Abstract
Autoantibodies are a hallmark in the diagnosis of many systemic autoimmune rheumatic diseases (SARD) including idiopathic inflammatory myopathies (IIM). Based on their specificity, autoantibodies in IIM are grouped into myositis specific (MSA) and myositis associated autoantibodies (MAA). Among the MSA, autoantibodies against aminoacyl-tRNA synthetases (ARS) represent the most common antibodies and can be detected in 25–35 % of patients. The presence of ARS and other autoantibodies have become a key feature for classification and diagnosis of IIM and are increasingly used to define clinically distinguishable IIM subsets. For example, anti-ARS autoantibodies are the key features of what has become known as anti-synthetase syndrome (aSS), characterized by multiple organ involvement, primarily interstitial lung disease, often accompanied by myositis, non-erosive arthritis, Raynaud’s phenomenon, fever, and “mechanic’s hands”. Autoantibodies directed to eight different ARS have been described: Jo-1 (histidyl), PL-7 (threonyl), PL-12 (alanyl), OJ (isoleucyl), EJ (glycyl), KS (asparaginyl), Zo (phenylalanyl) and Ha (tyrosyl). Each anti-ARS antibody seems to define a distinctive clinical phenotype. Although several research methods and commercial tests are available, routine testing for anti-ARS autoantibodies (other than anti-Jo-1/histidyl-tRNA synthetase) is not widely available, sometimes leading to delays in diagnosis and poor disease outcomes.
Keywords: autoantibodies, myositis, immunoassay, Jo-1, synthetase
1. Introduction
Autoantibodies are a hallmark in the diagnosis of many systemic autoimmune rheumatic diseases (SARD) including idiopathic inflammatory myopathies (IIM) [1,2]. These autoantibodies are typically directed to intracellular proteins, including nuclear and cytoplasmic antigens, and based on their specificity, autoantibodies in IIM can be grouped into myositis specific (MSA) and myositis associated autoantibodies (MAA) [3,4]. The presence of MSA and MAA has become a key feature for classification and diagnosis of IIM and are increasingly used to define clinically distinguishable IIM subsets. Among the MSA, autoantibodies against aminoacyl-tRNA synthetases (ARS) were detected in 25–35 % of IIM patients [5]. Other autoantibodies in IIM are directed to the signal recognition particle (SRP), 3-hydroxy-3-methylglutaryl coenzyme (HMGCR), chromodomain helicase DNA binding protein 4 (Mi-2), SAE/small ubiquitin-related modifier (SUMO-1), MJ/ nuclear matrix protein 2 (NXP2), melanoma differentiation-associated gene 5 (MDA5)/clinically amyopathic dermatomyositis p140 (CADM-140), and transcription intermediary factor (TIF)1-gamma (p155/140)[1,2]. The nomenclature of anti-ARS, like many other autoantibodies, is primarily based on the initials or the name of the index patient [6]. Anti-Jo-1 antibody is the most common, predominantly found in 15–30 % of patients with polymyositis (PM) and in 60–70 % of those with interstitial lung disease (ILD) [6]. Autoantibodies directed towards other ARS are less common, each reaching less than 5% prevalence in IIM. This chapter on the clinical and serological aspects of IIM is focused on ARS, including the biochemical properties and the current detection methods.
