FLAIR sequence and 151 subjects had a lesion volume less than 5 mL. The final FR cohort consisted of 180 subjects with an evaluable T2 FLAIR sequence and available MMP-9. There were no baseline differences in the demographics between the original cohort and that used for the final analysis, except for stroke severity (Table 1). Accordingly, the FR cohort had a higher NIH stroke scale (NIHSS) score and a higher acute stroke volume (p<0.01) compared to the original cohort, both of which were expected based on the selection criteria. The mean FR was 1.40 ± 0.23 obtained from acute brain MRI that occurred at 5.6 ± 4.3 hours from the last seen well time. On average, MMP-9 blood samples were collected 7.7 ± 4.0 hours after the last seen well time.
Table 1. Characteristics of the patients.
| Entire cohort | Flair Ratio cohort | P value | |
|---|---|---|---|
| n = 522 | n = 180 | ||
| Age (years), mean ± SD | 69.9 ± 15.2 | 70.2 ± 14.2 | 0.75 |
| Sex (female, %) | 229 (44%) | 74 (41%) | 0.54 |
| Comorbidities (%) | |||
| Diabetes | 122 (23%) | 37 (21%) | 0.47 |
| Hypertension | 380 (73%) | 130 (72%) | 0.85 |
| Hyperlipidemia | 255 (49%) | 89 (49%) | 0.93 |
| Coronary Artery Disease | 143 (28%) | 44 (24%) | 0.49 |
| Atrial fibrillation | 165 (32%) | 65 (36%) | 0.31 |
| Admission NIHSS, median [IQR] | 6 [3, 13] | 10 [5, 16] | <0.01 |
| IV tPA (%) | 196 (43%) | 77 (51%) | 0.13 |
| Admission glucose, median [IQR] | 121 [104, 145] | 123 [106, 149] | 0.36 |
| Hemorrhagic Transformation (%) | 65 (19%) | 29 (20%) | 0.80 |
| Time from LSW to blood draw (hrs), mean ± SD | 7.4 ± 3.7 | 7.7 ± 4.0 | 0.28 |
| MMP-9 level (ng/ml), median [IQR] | 173 [98, 309] | 200 [110, 322] | 0.15 |
| Time from LSW to MRI (hrs), mean ± SD | - | 5.6 ± 4.3 | |
| Time between MRI and blood draw (hrs), mean ± SD | - | 2.9 ± 4.6 | |
| DWI volume (mL), median [IQR] | 5 [1, 21] | 21 [10, 50] | <0.01 |
| FLAIR Ratio, mean ± SD | - | 1.40 ± 0.23 |
SD denotes standard deviation, IQR interquartile range, LSW last seen well, DWI diffusion-weighted image, FLAIR fluid-attenuated inversion recovery.
FLAIR ratio and MMP-9
Univariate associations with FR hyperintensity are shown in Table 2 (lefthand column). The time to MRI (Pearson r=0.17, p=0.027) and MMP-9 level (Pearson r=0.29, p<0.001 and Supplemental Figure I) were both associated with FR, but age, sex, intravenous (IV) tissue plasminogen activator (tPA) treatment and DWI volume were not. We also found that MMP-9 elevation was associated with DWI volume (Pearson r=0.15, p=0.002) and IV tPA treatment (p=0.012),8, 10, 14, 15 but not with the time from stroke onset to the blood draw (p=0.74). On the other hand, MMP-2, a related gelatinase that may also be upregulated in stroke,16, 17 did not demonstrate an association with FR (Pearson r=-0.06, p=0.42 and Table 2).
Table 2. Univariate and Multivariate factors associated with FLAIR ratio hyperintensity.