2. Clinical aspects of the anti-synthetase syndrome
Earlier studies only found anti-ARS autoantibodies in patients with IIM, but not in other SARD, and it was concluded that anti-ARS autoantibodies are myositis specific. Later on, it became evident that anti-ARS autoantibodies characterize their own clinical IIM phenotype that has become known as the anti-Synthetase Syndrome (aSS) and can sometimes occur as an overlap syndrome with other autoimmune diseases. Histological studies suggested that the aSS is a separate disease entity within the spectrum of IIM (reviewed in [4]). Myopathological changes in the aSS including perimysial connective tissue fragmentation and inflammation and muscle fiber pathology in neighboring perifascicular regions have been documented. Anti-ARS autoantibodies are the hallmarks of the aSS, which is characterized by multiple organ involvement, primarily ILD, and is often accompanied by myositis, non-erosive arthritis, Raynaud’s phenomenon, “mechanic’s hands”, skin rashes, sicca syndrome and constitutional symptoms, such as fever. Besides the clear nosographic classification, diagnosis and management of aSS are still challenging due to often masked and/or non-specific symptoms at the disease onset [7]. Each anti-ARS seems to be associated with heterogeneous disease expression and severity [8], in which lung and joint involvement could be prominent at early disease stages. Disease progression and prognosis are predominantly affected by lung involvement and myositis may remain on a subclinical level in a significant number of patients in the non-Jo-1 groups [4]. In idiopathic interstitial pneumonias, anti-ARS autoantibodies have been reported in about 7 % of patients, thus contributing to the definition of “idiopathic” ILD. Whether such autoantibodies could also have a predictive value for immune mediated ILD has to be further elucidated [9] . The vast majority of anti-ARS patients have ILD, whereas it is estimated that one- to two-thirds of patients with myositis and ILD are positive for any anti-ARS antibody. Anti-ARS autoantibodies are rarer in dermatomyositis (DM) and juvenile PM, DM and in other SARD [4]. Anti-ARS can also be associated with necrotizing myopathy (anti-PL-12 autoantibodies) or pericarditis (anti-PL-7 autoantibodies) [10,11] . Recently studies have indicated that patients with anti-ARS autoantibodies other than those directed to Jo-1 have a different clinical outcome [8,12–14]. Patients with anti-PL-7 and anti-PL-12 autoantibodies frequently have ILD [15], gastrointestinal manifestations and less frequently have myositis compared to anti-Jo-1 positive patients [16] . It has been speculated that this might be attributed to the delayed detection due to lack of routine testing for those autoantibodies [4]. Recently, the importance of making a diagnosis based on anti-ARS serology has been illustrated by a comprehensive case report describing a 21-year-old man with fever, arthralgia and pulmonary infiltrates [17]. Since another recent case report of two anti-OJ positive patients did not confirm the poor prognosis of these patients [18], future studies are needed to verify these observations. Autoantibodies have been shown to be present in the pre-clinical phase and can predict the outcome of a certain diseases [19] and this is true for the anti-ARS as well [20]. Larger studies are needed to understand the utility of anti-ARS autoantibodies for patient stratification and risk management of those patients. As shown for systemic sclerosis [21], autoantibodies have the potential to classify patients with a specific clinical phenotype, which might support personalized medicine.
A recent international study of 430 juvenile idiopathic inflammatory myopathy (JIIM) patients emphasized that the clinical and serological spectrum of IIM in children is not a mirror image of adult disease [3]. Like adult IIM, JIIM are also characterized by skeletal muscle weakness, characteristic rashes, and other systemic features. In this study, 68% had a single myositis autoantibody and 32% had no identified myositis autoantibodies. Anti-p155/140 autoantibodies were the most frequent serological subgroup, present in 32% of patients with juvenile dermatomyositis (JDM) or overlap myositis with JDM, followed by anti-MJ autoantibodies, which were seen in 20% of JIIM patients, primarily in JDM. And unlike adult IIM, other MSAs, including anti-synthetase, anti-signal recognition particle (SRP), and anti-Mi-2, were present in only 10% of JIIM. The key conclusion of the study was that juvenile myositis is a heterogeneous group of illnesses that can be classified on the basis of distinct autoantibody phenotypes.
3. Classification Criteria
Classification criteria for IIM date back almost 35 years to initial publications by Medsger et al. [22] to more current criteria proposed by Dalakas and Hohlfeld [23] and Hoogendijk et al. [24] (Table 1). Each set of proposed criteria have advantages and disadvantages, but the emphasis in establishing clinically valid criteria has more recently incorporated MSAs, starting with anti-Jo-1 and the aSS nomenclature (reviewed in [8,25]) and progressing to a wider spectrum of MSA as the basis for meaningful clinical phenotypes, particularly in JIIM [3].
Table 1.