| Univariate analysis of FLAIR ratio | Multivariate analysis of FLAIR ratio Adjusted |
|||||
|---|---|---|---|---|---|---|
| β coefficient* | 95% CI | P value | β coefficient* | 95% CI | P value | |
|
|
|
|||||
| Age | 0.001 | (−0.002 to 0.003) | 0.69 | -- | -- | |
| Sex (female) | −0.011 | (−0.045 to 0.023) | 0.52 | -- | -- | |
| IV tPA treatment (Y) | 0.004 | (−0.029 to 0.037 | 0.82 | -- | -- | |
| Time from last seen well to MRI (hrs) | 0.009 | (0.001 to 0.016) | 0.027 | 0.010 | (0.003 to 0.017) | 0.01 |
| DWI volume | −0.002 | (−0.077 to 0.073) | 0.95 | -- | -- | |
| MMP-2 | −0.078 | (−0.270 to 0.114) | 0.42 | -- | -- | |
| MMP-9 | 0.155 | (0.078 to 0.232) | <0.001 | 0.162 | (0.086 to 0.238) | <0.001 |
The β coefficient is the magnitude change in FLAIR ratio per unit or category. IV tPA denotes intravenous tissue plasminogen activator. DWI, diffusion weighted image. MMP, matrix metalloproteinase.
Multivariate linear regression confirmed that MMP-9 was independently associated with FR hyperintensity (p<0.001, Table 2 righthand column). Time from last seen well to MRI also remained significant (p=0.01). The inclusion of additional non-significant univariate factors in the model as potential confounders, including thrombolytic therapy, did not alter the independent association of MMP-9 with FR (p<0.001).
FLAIR Ratio and Hemorrhagic Transformation
Prior studies have highlighted that pre-treatment MMP-9 is associated with HT and symptomatic hemorrhage (sICH).10, 14, 18 Given the association of MMP-9 and FR, we hypothesized that FR may also correlate directly with HT and sICH. In our cohort, 29 patients exhibited HT (25 with hemorrhagic infarction and 4 with parenchymal hematomas). Figure 2A demonstrates that acute FR was elevated in those subjects who subsequently developed HT (p=0.013). Because not all HT may be clinically relevant,19 we also evaluated the association of FR with asymptomatic HT compared to sICH. Figure 2B demonstrates a stepwise increase in FR in patients with no hemorrhage (1.38 ± 0.22, n=115), compared to those with asymptomatic HT (1.49 ± 0.27, n=26) and those with sICH (1.68 ± 0.21, n=3) (ANOVA, p=0.012).
Figure 2. FLAIR ratio hyperintensity predicts symptomatic hemorrhage.
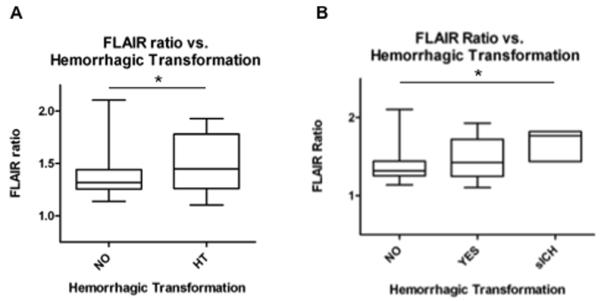
A) FLAIR ratio is elevated in subjects who subsequently develop hemorrhagic transformation (HT) (p=0.013). HT designations were based on the ECASS definition. B) FLAIR ratio also demonstrates a stepwise association between subjects with no hemorrhage, asymptomatic HT (aHT) and sICH (ANOVA, p=0.012). sICH was defined by the ECASS III definition.
We next evaluated previously reported predictors of HT in univariate logistic regression analysis (Table 3, lefthand column).20 Older age and increasing FR were associated with HT, Admission NIHSS and IV tPA treatment demonstrated a trend towards increased risk of HT in the univariate analysis. MMP-9 level, admission blood glucose and DWI volume did not. Additionally, a history of hypertension, diabetes or stroke subtype were not associated with risk of HT (p=0.36, p=0.13, p=0.41, respectively). Multivariate adjustment confirmed the independent effect of FR on HT (p=0.014, Table 3, righthand column). In this multivariate model, IV tPA was also a significant predictor of HT (p=0.018). The independent effect of FR (p=0.014) remained regardless of which variables were included in the model.
Table 3. Univariate and Multivariate predictors of hemorrhagic transformation.
| Univariate analysis of HT | Multivariate analysis of HT | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | Adjusted OR | 95% CI | P Value | |
|
|
|
|||||
| Age | 1.03 | 1.00-1.07 | 0.03 | 1.04 | 1.0-1.1 | 0.07 |
| Admission NIHSS | 1.06 | 0.99-1.12 | 0.06 | 1.07 | 0.98-1.18 | 0.14 |
| IV tPA treatment (Y) | 2.06 | 0.80-5.34 | 0.13 | 4.32 | 1.32-15.5 | 0.018 |
| MMP-9 | 1.48 | 0.58-3.69 | 0.40 | - | - | - |
| Admission glucose | 5.79 | 0.58-54.8 | 0.13 | 1.004 | 0.98-1.006 | 0.48 |
| DWI volume | 1.9 | 0.74-4.91 | 0.18 | 1.04 | 0.24-4.38 | 0.96 |
| FLAIR ratio | 7.97 | 1.56-47.4 | 0.013 | 19.9 | 1.92-242.9 | 0.014 |
Four patients were designated to have HT based solely on MRI: two had HI1 and the other two had HI2. Excluding these patients from the univariate analysis yielded similar results (see Supplementary Material). Multivariate analysis of CT-defined HT demonstrated that FR again remained an independent predictor of HT (adjusted OR 49, p=0.002) as did IV tPA treatment (adjusted OR 5.3, p=0.008) (see Supplemental Table I). Inspection of the receiver operator characteristic curve identified the optimal FR threshold of 1.54 for the prediction of HT (p=0.01). With this threshold, the sensitivity for HT was 0.48, the specificity 0.85, with a positive predictive value of 0.45 and a negative predictive value of 0.87.
Discussion
The blood-brain barrier (BBB) is susceptible to dysfunction in the setting of ischemia, which is partly mediated by matrix metalloproteinases such as MMP-9. MMP-9 degrades the basal lamina and when the disruption is severe enough, contributes to HT.21, 22 The underlying physiologic basis for FLAIR hyperintensity is poorly understood. Several theories have been explored including a blood oxygen-level dependent effect, temperature and viscosity, although the anticipated influence on T2 prolongation for these factors is minor.23, 24 More commonly, T2 prolongation is observed hours after stroke and is thought to be due to increasing water content in the ischemic tissue, representing vasogenic edema.5, 25
While our analysis does not resolve the biophysical basis for FLAIR hyperintensity, nor supports a causal relationship between MMP-9 and FR, the correlation between the two variables points toward a common association with BBB integrity. Our study has three primary findings that cumulatively strengthen the hypothesis that FLAIR hyperintensity is associated with impairment of BBB integrity: 1) a higher FR is correlated with elevated MMP-9 levels, 2) elevated FR is correlated with HT, and 3) there is a progressive increase in FR along the spectrum of patients without HT, asymptomatic HT and sICH. This proposed model suggests that vasogenic edema and HT represent a spectrum of BBB impairment, and that FLAIR hyperintensity is correlated (either directly or indirectly) with this phenomenon.
Some of the previously reported predictors of HT were not replicated in our cohort, including DWI volume and MMP-9 level. Differences in the cohort design and the timing of the blood sampling may account for these discrepancies. Large DWI volumes in the setting of thrombolysis are associated with increased risk of HT.26 Our cohort consisted of few patients with this size stroke, although all three patients with stroke lesions >100mL who developed HT were also treated with IV thrombolysis. Intriguingly, MMP-9 level did not predict HT in our cohort. However, prior studies reporting this association measured pre-treatment MMP-9 at very early time points (0-3 hours).14 In contrast, our cohort contained patients with MMP-9 blood samples drawn after IV tPA treatment, which itself can influence MMP-9 level.8, 27 Moreover, our cohort also included patients that did not receive thrombolysis which could further account for the discrepant findings.
Nevertheless, our data demonstrate that FR is associated with HT, which occurs in the setting of extensive physical breakdown of the BBB, allowing erythrocytes to extravasate into the brain parenchyma.27 Several studies have reported a similar finding,1-3 although this has not been replicated in every study.4 Differences in the timing of MRI, qualitative versus quantitative measurement of FLAIR hyperintensity, and/or differences in the definition of hemorrhagic transformation may account for the discrepancy. For example, 38% of patients in our cohort were imaged >6 hours from last seen well time, which is in contrast to earlier imaging time points in other studies.4 It is also important to note that HT and sICH are uncommon, and the relatively small sample sizes in prior studies may have limited the power to detect an association. Whether or not FLAIR hyperintensity could be ultimately used to guide thrombolytic treatment is uncertain. Future prospective studies with a larger sample size would be necessary to definitively establish a relationship between FR and sICH. Nevetheless, patients with elevated FR may warrant closer observation for subsequent HT.