IIM Features Included in Proposed Criteria
| Criteria Proponents | MW | MPT | MBx | EMG | ENZ | CUT | DEF | MSA | NDA | SIS | SCF | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medsger | X | X | X | X | [22] | |||||||
| DeVere & Bradley | X | X | X | X | X | [37] | ||||||
| Bohan & Peter | X | X | X | X | X | X | [26] | |||||
| Dalakas | X | X | X | X | X | X | X | [38] | ||||
| Tanimoto | X | X | X | X | X | X | X | X | X | X | X | [39] |
| Targoff | X | X | X | X | X | X | X | [40] | ||||
| Van der Meulen | X | X | X | X | X | [41] | ||||||
| Dalakas & Hohlfeld | X* | X | X | X | X | X** | [23] | |||||
| Hoogendijk, et al. *** | X | X**** | X | X | X | X | [24] |
Abbreviations: CUT, cutaneous features (V sign, shawl sign, Gottron’s papules, heliotrope); DEF, disease definition (i.e. definite probable, possible); EMG, electromyogram; ENZ, elevated muscle enzymes (i.e. creatine kinase, aldolase); MBx, inflammation observed in muscle biopsy; MPT, muscle pain & tenderness; MSA, myositis specific autoantibody (i.e. Jo-1); MW, proximal muscle weakness; NDA, non-destructive arthritis/arthralgia; Ref, references; SCF, sub-classification (i.e. dermatomyositis, inclusion body myositis, polymyositis, cancer-associated, unspecified, isolated, overlap, amyopathic); SIS, signs of systemic inflammation (i.e. fever, elevated ESR/CRP).
myopathic muscle weakness based on a number of exclusions and inclusions: affecting proximal muscles more than distal ones; sparing eye and facial muscles; characterised by a subacute onset (weeks to months); rapid progression in patients who have no family history of neuromuscular disease; no exposure to myotoxic drugs or toxins; no signs of biochemical muscle disease; pattern distinct from inclusion-body myositis
amyopathic dermatomyositis a separate category
detailed inclusion and exclusion criteria
detailed pathological features
For many years, the Bohan & Peter criteria [26] were the touchstone, but it was known that this schema had limitations because it was observer dependent (subjective), based on experience in a single institution, the rashes of DM were not specified, and no direction was provided on how to rule out other myopathies. In a study where the specialist consultant diagnosis was considered the gold standard, the 2003 criteria of Dalakas agreed best with specialist consultant diagnosis and the criteria of Bohan and Peter demonstrated very poor specificity [27]. Prospective studies are required to develop improved classification criteria.
4. Biological function and biochemical properties of synthetases
Aminoacyl-tRNA synthetases catalyze the ATP-dependent binding of a single amino acid to its specific tRNA during protein synthesis. Autoantibodies to Jo-1 (histidyl), PL-7 (threonyl), PL-12 (alanyl), OJ (isoleucyl), EJ (glycyl), KS (asparaginyl), Zo (phenylalanyl) and Ha (tyrosyl) have been described [4]. Although the biological significance remains unknown, many of the anti-ARS autoantibodies have been shown to inhibit the function of their target autoantigen in vitro [28].
5. Co-existence of anti-ARS and anti-Ro52 autoantibodies
Of high interest, the majority of IIM patients, especially those with the aSS also have anti-Ro52/TRIM21 autoantibodies [29]. Ro52, also known as TRIM21, is an E3 ligase that interacts with many proteins [29]. Patients with both anti-Ro52/TRIM21 and anti-ARS displayed a different clinical phenotype characterized by severe myositis and joint impairment. Moreover, the coexistence of anti-Ro52/TRIM21 autoantibodies seems to be associated with an increased risk of cancer [30].
6. Detection methods
Anti-ARS autoantibodies can be identified by several methods. Initially, these autoantibodies were defined by immunoprecipitation in which the anti-ARS was found to co-immunoprecipitate the synthetase along with the isoaccepting tRNA, and confirmation thus included separate protein and RNA immunoprecipitation approaches [28]. Many patients also have a strongly positive cytoplasmic staining pattern in indirect immunofluorescence (IIF). However, with the availability of novel methods for the detection of anti-ARS it was recognized that the IIF alone significantly lacks sensitivity [31]. While anti-Jo-1 autoantibody testing for clinical purposes is available using ELISA methods, addressable laser bead immunoassay (ALBIA) [32] and chemiluminescent immunoassay (CIA) [33], there is little information about the comparative sensitivity, specificity and consistency of these assays compared to the gold standard immunoprecipitation assays (Table 2). Also, autoantibodies to other synthetases are rarely detected by these assays [4], so protein and RNA immunoprecipitation remains the only commercial assay available that allows for a full enumeration of all the anti-ARS and other myositis autoantibodies. Line immunoassays (LIA) [7,34,35] became a popular tool to detect many MSA and MAA as a panel. Recently, a screening ELISA (research use only, RG7840RRG 7840R, MBL, Japan) has been developed for the detection of anti-ARS autoantibodies. The antigen composition of this assay contains Jo-1, PL-7, PL-12, EJ and KS.