Given our finding that FLAIR hyperintensity reflects circulating MMP-9 level, FR may also serve as a useful imaging biomarker for future clinical trials designed to prevent HT. In this context, lower FLAIR ratio and MMP-9 were both observed in an exploratory analysis in the GAMES-Pilot trial,11 a finding that is concordant with animal and human retrospective studies.27, 28 Although a double-blind, placebo-controlled trial is required to validate any candidate in preventing HT, our current work highlights the potential utility of imaging biomarkers for evaluating a candidate pharmaceutical agent.
The strengths of this study include a systematic collection of consecutive stroke patients from two centers. FLAIR hyperintensity was measured quantitatively through a rapid bedside method, rather than qualitatively.29 This is also the largest cohort to our knowledge with imaging and plasma sampling obtained within an average of 2.9 hours from each other.
There are several limitations. This was a retrospective analysis and in spite of the relatively large sample size, the number of patients with sICH was low. Our findings may not be generalizable to all stroke patients since we excluded subjects with small infarcts <5 mL, technical reasons, and since there may be a selection bias based on the ability to tolerate an acute MRI. Moreover, the use of FLAIR ratio is not applicable to centers where MRIs may not be routinely obtained in the acute setting of stroke evaluations.
Finally, although we and others have found an association with FLAIR ratio and HT, our data do not provide sufficient specificity to propose the use of FLAIR ratio to exclude patients for thrombolysis who would otherwise meet current criteria for IV thrombolysis in the 0-4.5 hour time window. On the other hand, in those patients with an unclear time of stroke onset, FLAIR hyperintensity has been proposed as potentially representing a “tissue clock”.30-32 Our data offer a new dimension to the interpretation of this signal, suggesting that FLAIR hyperintensity is associated with BBB breakdown and risk for HT. Further prospective studies are warranted to evaluate this relationship and determine whether it may be used to guide treatment decisions.
Conclusion
We report a novel relationship between acute FR and MMP-9 levels. This finding in combination with the association with HT suggests that acute FR may be a bedside radiographic marker that is associated with BBB integrity. Future studies comparing FLAIR hyperintensity to other markers of BBB integrity such as HARM (hyperintense acute reperfusion marker)33 or evaluating the prognostic value of FR in predicting symptomatic hemorrhagic transformation are warranted.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported in part by NIH 1K23NS076597 (W.T.K.). The original enrollment of the subjects and collection of the samples was supported by NIH 5P50NS05134307 (K.L.F.).
Footnotes
Disclosures
Dr. Lorenzano discloses a consultant relationship with Boehringer Ingelheim. Drs. Sheth and Kimberly disclose a research grant (GAMES-RP trial) from Remedy Pharmaceuticals, Inc.
Authorship contributions
R.J. and W.T.K. conceived of the study. R.J., T.W.K.B., L.P. and S.L. performed the imaging and plasma analysis. R.J. and W.T.K. performed statistical analyses. R.J. and W.T.K. wrote the manuscript. T.W.K.B, L.P., S.L., K.L.F. and K.N.S. critically edited the manuscript for intellectual content. All aspects of the study were supervised by W.T.K.