Table 2.
Overview of molecular targets of anti-synthetase autoantibodies and available testing
| Autoantigen name | Biochemical aminoacyl-tRNA synthetase target | Prevalence in myositis (%) | A-1 | A-2 | A-3 | A-4 | A-5 | IPP | IP |
|---|---|---|---|---|---|---|---|---|---|
| Jo-1 | Histidyl | 15–30 | x | x | x | x | x | x | x |
| PL-7 | Threonyl | 2–5 | x | x | x | x | x | x | x |
| PL-12 | Alanyl | 2–5 | x | x | x | x | x | x | x |
| EJ | Glycyl | 2–5 | x | x | x | x | x | x | |
| OJ | Isoleucyl | <2 | x | x | x | x | x | ||
| KS | Asparaginyl | <2 | x | ||||||
| Ha | Tyrosyl | <1 | |||||||
| Zo | Phenylalanyl | <1 |
A-1 = EUROLINE Myositis Profile 3 (IgG) (Euroimmun, Germany)
A-2 = Myositis plus (Orgentec, Germany);
A-3 = Anti-Synthetase ELISA Kit (MBL, Japan);
A-4 = ImmcoStripe Myositis (Immco, US);
A-5 = Polymyositis/Scleroderma12 IgG BlueDot (D-Tek, Belgium);
IPP = Protein and RNA immunoprecipitation (commercially available at http://omrf.org/research-faculty/core-facilities/myositis-testing/)
IP = Protein immunoprecipitation (commercially available at http://www.rdlinc.com/rdlinc/wp-content/uploads/2013/01/MyoMarker_Panels_web2012.pdf
x= antigen included
However, at the time of this writing no test for the detection of anti-ARS (other than Jo-1) has been cleared by the Food and Drug Administrative (FDA). In the USA, few reference laboratories offer autoantibody profiling for MSA and MAA, including but not limited to Oklahoma Medical Research Foundation clinical immunology laboratory (Oklahoma City, Oklahoma) and RDL Reference laboratory (Los Angeles, California). Most of their testing is based on IP which involves a long turnaround time (>2 weeks), Therefore, there is a strong need for cost-effective commercial assays for the detection of anti-ARS autoantibodies [8]. Ideally, those tests should be based on automated systems that allow for random access testing and have high sensitivity and specificity.
Autoantibodies to ARS are only seen in a minority of patients with IIM, which is by itself a rare condition [36]. Therefore, the development and application of immunoassays for the detection of these autoantibodies presents an economic challenge. An additional hurdle to overcome is the availability of control samples for development, manufacturing and for the quality control of such assays.
7. Concluding remarks
Anti-ARS autoantibodies are a defining feature in the diagnosis and prognosis of the aSS. Except for anti-Jo-1, most anti-ARS autoantibodies are rarely tested in routine diagnosis due to the lack of reliable, cost-effective, easy to use and regulatory body approved diagnostic assays.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. We thank Drs. Ejaz Shamim and Mark Gourley for useful critical comments on the manuscript, and Dr. Minoru Satoh for supplying Figures 1B and 1C.
Figure 1.
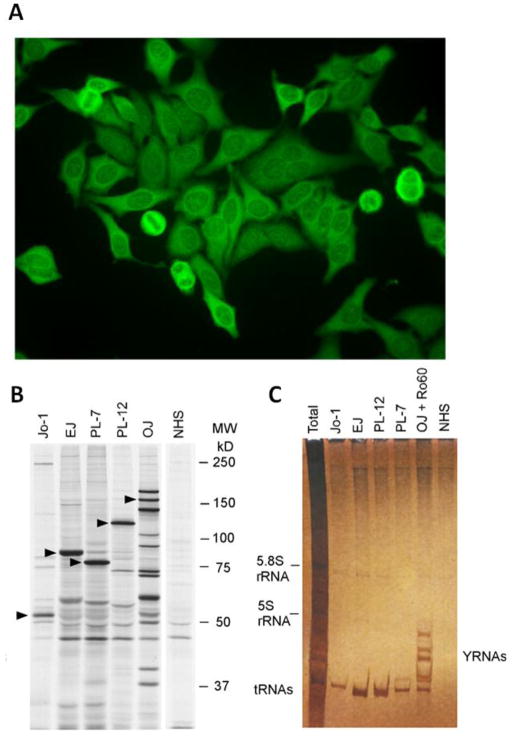
Detection of anti-ARS autoantibodies. A.) The classical indirect immunofluorescence (IIF) pattern of an anti-Jo-1 autoantibody positive sample is characterized by diffuse cytoplasmic staining on HEp-2 cells. Autoantibodies to other synthetases can show similar staining patterns. To differentiate the pattern from other cytoplasmic patterns (i.e. anti-ribososmal P autoantibodies), the absence of nucleolar staining is an important discriminator. It should be noted that less than half of the sera that have identifiable anti-Jo-1 will demonstrate this typical IIF pattern. The specific proteins identifying each autoantibody are marked with triangles. B.) Characterization of various anti-ARS autoantibodies (anti-Jo-1, PL-7, PL-12, OJ, EJ) by protein immunoprecipitation using 35S-methionine-labeled K562 cell lysates, SDS-PAGE and autoradiography is shown (courtesy of Dr. Minoru Satoh). C.) Immunoprecipitation profile of cytoplasmic RNAs using phenol/chloroform extraction and urea-PAGE followed by silver staining demonstrating the typical tRNAs associated with each of the anti-ARS autoantibodies (courtesy of Dr. Minoru Satoh).
Abbreviations
- ALBIA
addressable laser bead immunoassay
- ARS
aminoacyl-tRNA synthetases
- aSS
anti-synthetase syndrome
- IIF
indirect immunofluorescence
- DM
dermatomyositis
- IIM
idiopathic inflammatory myopathies
- IP
immunoprecipitation of antigens as assessed by protein electrophoresis
- IPP
immunoprecipitation of antigens as assessed by protein and RNA electrophoresis
- JDM
juvenile dermatomyositis
- JIIM
juvenile idiopathic inflammatory myopathy
- LIA
line immunoassay
- MAA
myositis associated autoantibodies
- MSA
myositis specific autoantibodies
- aSS
anti-synthetase syndrome
- PM
polymyositis
- SARD
systemic autoimmune rheumatic disease
- SSc
systemic sclerosis
Footnotes
Competing interests
M. Mahler, is employed at INOVA Diagnostics a manufacturer of autoantibody kits and assays. M.J. Fritzler is a paid consultant of ImmunoConcepts Inc. and INOVA Diagnostics and has received diagnostics gifts in kind from Euroimmun.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mammen AL. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nat Rev Neurol. 2011;7:343–354. doi: 10.1038/nrneurol.2011.63. [DOI] [PubMed] [Google Scholar]
- 2.Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209. doi: 10.1186/ar3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rider LG, Shah M, Mamyrova G, Huber AM, Rice MM, Targoff IN, et al. The Myositis Autoantibody Phenotypes of the Juvenile Idiopathic Inflammatory Myopathies. Medicine (Baltimore ) 2013;92:223–243. doi: 10.1097/MD.0b013e31829d08f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghirardello A, Bassi N, Palma L, Borella E, Domeneghetti M, Punzi L, et al. Autoantibodies in polymyositis and dermatomyositis. Curr Rheumatol Rep. 2013;15:335–0335. doi: 10.1007/s11926-013-0335-1. [DOI] [PubMed] [Google Scholar]
- 5.Koenig M, Fritzler MJ, Targoff IN, Troyanov Y, Senecal JL. Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights into clinical features and outcomes. Arthritis Res Ther. 2007;9:R78. doi: 10.1186/ar2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahler M, Fritzler MJ. Epitope specificity and significance in systemic autoimmune diseases. Ann N Y Acad Sci. 2010;1183:267–287. doi: 10.1111/j.1749-6632.2009.05127.x. [DOI] [PubMed] [Google Scholar]