References
- 1.Cho AH, Kim JS, Kim SJ, Yun SC, Choi CG, Kim HR, et al. Focal fluid-attenuated inversion recovery hyperintensity within acute diffusion-weighted imaging lesions is associated with symptomatic intracerebral hemorrhage after thrombolysis. Stroke. 2008;39:3424–3426. doi: 10.1161/STROKEAHA.108.516740. [DOI] [PubMed] [Google Scholar]
- 2.Chung JW, Kim KJ, Noh WY, Jang MS, Yang MH, Han MK, et al. Validation of flair hyperintense lesions as imaging biomarkers to predict the outcome of acute stroke after intra-arterial thrombolysis following intravenous tissue plasminogen activator. Cerebrovasc Dis. 2013;35:461–468. doi: 10.1159/000350201. [DOI] [PubMed] [Google Scholar]
- 3.Kufner A, Galinovic I, Brunecker P, Cheng B, Thomalla G, Gerloff C, et al. Early infarct flair hyperintensity is associated with increased hemorrhagic transformation after thrombolysis. Eur J Neurol. 2013;20:281–285. doi: 10.1111/j.1468-1331.2012.03841.x. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Costello C, Christensen S, Ebinger M, Parsons MW, Desmond PM, et al. Fluid-attenuated inversion recovery hyperintensity in acute ischemic stroke may not predict hemorrhagic transformation. Cerebrovasc Dis. 2011;32:401–405. doi: 10.1159/000331467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayata C, Ropper AH. Ischaemic brain oedema. J Clin Neurosci. 2002;9:113–124. doi: 10.1054/jocn.2001.1031. [DOI] [PubMed] [Google Scholar]
- 6.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 8.Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, et al. Association between tpa therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 9.Inzitari D, Giusti B, Nencini P, Gori AM, Nesi M, Palumbo V, et al. Mmp9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death. Stroke. 2013;44:2901–2903. doi: 10.1161/STROKEAHA.113.002274. [DOI] [PubMed] [Google Scholar]
- 10.Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2767. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- 11.Kimberly WT, Battey TW, Pham L, Wu O, Yoo AJ, Furie KL, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2013 doi: 10.1007/s12028-013-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 13.Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized mri stroke protocol: Comparison with ct in hyperacute intracerebral hemorrhage. Stroke. 1999;30:765–768. doi: 10.1161/01.str.30.4.765. [DOI] [PubMed] [Google Scholar]
- 14.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 15.Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: Influence of different therapies. Stroke. 2003;34:2165–2170. doi: 10.1161/01.STR.0000088062.86084.F2. [DOI] [PubMed] [Google Scholar]
- 16.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with bb-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, et al. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–1676. doi: 10.1161/01.STR.0000131656.47979.39. [DOI] [PubMed] [Google Scholar]
- 19.Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke. 2006;37:556–561. doi: 10.1161/01.STR.0000196942.84707.71. [DOI] [PubMed] [Google Scholar]
- 20.Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: A review of the risk factors. Cerebrovasc Dis. 2007;24:1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 21.Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: A focused review. J Cereb Blood Flow Metab. 2012;32:1699–1717. doi: 10.1038/jcbfm.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grohn OH, Kettunen MI, Penttonen M, Oja JM, van Zijl PC, Kauppinen RA. Graded reduction of cerebral blood flow in rat as detected by the nuclear magnetic resonance relaxation time t2: A theoretical and experimental approach. J Cereb Blood Flow Metab. 2000;20:316–326. doi: 10.1097/00004647-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen nmr relaxation times and relaxation mechanisms from 1-100 mhz: Dependence on tissue type, nmr frequency, temperature, species, excision, and age. Med Phys. 1984;11:425–448. doi: 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]
- 25.Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, et al. Serial mri after transient focal cerebral ischemia in rats: Dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–1972. doi: 10.1161/01.str.31.8.1965. discussion 1972-1963. [DOI] [PubMed] [Google Scholar]
- 26.Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63:52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- 27.Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, et al. Does inhibiting sur1 complement rt-pa in cerebral ischemia? Ann N Y Acad Sci. 2012;1268:95–107. doi: 10.1111/j.1749-6632.2012.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol. 2012;72:799–806. doi: 10.1002/ana.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler A, Ebinger M, Fiebach JB, Audebert HJ, Leistner S. Judgment of flair signal change in dwi-flair mismatch determination is a challenge to clinicians. J Neurol. 2012;259:971–973. doi: 10.1007/s00415-011-6284-6. [DOI] [PubMed] [Google Scholar]
- 30.Thomalla G, Rossbach P, Rosenkranz M, Siemonsen S, Krutzelmann A, Fiehler J, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–732. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 31.Cho AH, Sohn SI, Han MK, Lee DH, Kim JS, Choi CG, et al. Safety and efficacy of mri-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovasc Dis. 2008;25:572–579. doi: 10.1159/000132204. [DOI] [PubMed] [Google Scholar]
- 32.Ebinger M, Ostwaldt AC, Galinovic I, Rozanski M, Brunecker P, Nolte CH, et al. Clinical and radiological courses do not differ between fluid-attenuated inversion recovery-positive and negative patients with stroke after thrombolysis. Stroke. 2010;41:1823–1825. doi: 10.1161/STROKEAHA.110.583971. [DOI] [PubMed] [Google Scholar]
- 33.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