- 7.Ghirardello A, Bendo R, Rampudda ME, Bassi N, Zampieri S, Doria A. Commercial blot assays in the diagnosis of systemic rheumatic diseases. Autoimmun Rev. 2009 doi: 10.1016/j.autrev.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-201800. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takato H, Waseda Y, Watanabe S, Inuzuka K, Katayama N, Ichikawa Y, et al. Pulmonary manifestations of anti-ARS antibody positive interstitial pneumonia--with or without PM/DM. Respir Med. 2013;107:128–133. doi: 10.1016/j.rmed.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Labirua-Iturburu A, Selva-O'Callaghan A, Vincze M, Danko K, Vencovsky J, Fisher B, et al. Anti-PL-7 (Anti-Threonyl-tRNA Synthetase) Antisynthetase Syndrome: Clinical Manifestations in a Series of Patients From a European Multicenter Study (EUMYONET) and Review of the Literature. Medicine (Baltimore) 2012 doi: 10.1097/MD.0b013e318260977c. [DOI] [PubMed] [Google Scholar]
- 11.Mehndiratta P, Mehta S, Manjila SV, Kammer GM, Cohen ML, Preston DC. Isolated necrotizing myopathy associated with ANTI-PL12 antibody. Muscle Nerve. 2012;46:282–286. doi: 10.1002/mus.23383. [DOI] [PubMed] [Google Scholar]
- 12.Burford B, Gentry-Maharaj A, Graham R, Allen D, Pedersen JW, Nudelman AS, et al. Autoantibodies to MUC1 glycopeptides cannot be used as a screening assay for early detection of breast, ovarian, lung or pancreatic cancer. Br J Cancer. 2013:10. doi: 10.1038/bjc.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruellas MG, Viana VS, Levy-Neto M, de Souza FH, Shinjo SK. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics (Sao Paulo) 2013;68:909–914. doi: 10.6061/clinics/2013(07)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri M, Sahn SA, Oddis CV, Gharib SL, Christopher-Stine L, Danoff SK, et al. Clinical profile of anti-PL-12 autoantibody. Cohort study and review of the literature. Chest. 2009;135:1550–1556. doi: 10.1378/chest.08-2233. [DOI] [PubMed] [Google Scholar]
- 15.Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C, et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev. 2012;11:739–745. doi: 10.1016/j.autrev.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Hervier B, Devilliers H, Stanciu R, Meyer A, Uzunhan Y, Masseau A, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev. 2012;12:210–217. doi: 10.1016/j.autrev.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Christopher-Stine L, Robinson DR, Wu CC, Mark EJ. Case records of the Massachusetts General Hospital. Case 37–2012. A 21-year-old man with fevers, arthralgias, and pulmonary infiltrates. N Engl J Med. 2012;367:2134–2146. doi: 10.1056/NEJMcpc1208147. [DOI] [PubMed] [Google Scholar]
- 18.Kunimasa K, Arita M, Nakazawa T, Tanaka M, Tsubouchi K, Konishi S, et al. The clinical characteristics of two anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibody-positive interstitial lung disease patients with polymyositis/dermatomyositis. Intern Med. 2012;51:3405–3410. doi: 10.2169/internalmedicine.51.7452. [DOI] [PubMed] [Google Scholar]
- 19.Fritzler MJ. Challenges to the use of autoantibodies as predictors of disease onset, diagnosis and outcomes. Autoimmun Rev. 2008;7:616–620. doi: 10.1016/j.autrev.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Miller FW, Waite KA, Biswas T, Plotz PH. The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc Natl Acad Sci USA. 1990;87:9933–9937. doi: 10.1073/pnas.87.24.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehra S, Walker J, Patterson K, Fritzler MJ. Autoantibodies in systemic sclerosis. Autoimmun Rev. 2013;12:350–354. doi: 10.1016/j.autrev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Medsger TA, Jr, Dawson WN, Jr, Masi AT. The epidemiology of polymyositis. Am J Med. 1970;48:715–723. doi: 10.1016/s0002-9343(70)80006-7. [DOI] [PubMed] [Google Scholar]
- 23.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 24.Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14:337–345. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Hervier B, Benveniste O. Clinical heterogeneity and outcomes of antisynthetase syndrome. Curr Rheumatol Rep. 2013;15:349–0349. doi: 10.1007/s11926-013-0349-8. [DOI] [PubMed] [Google Scholar]
- 26.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 27.Linklater H, Pipitone N, Rose MR, Norwood F, Campbell R, Salvarani C, et al. Classifying idiopathic inflammatory myopathies: comparing the performance of six existing criteria. Clin Exp Rheumatol. 2013 [PubMed] [Google Scholar]
- 28.Targoff IN, Trieu EP, Miller FW. Reaction of anti-OJ autoantibodies with components of the multi-enzyme complex of aminoacyl-tRNA synthetases in addition to isoleucyl-tRNA synthetase. J Clin Invest. 1993;91:2556–2564. doi: 10.1172/JCI116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632–637. doi: 10.1016/j.autrev.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF, et al. Short-Term and Long-Term Outcome of Anti-Jo1-Positive Patients with Anti-Ro52 Antibody. Semin Arthritis Rheum. 2011 doi: 10.1016/j.semarthrit.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman IE, Peene I, Veys EM, De KF. Detection of specific antinuclear reactivities in patients with negative anti-nuclear antibody immunofluorescence screening tests. Clin Chem. 2002;48:2171–2176. [PubMed] [Google Scholar]
- 32.Fritzler MJ, Fritzler ML. The emergence of multiplexed technologies as diagnostic platforms in systemic autoimmune diseases. Curr Med Chem. 2006;13:2503–2512. doi: 10.2174/092986706778201639. [DOI] [PubMed] [Google Scholar]
- 33.Bentow C, Swart A, Wu J, Seaman A, Manfredi M, Infantino M, et al. Clinical performance evaluation of a novel rapid response chemiluminescent immunoassay for the detection of autoantibodies to extractable nuclear antigens. Clin Chim Acta. 2013 doi: 10.1016/j.cca.2013.05.011. in press. [DOI] [PubMed] [Google Scholar]
- 34.Ronnelid J, Helmers SB, Storfors H, Grip C, Ronnblom L, Larsson KF, et al. Use of a commercial line blot assay as a screening test for autoantibodies in inflammatory myopathies. Autoimmun Rev. 2009;9:58–61. doi: 10.1016/j.autrev.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Ghirardello A, Rampudda M, Ekholm L, Bassi N, Tarricone E, Zampieri S, et al. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology (Oxford) 2010;49:2370–2374. doi: 10.1093/rheumatology/keq281. [DOI] [PubMed] [Google Scholar]
- 36.Bernatsky S, Joseph L, Pineau CA, Belisle P, Boivin JF, Banerjee D, et al. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: age, sex and regional differences. Ann Rheum Dis. 2009;68:1192–1196. doi: 10.1136/ard.2008.093161. [DOI] [PubMed] [Google Scholar]
- 37.DeVere R, Bradley WG. Polymyositis: its presentation, morbidity and mortality. Brain. 1975;98:637–666. doi: 10.1093/brain/98.4.637. [DOI] [PubMed] [Google Scholar]
- 38.Dalakas MC. Polymyositis, dermatomyositis, and inclusion-body myositis. N Engl J Med. 1991;325:1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- 39.Tanimoto K, Nakano K, Kano S, Mori S, Ueki H, Nishitani H, et al. Classification criteria for polymyositis and dermatomyositis. J Rheumatol. 1995;22:668–674. [PubMed] [Google Scholar]
- 40.Targoff IN, Miller FW, Medsger TA, Jr, Oddis CV. Classification criteria for the idiopathic inflammatory myopathies. Curr Opin Rheumatol. 1997;9:527–535. doi: 10.1097/00002281-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Van Der Meulen MF, Bronner IM, Hoogendijk JE, Burger H, Van Venrooij WJ, Voskuyl AE, et al. Polymyositis: An overdiagnosed entity. Neurology. 2003;61:316–321. doi: 10.1212/wnl.61.3.316. [DOI] [PubMed] [Google Scholar]